Label-Free Biomedical Imaging Using High-Speed Lock-In Pixel Sensor for Stimulated Raman Scattering
Abstract
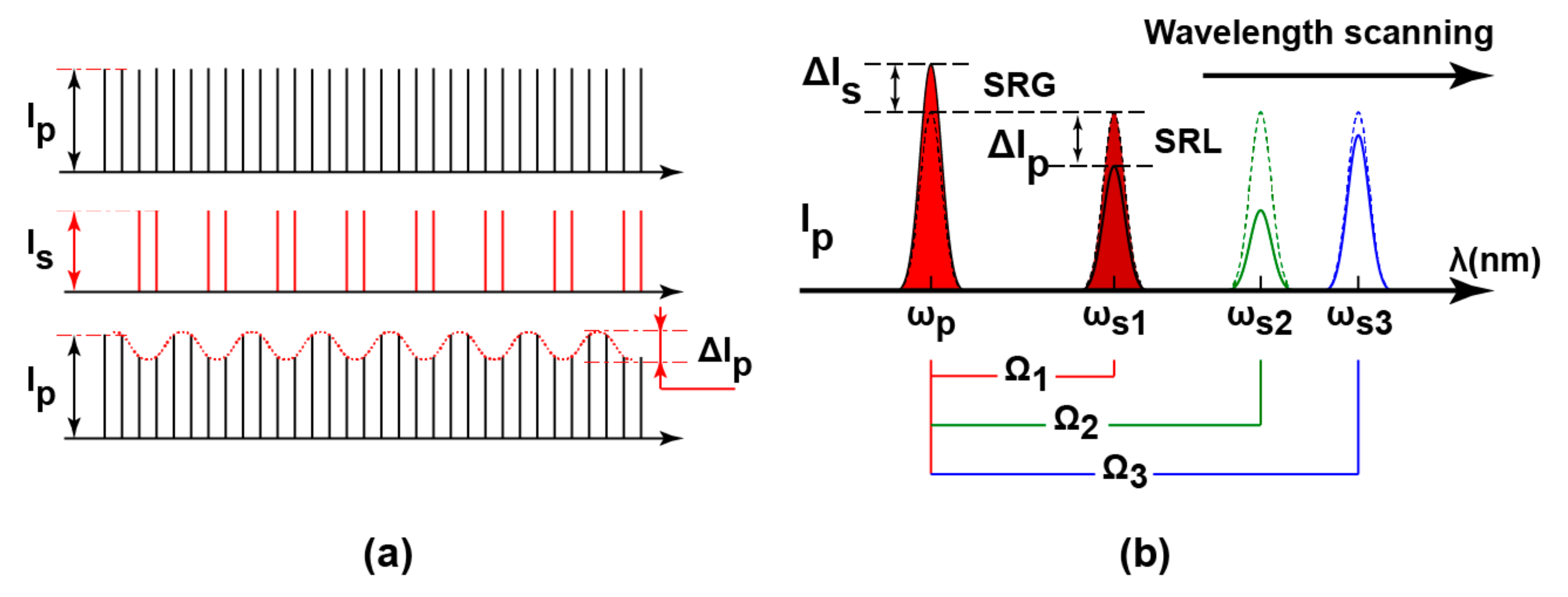
:1. Introduction
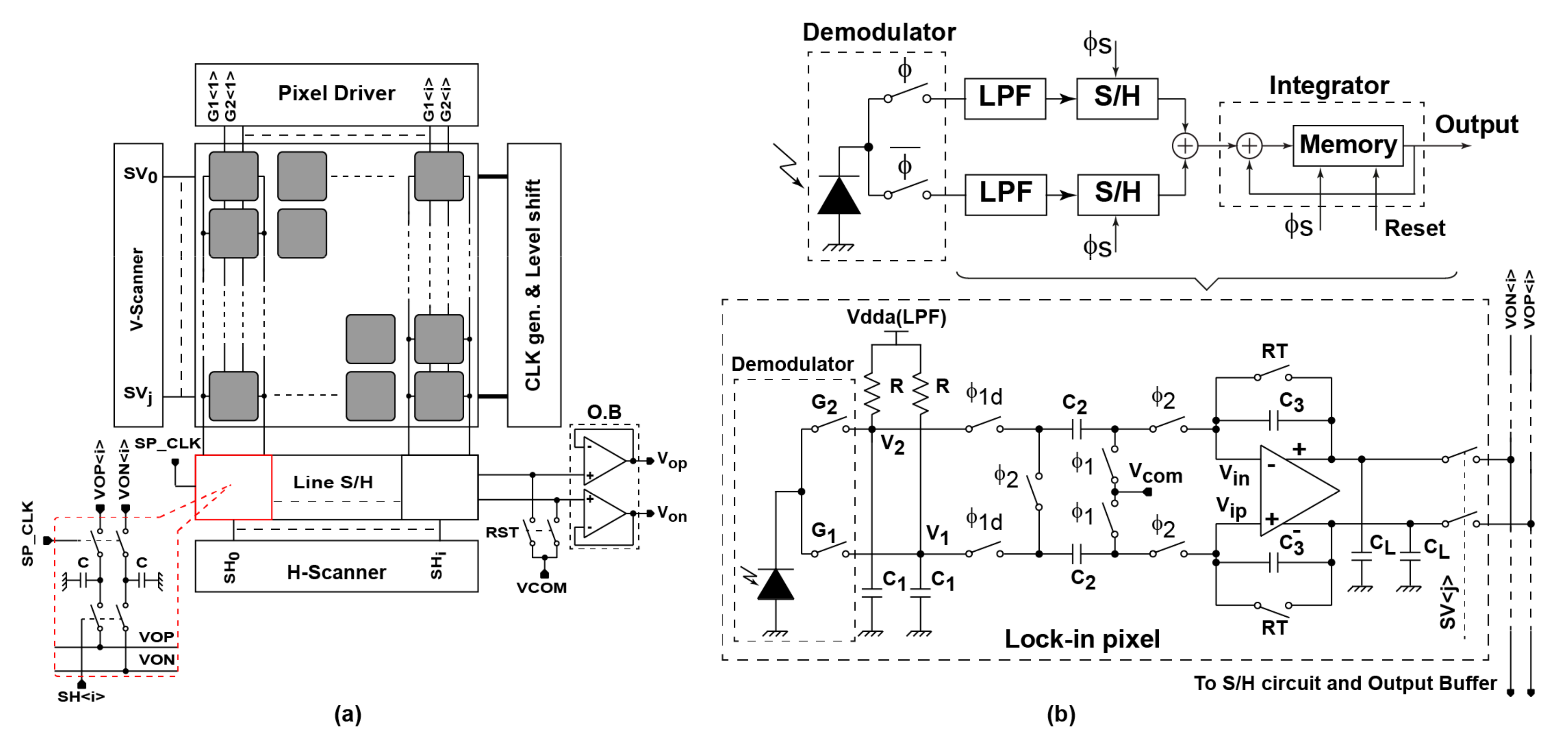
2. SRS Lock-In Pixel Design
2.1. Large Area Demodulator Design
2.2. Lock-In Pixel, Readout Circuit and Their Operations
3. Measurement Results and Discussion
3.1. Chip Implementation
3.2. Experimental Setup
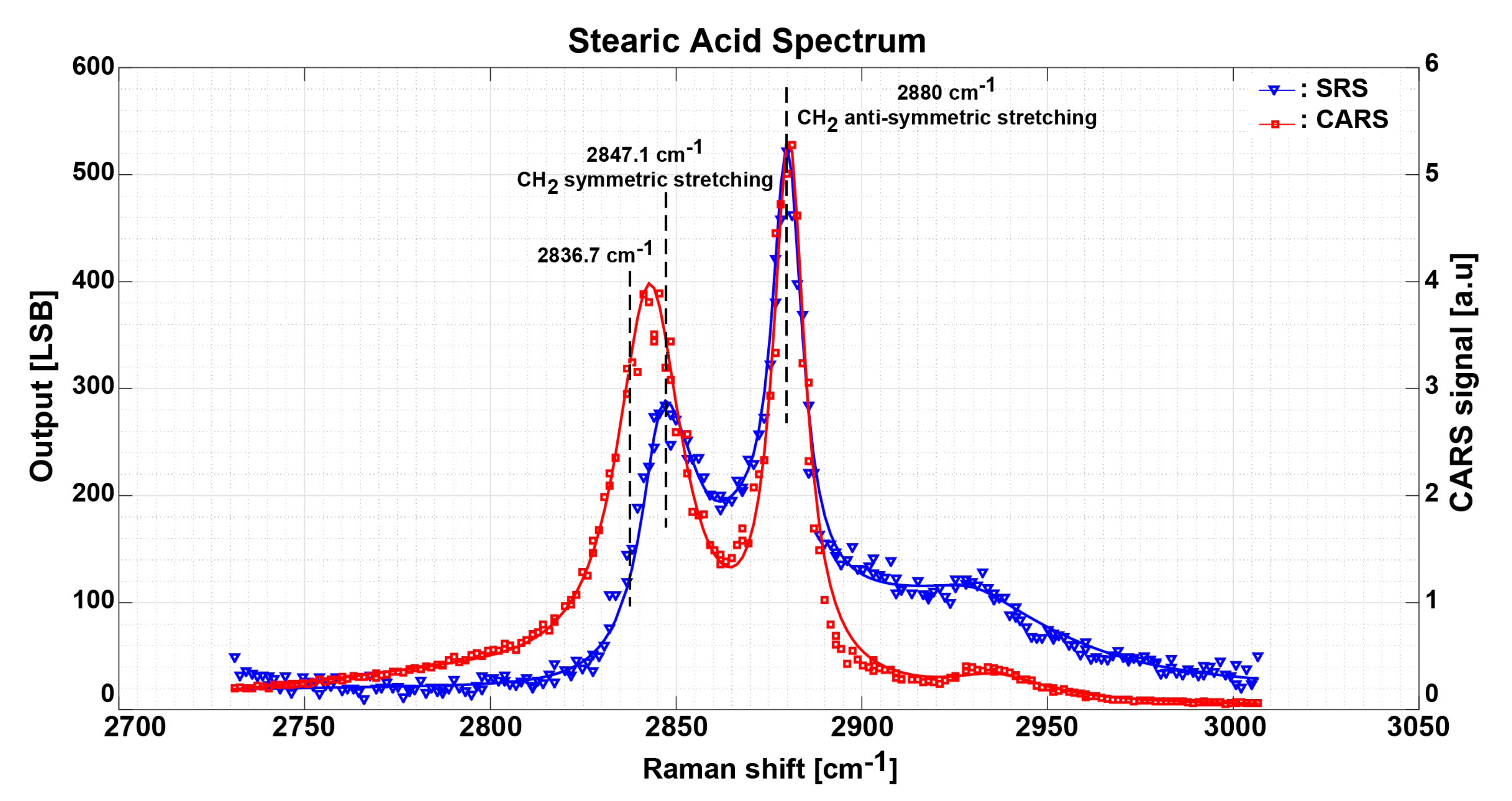
