A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
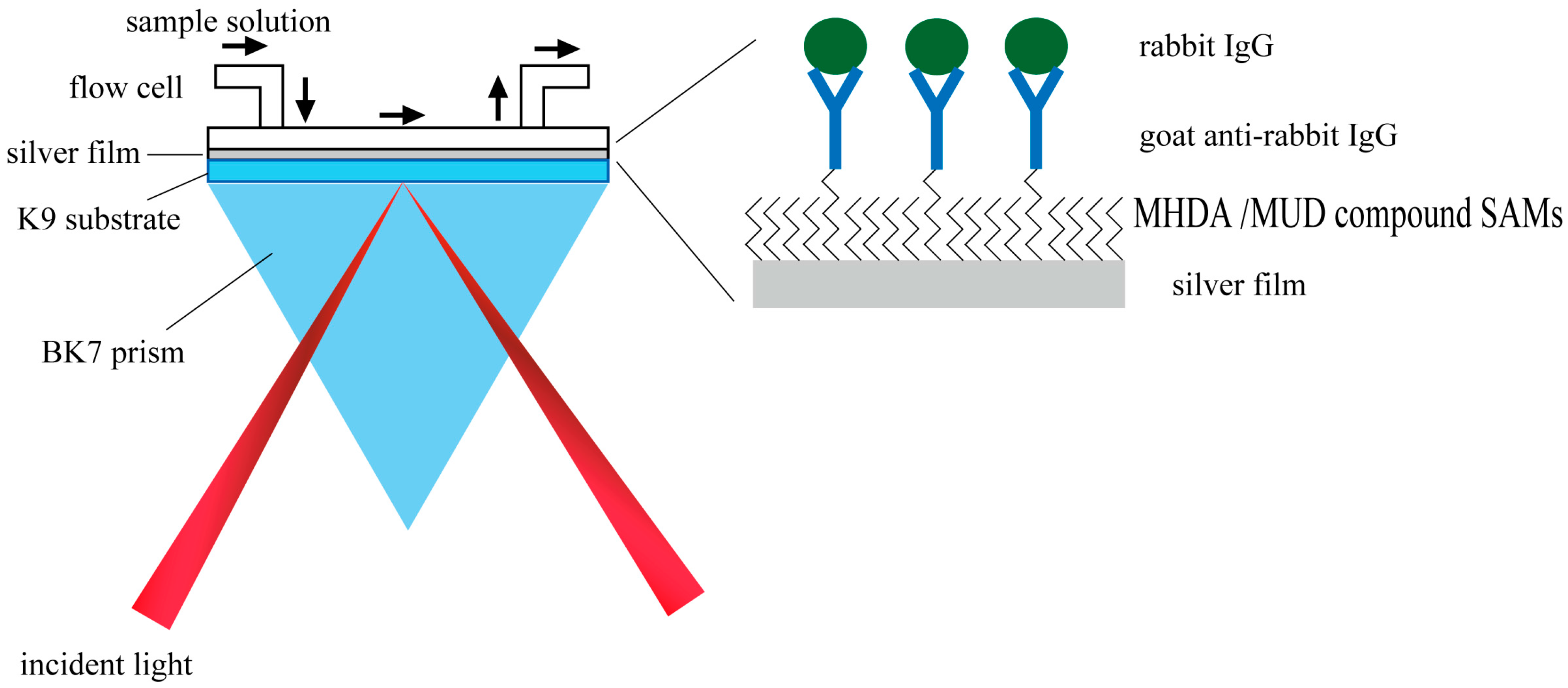
2.2.1. Sputtering Silver or Gold Film on the K9 Substrate
2.2.2. Formation of SAM and Mixed SAMs on the Metal Film
2.2.3. Angular Spectrum Setup of Silver-Based SPR Sensor
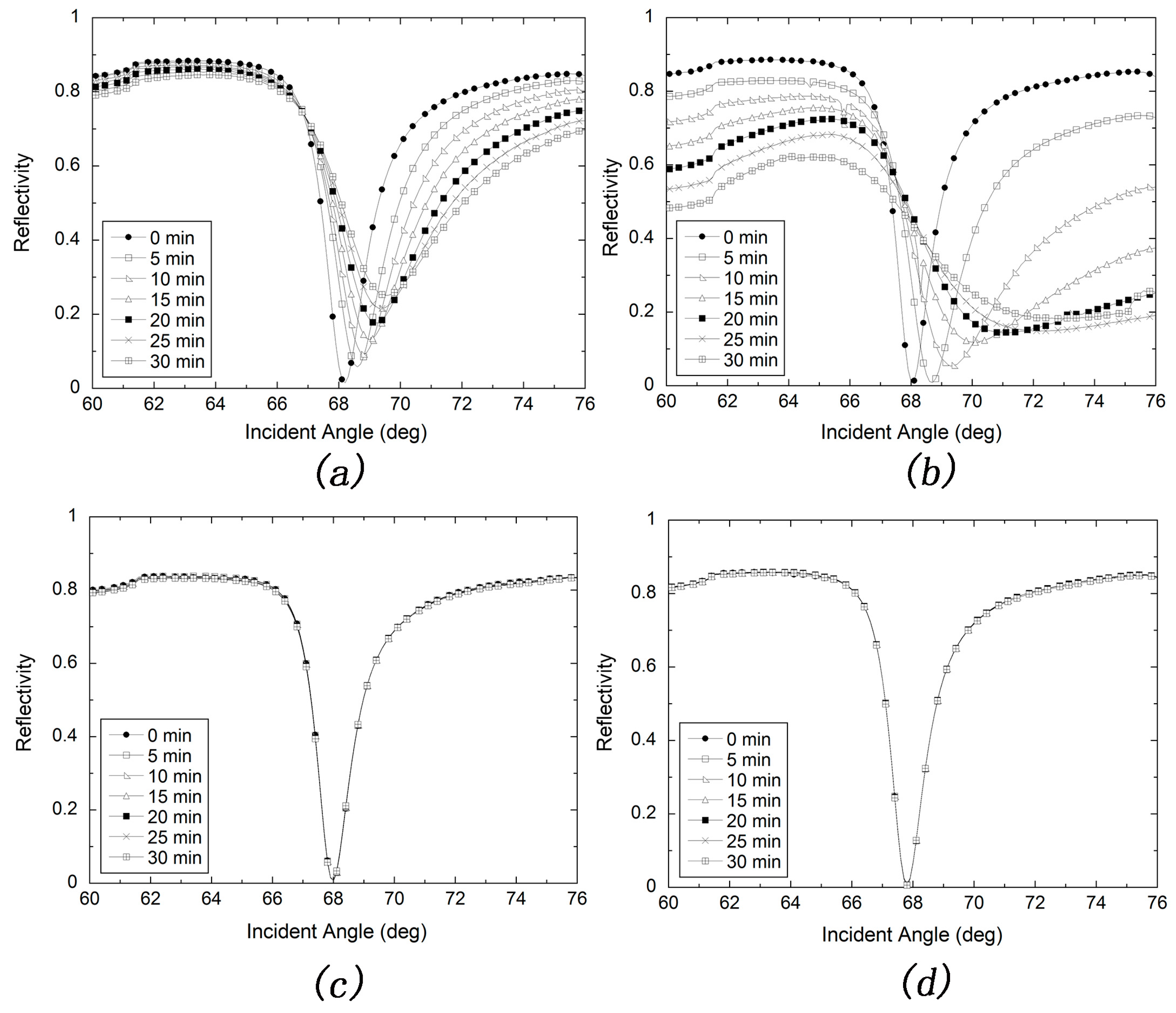
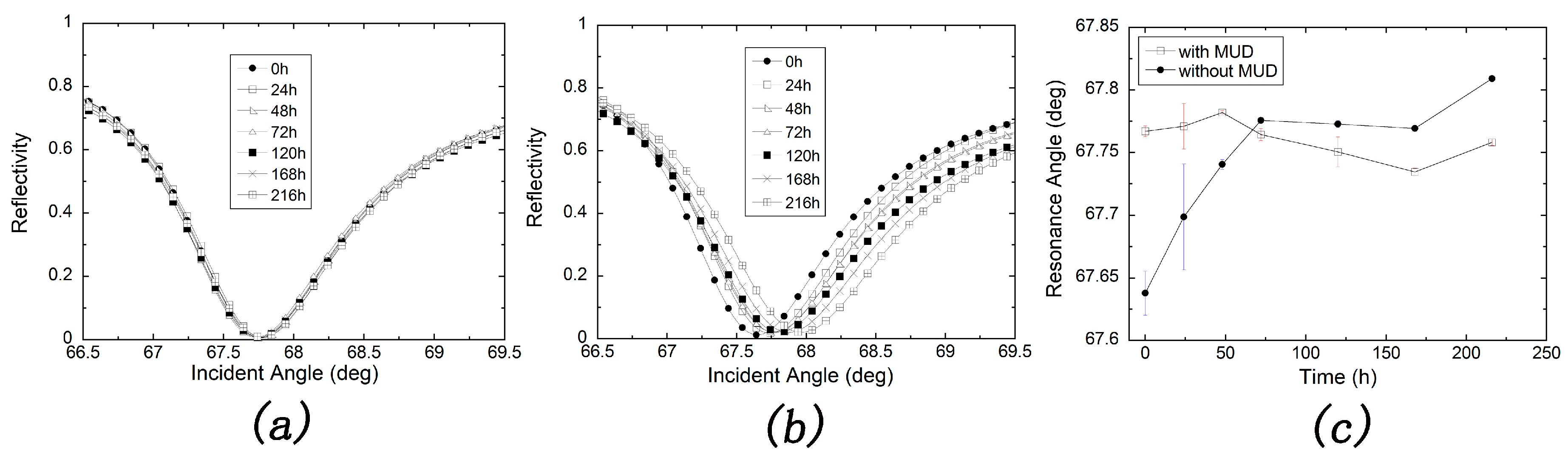
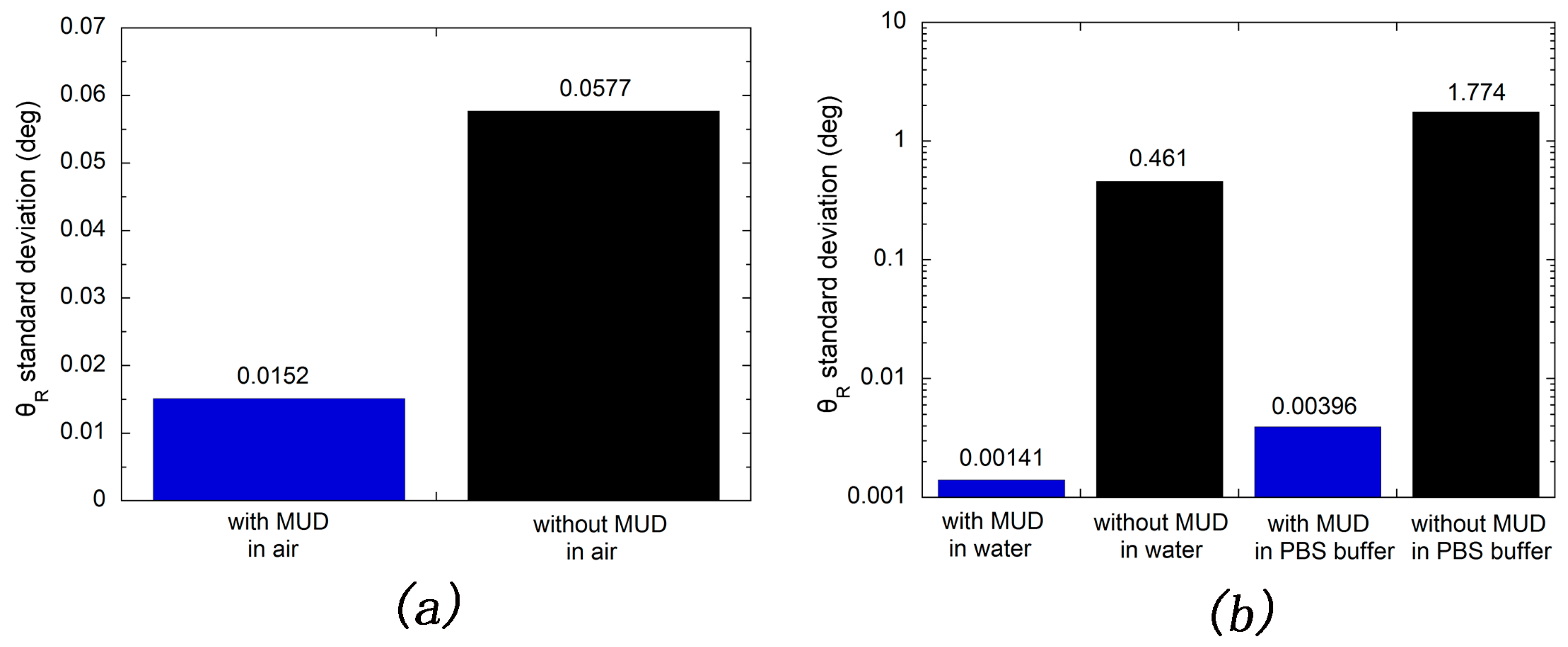
2.2.4. Stability Test for the MUD SAM-Protected and Bare Silver Films
2.2.5. Sensitivity and Limit of Detection Experiment
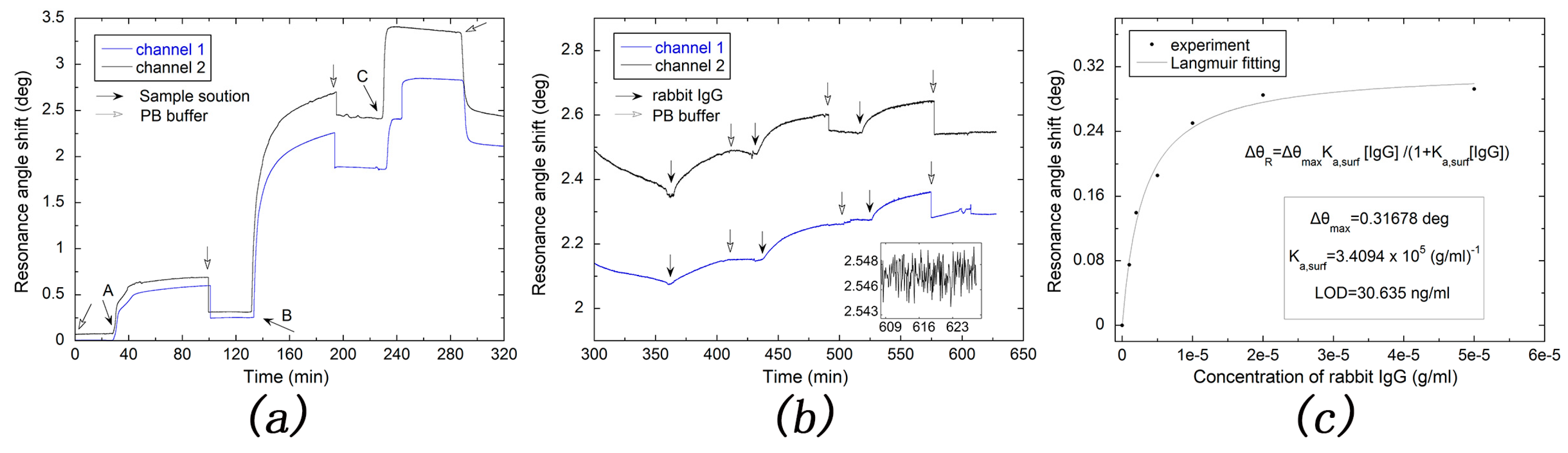
2.2.6. Immobilization of GAR IgG molecules on SAM-Modified Metal Film
2.2.7. Detection of Rabbit IgG Molecule
3. Results and Discussion
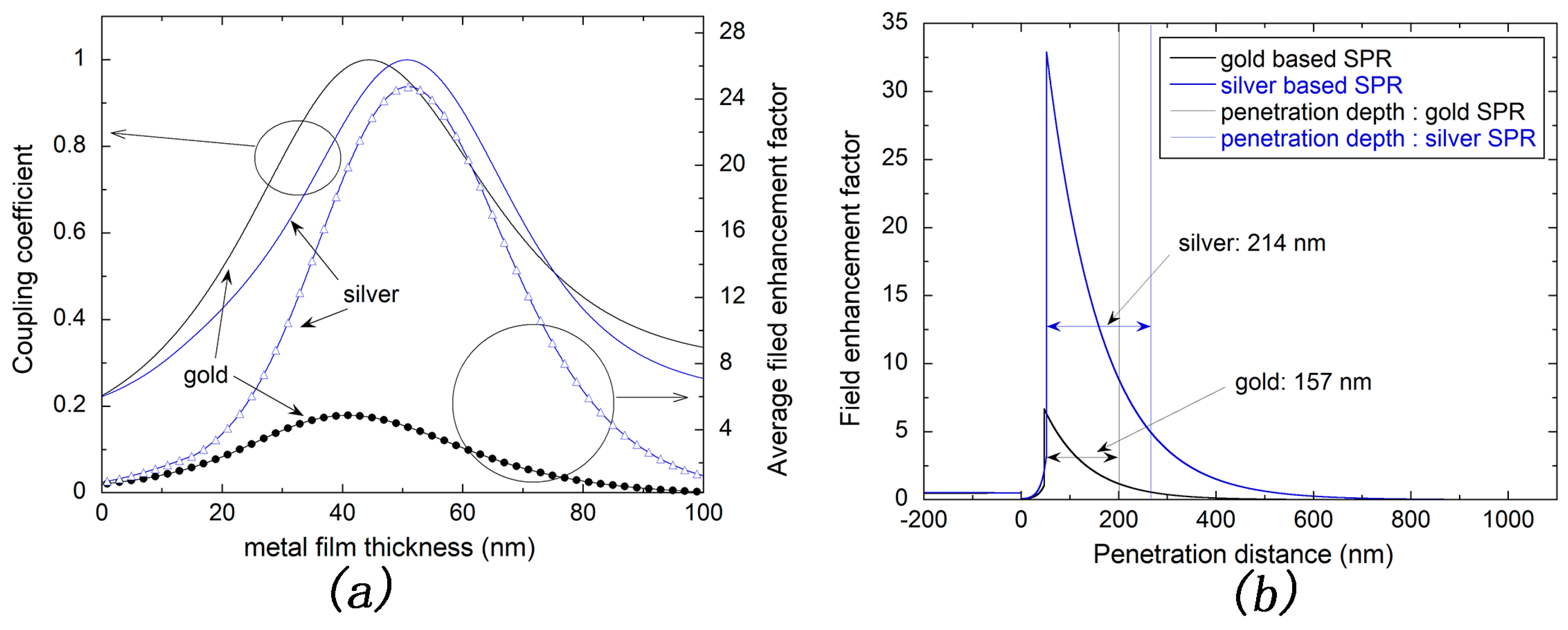
3.1. Optimization and Comparison of Silver and Gold Based SPR Sensors
3.2. Stability Test of Silver-Based SPR Sensor
3.3. Sensitity Factor (SF) and Limit of Detection (LOD) Tests
3.4. Real-Time Monitoring Biomolecule Interaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wood, R.W. XLII. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Phil. Mag. 1902, 4, 396–402. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Notizen: Radiative decay of non radiative surface plasmons excited by light. Z. Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Phys. 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Eng, L.; Nygren-Babol, L.; Hanning, A. Label-enhanced surface plasmon resonance applied to label-free interaction analysis of small molecules and fragments. Anal. Biochem. 2016, 510, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, D.-F.; Zhang, Z.; Liu, Q.; Qi, Z.