Biomimetic Sensors for the Senses: Towards Better Understanding of Taste and Odor Sensation
Abstract
:1. Introduction
2. Biological Mechanisms of Taste and Olfaction Sensation
3. Artificial Sensors for Taste and Smell
4. Biomimetic Sensors for Taste and Odor Sensation Mechanisms
5. In Vitro Biosensing Approaches
5.1. Tissue-Based In Vitro Biosensing Approaches
5.2. Cell-Based In Vitro Biosensing Approaches
5.3. Receptor-Based In Vitro Biosensing Approaches
6. In Vivo Biosensing Approaches
7. Conclusions and Prospects
Acknowledgments
Conflicts of Interest
Abbreviations
| ASIC2 | acid-sensing ion channel 2 |
| ATP | adenosine triphosphate |
| ATD | amino-terminal domains |
| CNG channel | cyclic nucleotide gated channel |
| EIS | electrochemical impedance spectroscopy |
| ENaC | epithelial Na channel |
| FET | field effect transistor |
| FEDs | field-effect devices |
| GPCRs | G-protein coupled receptors |
| GRK2 | G-protein-coupled receptor kinase 2 |
| HCNs | hyperpolarization-activated cyclic nucleotide-gated channels |
| HEK-293 | human embryonic kidney 293 |
| hERG | human ether-a-go-go related gene |
| LAPS | light-addressable potentiometric sensor |
| MCF-7 | Michigan cancer foundation (MCF) -7 |
| MEA | microelectrode array |
| MFT | 2-methyl-3-furanthiol |
| MOE | main olfactory epithelium |
| ORs | olfactory receptors |
| ORNs | olfactory receptor neurons |
| PCA | principal component analysis |
| PKD channels | polycystic kidney disease-like channels |
| QCM | quartz crystal microbalance |
| RAMPs | receptor activity-modifying proteins |
| REEPs | receptor expression enhancing proteins |
| RTPs | receptor transporting proteins |
| SAMs | self-assembled monolayers |
| SAW | surface acoustic wave |
| SPR | surface Plasmon resonance |
| TCM | traditional Chinese medicine |
| VFT | Venus flytrap |
| VNO | vomeronasal organ |
References
- Buck, L.; Axel, R. A novel multi gene family may encode odorant receptors-a molecular-basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Kay, L.M.; Stopfer, M. Information processing in the olfactory systems of insects and vertebrates. Semin. Cell Dev. Biol. 2006, 17, 433–442. [Google Scholar] [CrossRef] [PubMed]
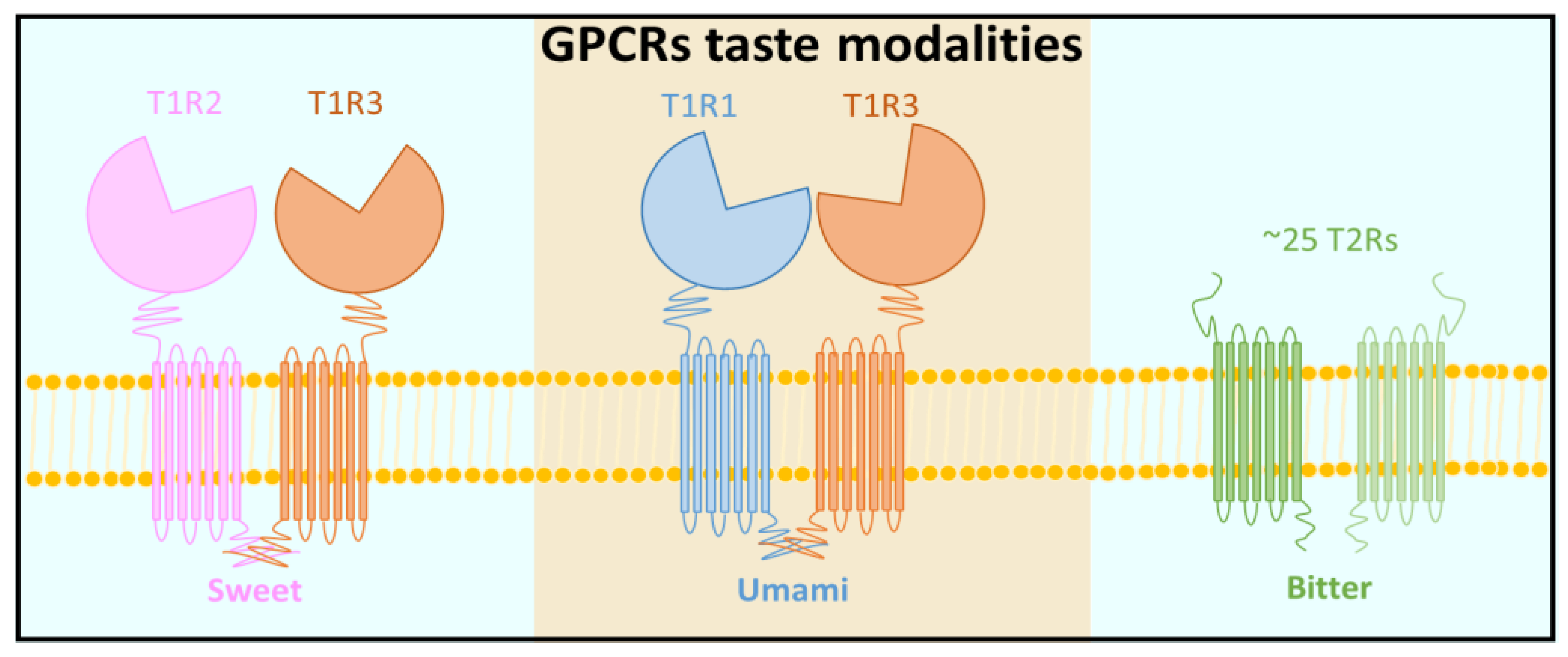
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.P.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef]
- Kim, U.K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Meyerhof, W. Oral and extraoral bitter taste receptors. Results Probl. Cell Differ. 2010, 52, 87–99. [Google Scholar] [PubMed]
- Depoortere, I. Taste receptors of the gut: Emerging roles in health and disease. Gut 2014, 63, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wiener, A.; Shudler, M.; Levit, A.; Niv, M.Y. Bitter DB: A database of bitter compounds. Nucleic Acids Res. 2012, 40, D413–D419. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Chi, Q.; Zhuang, H.; Matsunami, H.; Mainland, J.D. Odor coding by a mammalian receptor repertoire. Sci. Signal. 2009, 2, ra9. [Google Scholar] [CrossRef] [PubMed]
- Di Pizio, A.; Niv, M.Y. Promiscuity and selectivity of bitter molecules and their receptors. Bioorg. Med. Chem. 2015, 23, 4082–4091. [Google Scholar] [CrossRef] [PubMed]