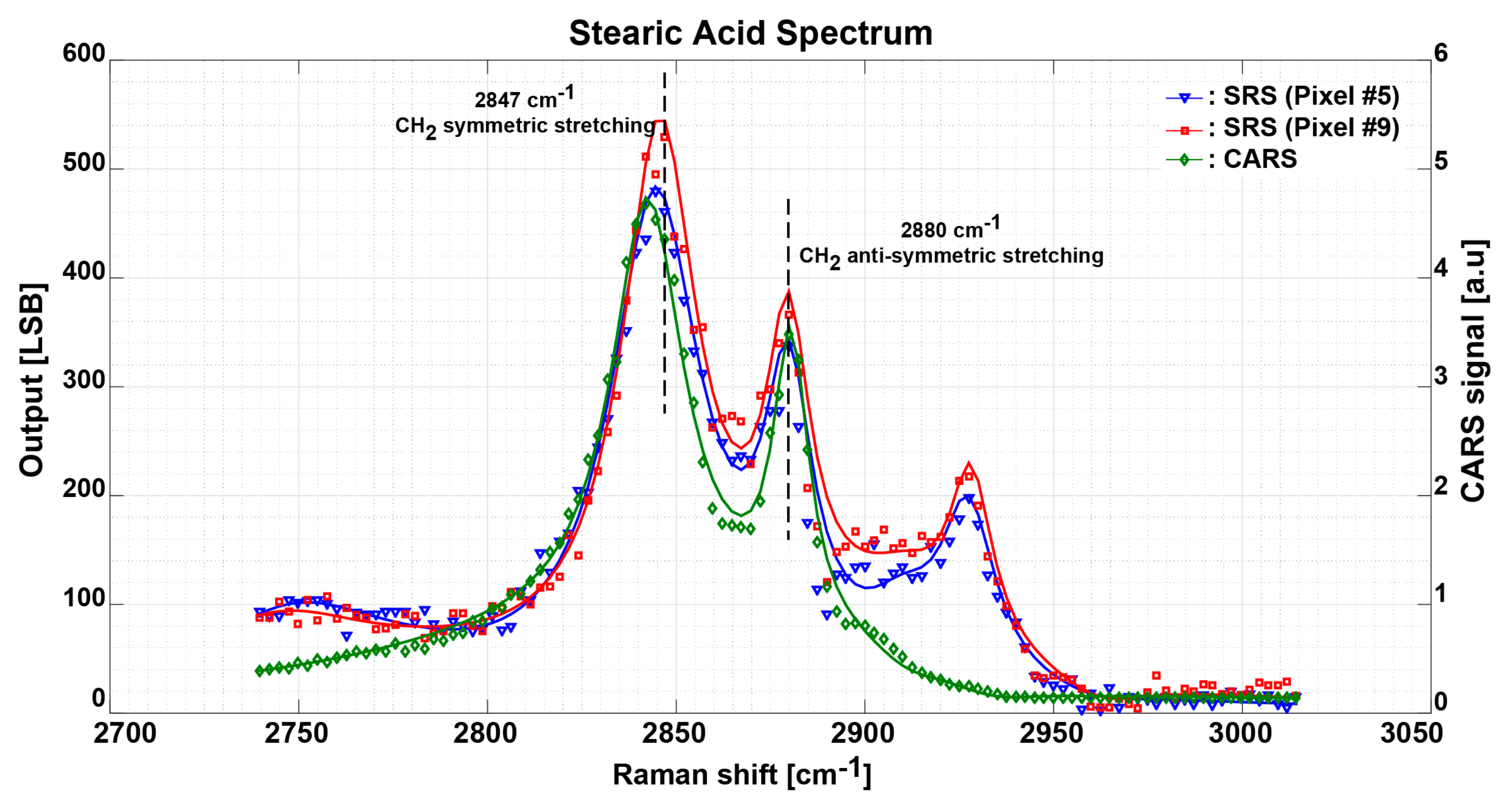
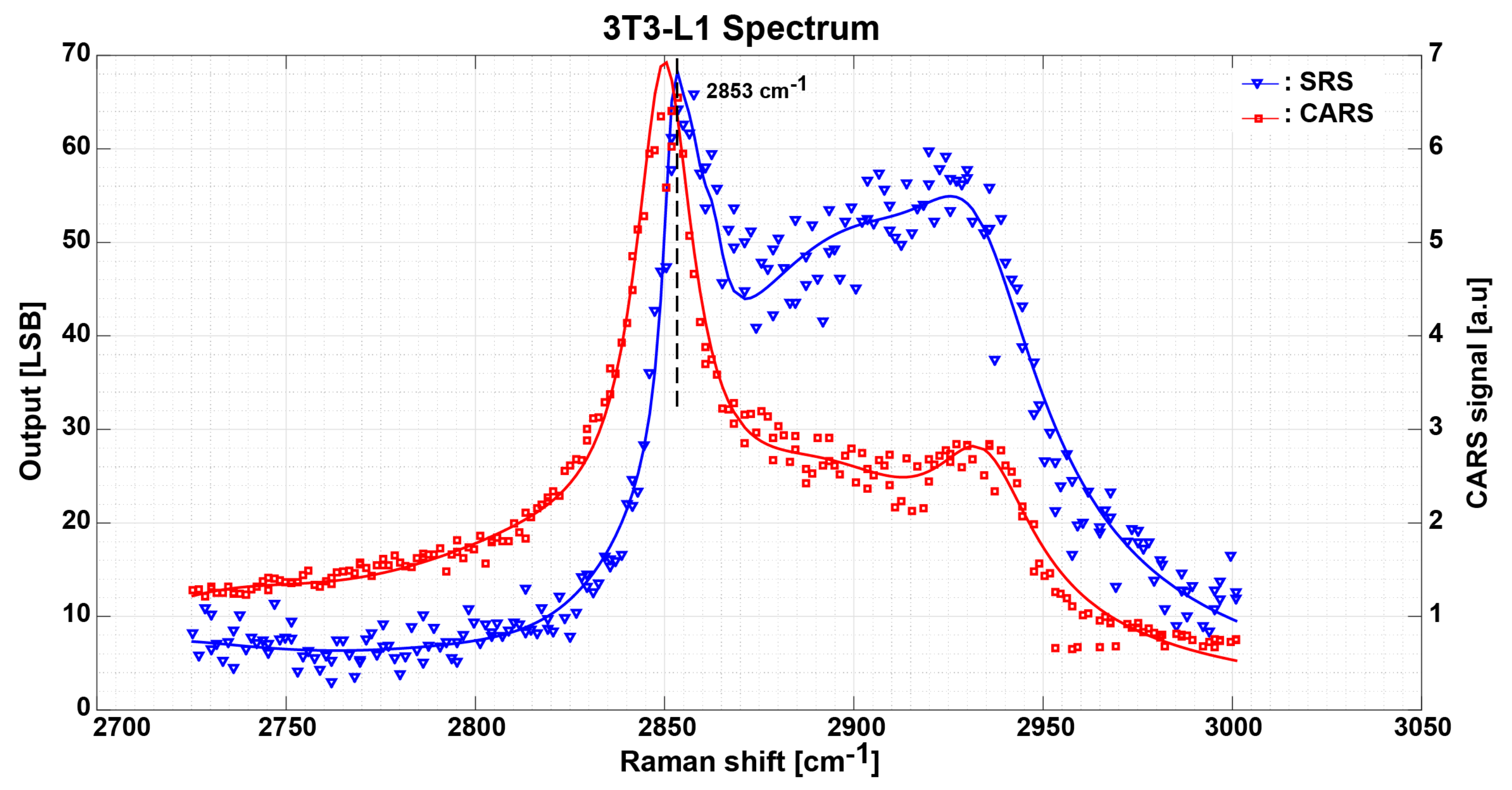
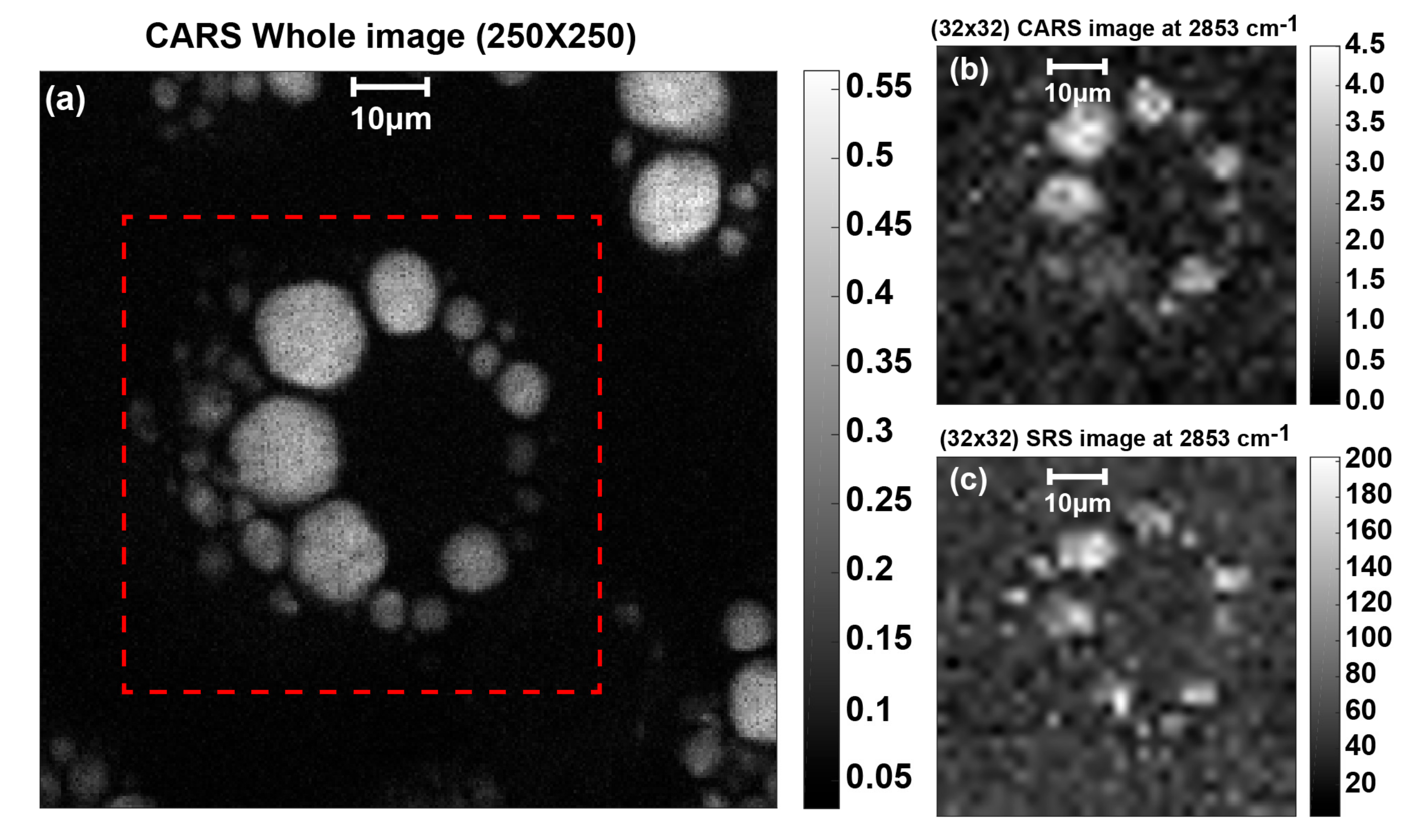
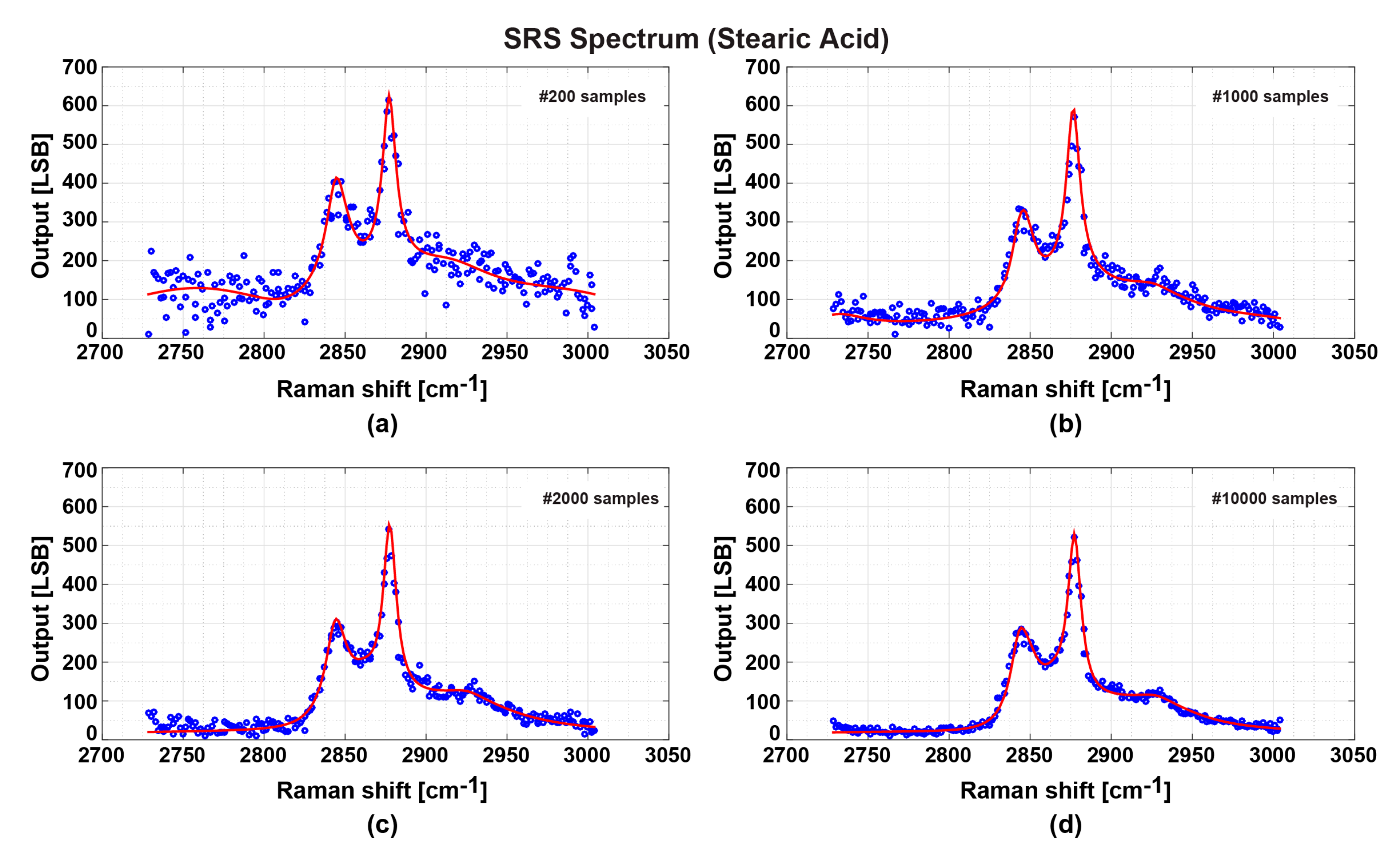
3.3. Measurement Results and Discussion
4. Outlook
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, Y.; Ramachandran, P.V.; Wang, M.C. Shedding new light on lipid functions with CARS and SRS microscopy. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Camp, C.H.C., Jr.; Lee, Y.J.; Heddleston, J.M.; Hartshorn, C.M.; Walker, A.R.H.; Rich, J.N.; Lathia, J.D.; Cicerone, M.T. High-speed