-M. Hierarchical mesoporous silica film modified near infrared SPR sensor with high sensitivities to small and large molecules. Sens. Actuators B Chem. 2014, 203, 690–696. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Pattnaik, P. Surface plasmon resonance. Appl. Biochem. Biotechnol. 2005, 126, 79–92. [Google Scholar] [CrossRef]
- Fabini, E.; Danielson, U.H. Monitoring drug–serum protein interactions for early ADME prediction through Surface Plasmon Resonance technology. J. Pharm. Biomed. Anal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.; Kullman-Magnusson, M.; Hämäläinen, M.D.; Remaeus, A.; Andersson, K.; Borg, P.; Gyzander, E.; Deinum, J. Biosensor analysis of drug–target interactions: Direct and competitive binding assays for investigation of interactions between thrombin and thrombin inhibitors. Anal. Biochem. 2000, 278, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nonobe, Y.; Yokoyama, T.; Kamikubo, Y.; Yoshida, S.; Hisajima, N.; Shinohara, H.; Shiraishi, Y.; Sakurai, T.; Tabata, T. Application of surface plasmon resonance imaging to monitoring G protein-coupled receptor signaling and its modulation in a heterologous expression system. BMC Biotechnol. 2016, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-H.; Chang, C.-C.; Chen, C.-C.; Chu-Su, Y.; Lin, C.-W. Development of an Au/ZnO thin film surface plasmon resonance-based biosensor immunoassay for the detection of carbohydrate antigen 15-3 in human saliva. Clin. Biochem. 2012, 45, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Ehler, T.T.; Noe, L.J. Surface plasmon studies of thin silver/gold bimetallic films. Langmuir 1995, 11, 4177–4179. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, B. On the performance of different bimetallic combinations in surface plasmon resonance based fiber optic sensors. J. Appl. Phys. 2007, 101, 093111. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Z.; Singh, V.; Zheng, Z.; Wang, Y.; Li, S.; Song, L.; Zhu, J. Stable and sensitive silver surface plasmon resonance imaging sensor using trilayered metallic structures. Anal. Chem. 2014, 86, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-T.; Lo, K.-C.; Chang, H.-Y.; Wu, H.-T.; Ho, J.H.; Yen, T.-J. Ag/Au bi-metallic film based color surface plasmon resonance biosensor with enhanced sensitivity, color contrast and great linearity. Biosens. Bioelectron. 2012, 36, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ong, B.H.; Yuan, X.C.; Tjin, S.C.; Zhang, J.W.; Ng, H.M. Optimised film thickness for maximum evanescent field enhancement of a bimetallic film surface plasmon resonance biosensor. Sens. Actuators B Chem. 2006, 114, 1028–1034. [Google Scholar] [CrossRef]
- Zynio, S.A.; Samoylov, A.V.; Surovtseva, E.R.; Mirsky, V.M.; Shirshov, Y.M. Bimetallic layers increase sensitivity of affinity sensors based on surface plasmon resonance. Sensors 2002, 2, 62–70. [Google Scholar] [CrossRef]
- Dash, J.N.; Jha, R. On the Performance of Graphene-Based D-Shaped Photonic Crystal Fibre Biosensor Using Surface Plasmon Resonance. Plasmonics 2015, 10, 1123–1131. [Google Scholar] [CrossRef]
- Manesse, M.; Sanjines, R.; Stambouli, V.; Boukherroub, R.; Szunerits, S. Preparation and characterization of antimony-doped SnO2 thin films on gold and silver substrates for electrochemical and surface plasmon resonance studies. Electrochem. Commun. 2008, 10, 1041–1043. [Google Scholar] [CrossRef]