- Di Pizio, A.; Levit, A.; Slutzki, M.; Behrens, M.; Karaman, R.; Niv, M.Y. Comparing Class A GPCRs to bitter taste receptors: Structural motifs, ligand interactions and agonist-to-antagonist ratios. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
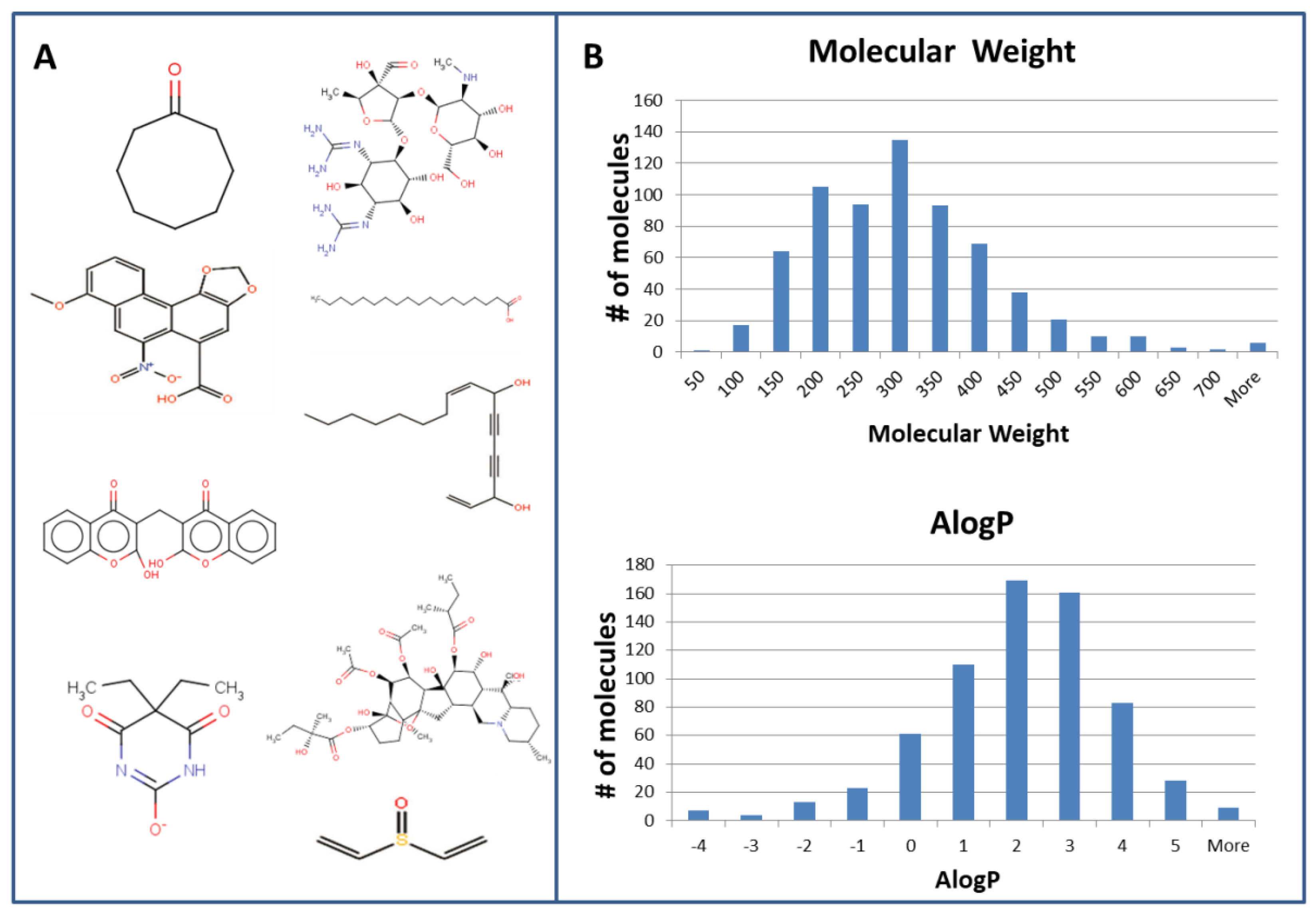
- Nissim, I.; Dagan-Wiener, A.; Niv, M.Y. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y. TCM Database@Taiwan: The world’s largest traditional Chinese medicine database for drug screening in silico. PLoS ONE 2011, 6, e15939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levit, A.; Nowak, S.; Peters, M.; Wiener, A.; Meyerhof, W.; Behrens, M.; Niv, M.Y. The bitter pill: Clinical drugs that activate the human bitter taste receptor TAS2R14. FASBE J. 2014, 28, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.A.; Liggett, S.B.; Munger, S.D. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. 2012, 26, 4827–4831. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.; Irwin, J.J. ZINC 15–ligand discovery for everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.S.; Nissim, I.; Niv, M.Y. Bitterness prediction in-silico: A step towards better drugs. Int. J. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dagan-Wiener, A.; Nissim, I.; Abu, N.B.; Borgonovo, G.; Bassoli, A.; Niv, M.Y. Bitter or not? BitterPredict, a tool for predicting taste from chemical structure. Sci. Rep. 2017, 7, 12074. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, B. Taste reception. Physiol. Rev. 1996, 76, 718–766. [Google Scholar] [PubMed]
- Temussi, P.A. Sweet, bitter and umami receptors: A complex relationship. Trends Biochem. Sci. 2009, 34, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ji, Q.Z.; Liu, Z.; Snyder, L.A.; Benard, L.M.J.; Margolskee, R.F.; Max, M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 2004, 279, 45068–45075. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, S.; Yamamoto, T.; Ueda, T.; Ishida, Y.; Inagaki, A.; Nishigaki, M.; Shimada, S. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J. Neurosci. 2003, 23, 3616–3622. [Google Scholar] [PubMed]
- Stevens, D.R.; Seifert, R.; Bufe, B.; Muller, F.; Kremmer, E.; Gauss, R.; Meyerhof, W.; Kaupp, U.B.; Lindemann, B. Hyperpolarizationactivated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature 2001, 413, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.A.; Dvoryanchikov, G.A.; Chaudhari, N.; Roper, S.D. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J. Neurophysiol. 2004, 92, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; Chen, X.; Hoon, M.A.; Chandrashekar, J.; Guo, W.; Trankner, D.; Ryba, N.J.; Zuker, C.S. The cells and logic for mammalian sour taste detection. Nature 2006, 442, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Inada, H.; Kubota, M.; Zhuang, H.; Tominaga, M.; Matsunami, H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.G.; Rousseau, D.; Duizer, L.; Cockburn, M.; Chiu, W.; Nielsen, D.; El-Sohemy, A. Genetic variation in putative salt taste receptors and salt taste perception in humans. Chem. Senses 2013, 38, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Menuz, K.; Carlson, R. Olfactory Perception: Receptors, Cells, and Circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Baum, M.J.; Brock, O.; Brennan, P.A.; Bakker, J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav. Brain Res. 2009, 200, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Pace, U.; Hanski, E.; Salomon, Y.; Lancet, D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature 1985, 316, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Dryer, L.; Berghard, A. Odorant receptors: A plethora of G-protein-coupled receptors. Trends Pharmacol. Sci. 1999, 20, 413–417. [Google Scholar] [CrossRef]
- Sullivan, S.L.; Ressler, K.; Buck, L.B. Spatial patterning and information coding in the olfactory system. Curr. Opin. Genet. Dev. 1995, 5, 516–523. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, L.C.; Dubourdieu, D. Which impact for beta-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Grosch, W. Evaluation of the key odorants of foods by Dilution Experiment, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.; Roper, J. A complex relationship among chemical concentration, detection threshold, and suprathreshold intensity of bitter compounds. Chem. Senses 2007, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Preissner, S.; Dunkel, M.; Worth, C.L.; Eckert, A.; Preissner, R. SuperSweet—A resource on natural and artificial sweetening agents. Nucleic Acids Res. 2011, 39, D377–D382. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, M.; Schmidt, U.; Struck, S.; Berger, L.; Gruening, B.; Hossbach, J.; Jaeger, I.S.; Effmert, U.; Piechulla, B.; Eriksson, R.; et al. SuperScent—A database of flavors and scents. Nucleic Acids Res. 2009, 37, D291–D294. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ko, H.J.; Park, T.H. Piezoelectric biosensor using olfactory receptor protein expressed in Escherichia coli. Biosens. Bioelectron. 2006, 21, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Fromherz, P.; Offenhausser, A.; Vetter, T.; Weis, J. A neuron-silicon junction: A Retzius cell of the leech on an insulated-gate field effect transistor. Science 1991, 252, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Schütz, S.; Schoning, M.J.; Schroth, P.; Malkocb, Ü.; Weißbecker, B.; Kordos, P.; Lüth, H.; Hummel, H.E. An insect-based biofet as a bioelectronic nose. Sens. Actuators B Chem. 2000, 65, 291–295. [Google Scholar] [CrossRef]
- Huotari, M.J. Biosensing by insect olfactory receptor neurons. Sens. Actuators B Chem. 2000, 71, 212–222. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Advances in surface plasmon resonance biosensor analysis. Curr. Opin. Biotechnol. 2000, 11, 54–61. [Google Scholar] [CrossRef]
- Du, L.P.; Zou, L.; Zhao, L.H.; Huang, L.Q.; Wang, P.; Wu, C.S. Label-free functional assays of chemical receptors using a bioengineered cell-based biosensor with localized extracellular acidification measurement. Biosens. Bioelectron. 2014, 54, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Mainland, J.D.; Keller, A.; Li, Y.R.; Zhou, T.; Trimmer, C.; Snyder, L.L.; Moberly, A.H.; Adipietro, K.A.; Liu, W.L.L.; Zhuang, H.; et al. The Missense of Smell: Functional Variability in the Human Odorant Receptor Repertoire. Nat. Neurosci. 2014, 17, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pizio, A.; Niv, M.Y. Computational Studies of Smell and Taste Receptors. Isr. J. Chem. 2014, 54, 1205–1218. [Google Scholar] [CrossRef]