- Manesse, M.; Sanjines, R.; Stambouli, V.; Jorel, C.; Pelissier, B.; Pisarek, M.; Boukherroub, R.; Szunerits, S. Preparation and Characterization of Silver Substrates Coated with Antimony-Doped SnO2 Thin Films for Surface Plasmon Resonance Studies. Langmuir 2009, 25, 8036–8041. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Guan, W.; Liu, C.; Xue, T.; Wang, Q.; Zheng, W.; Cui, X. A stable and high resolution optical waveguide biosensor based on dense TiO2/Ag multilayer film. Appl. Surf. Sci. 2016, 377, 207–212. [Google Scholar] [CrossRef]
- Lin, W.B.; Lacroix, M.; Chovelon, J.M.; Jaffrezic-Renault, N.; Gagnaire, H. Development of a fiber-optic sensor based on surface plasmon resonance on silver film for monitoring aqueous media. Sens. Actuators B Chem. 2001, 75, 203–209. [Google Scholar] [CrossRef]
- Touahir, L.; Niedziolka-Joensson, J.; Galopin, E.; Boukherroub, R.; Gouget-Laemmel, A.C.; Solomon, I.; Petukhov, M.; Chazalviel, J.-N.; Ozanam, F.; Szunerits, S. Surface Plasmon Resonance on Gold and Silver Films Coated with Thin Layers of Amorphous Silicon-Carbon Alloys. Langmuir 2010, 26, 6058–6065. [Google Scholar] [CrossRef] [PubMed]
- Manickam, G.; Gandhiraman, R.; Vijayaraghavan, R.K.; Kerr, L.; Doyle, C.; Williams, D.E.; Daniels, S. Protection and functionalisation of silver as an optical sensing platform for highly sensitive SPR based analysis. Analyst 2012, 137, 5265–5271. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, A.; Christoulaki, A.; Spiliopoulos, N.; Vradis, A.; Toprakcioglu, C.; Pispas, S. Complexationof Lysozyme with Adsorbed PtBS-b-SCPI Block Polyelectrolyte Micelles on Silver Surface. Langmuir 2015, 31, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, Z.; Gillespie, D.E.; Lausted, C.; Zheng, Z.; Yang, M.; Zhu, J. Plain silver surface plasmon resonance for microarray application. Anal. Chem. 2015, 87, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Elfassy, E.; Mastai, Y.; Salomon, A. Cysteine sensing by plasmons of silver nanocubes. J. Solid State Chem. 2016, 241, 110–114. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Malinsky, M.D.; Kelly, K.L.; Schatz, G.C.; Van Duyne, R.P. Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J. Am. Chem. Soc. 2001, 123, 1471–1482. [Google Scholar] [CrossRef]
- McFarland, A.D.; Van Duyne, R.P. Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity. Nano Lett. 2003, 3, 1057–1062. [Google Scholar] [CrossRef]
- Peterlinz, K.A.; Georgiadis, R. In situ kinetics of self-assembly by surface plasmon resonance spectroscopy. Langmuir 1996, 12, 4731–4740. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Wang, J.; Zhu, X.; Jia, F.; Cao, Y.; Wang, X.; Zhang, H.; Song, D. Sensitivity enhancement of SPR biosensor with silver mirror reaction on the Ag/Au film. Talanta 2009, 78, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Jung, L.S.; Campbell, C.T.; Chinowsky, T.M.; Mar, M.N.; Yee, S.S. Quantitative interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 1998, 14, 5636–5648. [Google Scholar] [CrossRef]