- Park, T.H. Bioelectronic Nose: Integration of Biotechnology and Nanotechnology; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-8-57-811079-6. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Advances in olfaction-inspired biomaterials applied to bioelectronic noses. Sens. Actuators B Chem. 2018, 257, 511–537. [Google Scholar] [CrossRef]
- Winquist, F.; Wide, P.; Lundström, I. An electronic tongue based on voltammetry. Anal. Chim. Acta 1997, 357, 21–31. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Peris, M. Review: Highlights in recent applications of electronic tongues in food analysis. Anal. Chim. Acta 2010, 665, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Krantz-Rülcker, C.; Stenberg, M.; Winquist, F.; Lundström, I. Electronic tongues for environmental monitoring based on sensor arrays and pattern recognition: A review. Anal. Chim. Acta 2001, 426, 217–226. [Google Scholar] [CrossRef]
- Liu, Q.J.; Wu, C.S.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-based biosensors and their application in biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Gebicki, J.; Kamysz, W. Bioelectronic nose : Current status and perspectives. Biosens. Bioelectron. 2017, 87, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Lee, J.Y.; Ko, H.J.; Park, T.H. Bioelectronic nose : An emerging tool for odor standardization. Trends Biotechnol. 2017, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs function as bitter taste receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef]
- Palmer, R.K.; Long, D.; Brennan, F.; Buber, T.; Bryant, R.; Salemme, F.R. A high throughput in vivo assay for taste quality and palatability. PLoS ONE 2013, 8, e72391. [Google Scholar] [CrossRef] [PubMed]
- Cheled-Shoval, S.; Behrens, M.; Korb, A.; Pizio, A.D.; Meyerhof, W.; Uni, Z.; Niv, M.Y. From cell to beak: In-vitro and in-vivo characterization of chicken bitter taste thresholds. Molecules 2017, 22, 821. [Google Scholar] [CrossRef] [PubMed]
- Bachmanov, A.A.; Tordoff, M.G.; Beauchamp, G.K. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem. Senses 2001, 26, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.T.; Hajnal, A. Male rats show an indifference-avoidance response for increasing concentrations of the artificial sweetener sucralose. Nutr. Res. 2005, 25, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Zhao, H.B. Complex evolutionary history of the vertebrate sweet/umami taste receptor genes. Chin. Sci. Bull. 2013, 58, 2198–2204. [Google Scholar] [CrossRef]
- Du, L.P.; Wu, C.S.; Liu, Q.J.; Huang, L.Q.; Wang, P. Recent advances in olfactory receptor-based biosensors. Biosens. Bioelectron. 2013, 42, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Du, L.P.; Zou, L.; Zhao, L.H.; Huang, L.Q.; Wang, P. Recent advances in taste cell- and receptor-based biosensors. Sens. Actuator. B Chem. 2014, 201, 75–85. [Google Scholar] [CrossRef]
- Freeman, W.J. Simulation of chaotic EEG patterns with a dynamic-model of the olfactory system. Biol. Cybern. 1987, 56, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, P.; Kikkawa, Y.; Winquist, F.; Krantz-Rulcker, C.; Hojer, N.E.; Hayashi, K.; Toko, K.; Lundstrom, I. Comparison of a voltammetric electronic tongue and a lipid membrane taste sensor. Anal. Chim. Acta 2001, 449, 59–68. [Google Scholar] [CrossRef]