- Yang, Z.P.; Frey, W.; Oliver, T.; Chilkoti, A. Light-activated affinity micropatterning of proteins on self-assembled monolayers on gold. Langmuir 2000, 16, 1751–1758. [Google Scholar] [CrossRef]
- Ding, Y.; Fan, Y.; Zhang, Y.; He, Y.; Sun, S.; Ma, H. Fabrication and optical sensing properties of mesoporous silica nanorod arrays. RSC Adv. 2015, 5, 90659–90666. [Google Scholar] [CrossRef]
- Fan, Y.; Ding, Y.; Ma, H.; Teramae, N.; Sun, S.; He, Y. Optical waveguide sensor based on silica nanotube arrays for label-free biosensing. Biosens. Bioelectron. 2015, 67, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ding, Y.; Zhang, Y.; Ma, H.; He, Y.; Sun, S. A SiO2-coated nanoporous alumina membrane for stable label-free waveguide biosensing. RSC Adv. 2014, 4, 62987–62995. [Google Scholar] [CrossRef]
- Fan, Y.; Hotta, K.; Yamaguchi, A.; Ding, Y.; He, Y.; Teramae, N.; Sun, S.; Ma, H. Highly sensitive real-time detection of DNA hybridization by using nanoporous waveguide fluorescence spectroscopy. Appl. Phys. Lett. 2014, 105, 031103. [Google Scholar] [CrossRef]
- Sensors and Actuators B: ChemicalAlvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Delivery Rev. 2013, 65, 1148–1171. [Google Scholar]
- Jain, K.; Gupta, U.; Jain, N.K. Dendronized nanoconjugates of lysine and folate for treatment of cancer. Eur. J. Pharm. Biopharm. 2014, 87, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, G.; Sun, S.; Tan, H.; He, Y. Digital immunoassay of a prostate-specific antigen using gold nanorods and magnetic nanoparticles. RSC Adv. 2017, 7, 27595–27602. [Google Scholar] [CrossRef]
- Hansen, W.N. Electric fields produced by the propagation of plane coherent electromagnetic radiation in a stratified medium. JOSA 1968, 58, 380–390. [Google Scholar] [CrossRef]