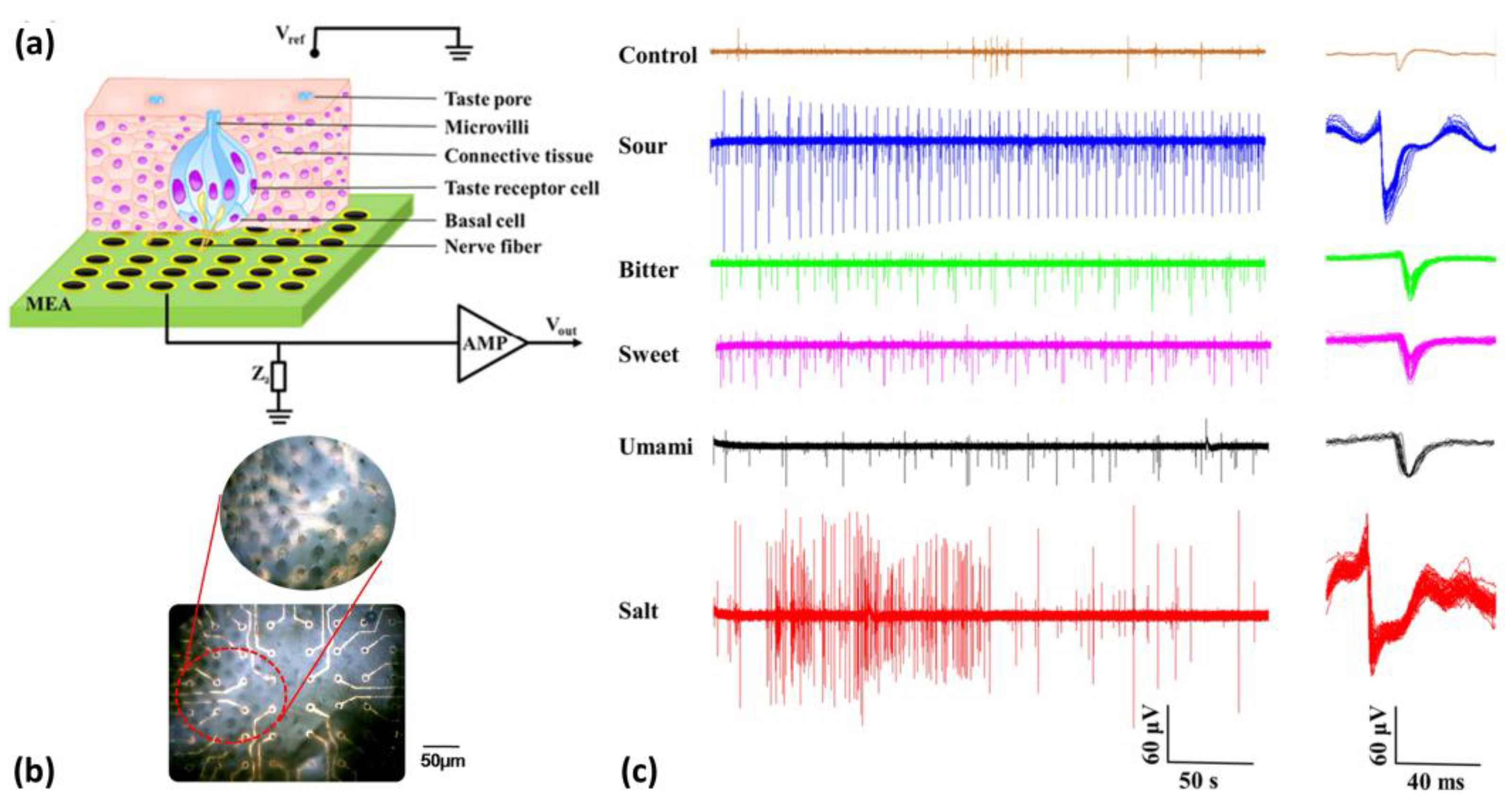
- Liu, Q.J.; Zhang, F.N.; Zhang, D.M.; Hu, N.; Hsia, K.J.; Wang, P. Extracellular potentials recording in intact taste epithelium by microelectrode array for a taste sensor. Biosens. Bioelectron. 2013, 43, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Zhang, F.N.; Zhang, Q.; Lu, Y.L.; Liu, Q.J.; Wang, P. Umami evaluation in taste epithelium on microelectrode array by extracellular. Biochem. Biophys. Res. Commun. 2013, 438, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.J.; Zhang, D.M.; Zhang, F.N.; Zhao, Y.; Hsia, K.J.; Wang, P. Biosensor recording of extracellular potentials in the taste epithelium for bitter detection. Sens. Actuators B Chem. 2013, 176, 497–504. [Google Scholar] [CrossRef]
- Liu, Q.J.; Hu, N.; Ye, W.W.; Cai, H.; Zhang, F.N.; Wang, P. Extracellular recording of spatiotemporal patterning in response to odors in the olfactory epithelium by microelectrode arrays. Biosens. Bioelectron. 2011, 27, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Micholta, E.; Jans, D.; Callewaert, G.; Bartic, C.; Lammertyn, J.; Nicolaï, B. Extracellular recordings from rat olfactory epithelium slices using micro electrode arrays. Sens. Actuators B Chem. 2013, 184, 40–47. [Google Scholar] [CrossRef]
- Liu, Q.J.; Ye, W.W.; Hu, N.; Cai, H.; Yu, H.; Wang, P. Olfactory receptor cells respond to odors in a tissue and semiconductor hybrid neuron chip. Biosens. Bioelectron. 2010, 26, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Schoning, M.J.; Schutz, S.; Schroth, P.; Weissbecker, B.; Steffen, A.; Kordos, P.; Hummel, H.E.; Luth, H. A BioFET on the basis of intact insect antennae. Sens. Actuators B Chem. 1998, 47, 235–238. [Google Scholar] [CrossRef]
- Weibecker, B.; Schütz, S. Insect Olfaction as a Natural Blueprint of Gas Sensors? Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zhang, W.; Li, Y.; Liu, Q.J.; Xu, Y.; Cai, H.; Wang, P. A novel experimental research based on taste cell chips for taste transduction mechanism. Sens. Actuators B Chem. 2008, 131, 24–28. [Google Scholar] [CrossRef]
- Chen, P.H.; Wang, B.Q.; Cheng, G.; Wang, P. Taste receptor cell-based biosensor for taste specific recognition based on temporal firing. Biosens. Bioelectron. 2009, 2515, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Liu, X.D.; Wang, B.Q.; Cheng, G.; Wang, P. A biomimetic taste receptor cell-based biosensor for electrophysiology recording and acidic sensation. Sens. Actuators B Chem. 2009, 139, 576–583. [Google Scholar] [CrossRef]
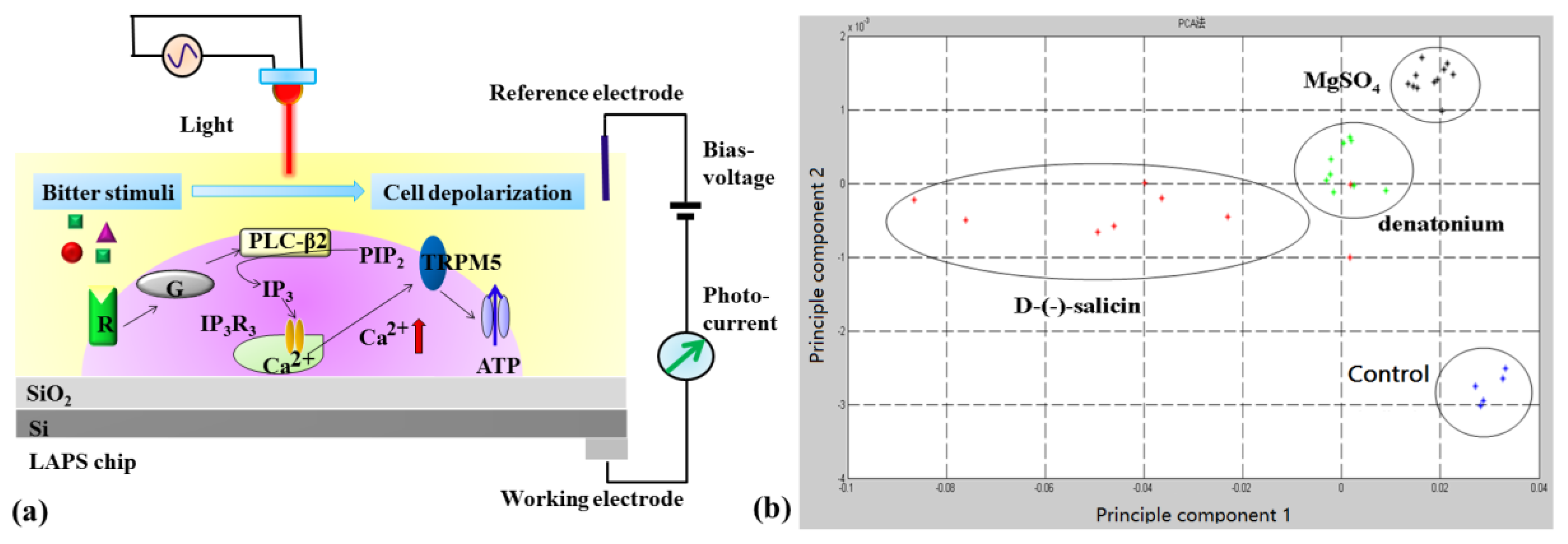
- Wu, C.S.; Du, L.P.; Mao, L.H.; Wang, P. A novel bitter detection biosensor based on light addressable potentiometric sensor. J. Innov. Opt. Health Sci. 2012, 5, 1250008. [Google Scholar] [CrossRef]
- Wu, C.S.; Du, L.P.; Hu, L.; Zhang, W.; Zhao, L.H.; Wang, P. A new acid biosensor for taste transduction based on extracellular recording of PKD channels. IEEE Sens. J. 2012, 12, 3113–3118. [Google Scholar] [CrossRef]
- Wang, T.H.; Hui, G.H.; Deng, S.P. A novel sweet taste cell-based sensor. Biosens. Bioelectron. 2010, 26, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Hui, G.H.; Mi, S.S.; Deng, S.P. Sweet and bitter tastants specific detection by the taste cell based sensor. Biosens. Bioelectron. 2012, 35, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Hui, G.H.; Mi, S.S.; Ye, S.Y.; Jin, J.J.; Chen, Q.Q.; Yu, Z. Tastant quantitative analysis from complex mixtures using taste cell-based sensor and double-layered cascaded series stochastic resonance. Electrochim. Acta 2014, 136, 75–88. [Google Scholar] [CrossRef]
- Xu, J.; Cao, J.; Iguchi, N.; Riethmacher, D.; Huang, L.Q. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 2013, 19, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, J.; Qin, Z.; Hu, N.; Zhou, M.; Huang, L.Q.; Wang, P. Detection of bitterness in vitro by a novel male mouse germ cell-based biosensor. Sens. Acuator. B Chem. 2016, 223, 461–469. [Google Scholar] [CrossRef]
- Liu, Q.J.; Cai, H.; Xu, Y.; Li, Y.; Li, R.; Wang, P. Olfactory cell-based biosensor: A first step towards a neurochip of bioelectronic nose. Biosens. Bioelectron. 2006, 22, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Chen, P.H.; Yu, H.; Liu, Q.J.; Zong, X.L.; Cai, H.; Wang, P. A novel biomimetic olfactory-based biosensor for single olfactory sensory neuron monitoring. Biosens. Bioelectron. 2009, 24, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Du, L.P.; Wu, C.S.; Peng, H.; Zhao, L.H.; Wang, P. Bioengineered olfactory sensor neuron-based biosensor for specific odorant detection. Biosens. Bioelectron. 2013, 40, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.C.; Gao, T.Y.; Liu, J.; Li, Y.Q.; Zhou, J.; Li, J.; Zhou, C.C.; Tu, C.L.; Han, F.; Ye, X.S. The fabrication of an olfactory receptor neuron chip based on planar multi-electrode array and its odor-response analysis. Biosens. Bioelectron. 2010, 26, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, S.B.; Ko, H.J.; Kim, S.J.; Park, T.H. Cell-based olfactory biosensor using microfabricated planar electrode. Biosens. Bioelectron. 2009, 24, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Mitsuno, H.; Kanzaki, R.; Takeuchi, S. Highly sensitive and selective odorant sensor using living cells expressing insect olfactory receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 15340–15344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Ko, H.J.; Park, T.H. Cell-based measurement of odorant molecules using surface plasmon resonance. Enzyme Microb. Technol. 2006, 39, 375–380. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, J.; Ahn, J.H.; Jin, H.J.; Hong, S.; Park, T.H. A peptide receptor-based bioelectronic nose for the real-time determination of seafood quality. Biosens. Bioelectron. 2013, 39, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Z. A piezoelectric biosensor as an olfactory receptor for odour detection: Electronic nose. Biosens. Bioelectron. 1999, 14, 9–18. [Google Scholar] [CrossRef]
- Wu, C.S.; Du, L.P.; Zou, L.; Huang, L.Q.; Wang, P. A biomimetic bitter receptor-based biosensor with high efficiency immobilization and purification using self-assembled aptamers. Analyst 2013, 138, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Park, T.H. Piezoelectric olfactory biosensor: Ligand specificity and dose-dependence of an olfactory receptor expressed in a heterologous cell system. Biosens. Bioelectron. 