- Palik, E.; Ghosh, G.; Prucha, E. Handbook of Optical Constants of Solids; Academic Press: New York, NY, USA, 1985. [Google Scholar]
- Xia, L.; Yin, S.; Gao, H.; Deng, Q.; Du, C. Sensitivity enhancement for surface plasmon resonance imaging biosensor by utilizing gold–silver bimetallic film configuration. Plasmonics 2011, 6, 245–250. [Google Scholar] [CrossRef]
- Quaroni, L.; Chumanov, G. Preparation of polymer-coated functionalized silver nanoparticles. J. Am. Chem. Soc. 1999, 121, 10642–10643. [Google Scholar] [CrossRef]
- Bahrami, F.; Maisonneuve, M.; Meunier, M.; Aitchison, J.S.; Mojahedi, M. An improved refractive index sensor based on genetic optimization of plasmon waveguide resonance. Opt. Express 2013, 21, 20863–20872. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Worek, W. Two-wavelength interferometric technique for measuring the refractive index of salt-water solutions. Appl. Opt. 1993, 32, 3992–4002. [Google Scholar] [CrossRef] [PubMed]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Opt. Express 2009, 17, 16505–16517. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; Chang, L.; Klein, W.L.; Van Duyne, R.P. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc. 2005, 127, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, M.-C.; Châteauneuf, I.; Guérin, M.; Lemieux, R. Sensitive detection of human IgG in ELISA using a monoclonal anti-IgG-peroxidase conjugate. Hybrid. Hybrid. 2004, 23, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Slayter, H.; Schurtenberger, P.; Chamberlin, R.; Colton, C.; Yarmush, M. Size and structure of antigen-antibody complexes. Electron microscopy and light scattering studies. Biophys. J. 1988, 54, 45–56. [Google Scholar] [CrossRef]