2005, 20, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Malach, E.; Shaul, M.E.; Peri, I.; Huang, L.Q.; Spielman, A.I.; Seger, R.; Naim, M. Membrane-permeable tastants amplify beta2-adrenergic receptor signaling and delay receptor desensitization via intracellular inhibition of GRK2’s kinase activity. Biochim. Biophys. Acta 2015, 1850, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Pioszak, A.A. Receptor activity-modifying proteins (RAMPs): New insights and roles. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Ilegems, E.; Iwatsuki, K.; Kokrashvili, Z.; Benard, O.; Ninomiya, Y.; Margolskee, R.F. REEP2 enhances sweet receptor function by recruitment to lipid rafts. J. Neurosci. 2010, 30, 13774–13783. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kubota, M.; Roberts, R.W.; Chi, Q.; Matsunami, H. RTP family members induce functional expression of mammalian odorant receptors. Cell 2004, 11, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ugawa, S.; Yamamura, H.; Imaizumi, Y.; Shimada, S. Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J. Neurosci. 2003, 23, 7376–7380. [Google Scholar] [PubMed]
- Wu, C.S.; Du, L.P.; Wang, D.; Wang, L.; Zhao, L.H.; Wang, P. A novel surface acoustic wave-based biosensor for highly sensitive functional assays of olfactory receptors. Biochem. Biophys. Res. Commun. 2011, 407, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Du, L.P.; Wang, D.; Zhao, L.H.; Wang, P. A biomimetic olfactory-based biosensor with high efficiency immobilization of molecular detectors. Biosens. Bioelectron. 2012, 31, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.; Jaffrezic-Renault, N.; Martelet, C.; Zhang, A.D.; Minic-Vidic, J.; Gorojankina, T.; Persuy, M.A.; Pajot-Augy, E.; Salesse, R.; Akimov, V.; et al. A novel detection strategy for odorant molecules based on controlled bioengineering of rat olfactory receptor 17. Biosens. Bioelectron. 2007, 22, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Alfinito, E.; Millithaler, J.F.; Pennetta, C.; Reggiani, L. A single protein based nanobiosensor for odorant recognition. Microelectro. J. 2010, 41, 718–722. [Google Scholar] [CrossRef]
- Alfinito, E.; Millithaler, J.F.; Reggiani, L.; Zine, N.; Jaffrezic-Renault, N. Human olfactory receptor 17–40 as an active part of a nanobiosensor: A microscopic investigation of its electrical properties. RSC Adv. 2011, 1, 123–127. [Google Scholar] [CrossRef]
- Alfinito, E.; Pennetta, C.; Reggiani, L. Olfactory receptor-based smell nanobiosensors: An overview of theoretical and experimental results. Sens. Actuators B Chem. 2010, 146, 554–558. [Google Scholar] [CrossRef]
- Katz, D.B.; Simon, S.A.; Nicolelis, M.A.L. Taste-specific neuronal ensembles in the gustatory cortex of awake rats. J. Neurosci. 2002, 22, 1850–1857. [Google Scholar] [PubMed]
- Ogawa, H.; Sato, M.; Yamashita, S. Multiple sensitivity of chorda tympani fibres of the rat and hamster to gustatory and thermal stimuli. J. Physiol. 1968, 199, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Smith, D.V. Gustatory sensitivities of the hamster’s soft palate. Chem. Senses 1992, 17, 37–51. [Google Scholar] [CrossRef]
- Gazit, I.; Terkel, J. Explosives detection by sniffer dogs following strenuous physical activity. Appl. Anim. Behav. Sci. 2003, 81, 149–161. [Google Scholar] [CrossRef]
- Fjellanger, R.; Andersen, E.; McLean, I.G. A training program for filter-search mine-detection dogs. Int. J. Comp. Psychol. 2002, 15, 278–287. [Google Scholar]
- Ehmann, R.; Boedeker, E.; Friedrich, U.; Sagert, J.; Dippon, J.; Friedel, G.; Walles, T. Canine scent detection in the diagnosis of lung cancer: Revisiting a puzzling phenomenon. Eur. Respir. J. 2012, 39, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J.K.; Moxon, K.A.; Markowitz, R.S.; Nicolelis, M.A.L. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat. Neurosci. 1999, 2, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Nicolelis, M.A.L.; Dimitrov, D.; Carmena, J.M.; Crist, R.; Lehew, G.; Kralik, J.D.; Wise, S.P. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl. Acad. Sci. USA 2003, 100, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Lehmkuhle, M.; Normann, R.; Maynard, E. High-resolution analysis of the spatio-temporal activity patterns in rat OB evoked by enantiomer odors. Chem. Senses 2003, 28, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Rinberg, D.; Koulakov, A.; Gelperin, A. Sparse odor coding in awake behaving mice. J. Neurosci. 2006, 26, 8857–8865. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, U.S.; Bower, J.M. Multiday recordings from OB neurons in awake freely moving rats: Spatially and temporally organized variability in odorant response properties. J. Comput. Neurosci. 1997, 4, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Davison, I.G.; Katz, L.C. Sparse and selective odor coding by mitral/tufted neurons in the main OB. J. Neurosci. 2007, 27, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- You, K.J.; Ham, H.G.; Lee, H.J.; Lang, Y.; Im, C.; Koh, C.S.; Kim, M.Y.; Shin, H.C.; Shin, H.C. Odor discrimination using neural decoding of the main OB in rats. IEEE Trans. Biomed. Eng. 2011, 58, 1208–1215. [Google Scholar] [PubMed]
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004, 7, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Du, L.P.; Zhuang, L.J.; Li, R.; Liu, Q.J.; Wang, P. A novel bioelectronic nose based on brain-machine interface using implanted electrode recording in vivo in olfactory bulb. Biosens. Bioelectron. 2013, 49, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.J.; Guo, T.T.; Cao, D.X.; Ling, L.Q.; Su, K.Q.; Hu, N.; Wang, P. Detection and classification of natural odors with an in vivo bioelectronic nose. Biosens. Bioelectron. 2015, 67, 694–699. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Du, Y.-W.; Huang, L.; Ben-Shoshan Galeczki, Y.; Dagan-Wiener, A.; Naim, M.; Niv, M.Y.; Wang, P. Biomimetic Sensors for the Senses: Towards Better Understanding of Taste and Odor Sensation. Sensors 2017, 17, 2881. https://doi.org/10.3390/s17122881
Wu C, Du Y-W, Huang L, Ben-Shoshan Galeczki Y, Dagan-Wiener A, Naim M, Niv MY, Wang P. Biomimetic Sensors for the Senses: Towards Better Understanding of Taste and Odor Sensation. Sensors. 2017; 17(12):2881. https://doi.org/10.3390/s17122881
Chicago/Turabian StyleWu, Chunsheng, Ya-Wen Du, Liquan Huang, Yaron Ben-Shoshan Galeczki, Ayana Dagan-Wiener, Michael Naim, Masha Y. Niv, and Ping Wang. 2017. "Biomimetic Sensors for the Senses: Towards Better Understanding of Taste and Odor Sensation" Sensors 17, no. 12: 2881. https://doi.org/10.3390/s17122881