- Yu, C.; Irudayaraj, J. Quantitative evaluation of sensitivity and selectivity of multiplex nanoSPR biosensor assays. Biophys. J. 2007, 93, 3684–3692. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.B.; Jaffrezic-Renault, N.; Chovelon, J.M.; Lacroix, M. Optical fiber as a whole surface probe for chemical and biological applications. Sens. Actuators B Chem. 2001, 74, 207–211. [Google Scholar] [CrossRef]
- Guner, H.; Ozgur, E.; Kokturk, G.; Celik, M.; Esen, E.; Topal, A.E.; Ayas, S.; Uludag, Y.; Elbuken, C.; Dana, A. A smartphone based surface plasmon resonance imaging (SPRi) platform for on-site biodetection. Sens. Actuators B Chem. 2017, 239, 571–577. [Google Scholar] [CrossRef]


| Silver-based SPR | SF () | SM () | () |
|---|---|---|---|
| Experimental data | 130.4539 | 0.6503 | 84.8342 |
| Theoretical value | 115.959 | 0.7398 | 85.7865 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Wang, C.; Yang, R.; Liu, W.; Sun, S. A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film. Sensors 2017, 17, 2777. https://doi.org/10.3390/s17122777
Wang G, Wang C, Yang R, Liu W, Sun S. A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film. Sensors. 2017; 17(12):2777. https://doi.org/10.3390/s17122777
Chicago/Turabian StyleWang, Guiqiang, Chunnan Wang, Rui Yang, Wenlan Liu, and Shuqing Sun. 2017. "A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film" Sensors 17, no. 12: 2777. https://doi.org/10.3390/s17122777