coherent Raman fingerprint imaging of biological tissues. Nat. Photonics 2014, 8, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Kostamovaara, J.; Tenhunen, J.; Kögler, M.; Nissinen, I.; Nissinen, J.; Keränen, P. Fluorescence suppression in Raman spectroscopy using a time-gated CMOS SPAD. Opt. Express 2013, 21, 31632–31645. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-X.; Xie, X.S. Vibrational spectroscopic imaging of living systems: An emerging platform for biology and medicine. Science 2015, 350, 8870. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-S.; Slipchenko, M.N.; Wang, P.; Li, J.; Lee, S.-Y.; Oglesbee, R.A.; Cheng, J.-X. Microsecond scale vibrational spectroscopic imaging by multiplex stimulated Raman scattering microscopy. Light Sci. Appl. 2015, 4, e265. [Google Scholar] [CrossRef] [PubMed]
- Saar, B.G.; Zeng, Y.; Freudiger, C.W.; Liu, Y.S.; Himmel, M.E.; Xie, X.S.; Ding, S.Y. Label-free, real-time monitoring of biomass processing with stimulated raman scattering microscopy. Angew. Chem. Int. Ed. 2010, 49, 5476–5479. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Volkmer, A.; Book, L.D.; Xie, X.S. Multiplex coherent anti-stokes Raman scattering microspectroscopy and study of lipid vesicles. J. Phys. Chem. B 2002, 106, 8493–8498. [Google Scholar] [CrossRef]
- Slipchenko, M.N.; Le, T.T.; Chen, H.; Cheng, J.-X. High-Speed Vibrational Imaging and Spectral Analysis of Lipid Bodies by Compound Raman Microscopy. J. Phys. Chem. B 2009, 113, 7681–7686. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.L.; Xie, X.S. Coherent anti-Stokes Raman scattering microscopy: Chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef] [PubMed]
- Day, J.P.R.; Domke, K.F.; Rago, G.; Kano, H.; Hamaguchi, H.; Vartiainen, E.M.; Bonn, M. Quantitative Coherent Anti-Stokes Raman Scattering (CARS) Microscopy. J. Phys. Chem. B 2011, 115, 7713–7725. [Google Scholar] [CrossRef] [PubMed]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moester, M.J.B.; Ariese, F.; de Boer, J.F. Optimized signal-to-noise ratio with shot noise limited detection in stimulated Raman scattering microscopy. J. Eur. Opt. Soc. Rapid 2015, 10, 15022. [Google Scholar] [CrossRef]
- Ozeki, Y.; Dake, F.; Kajiyama, S.; Fukui, K.; Itoh, K. Analysis and experimental assessment of the sensitivity of stimulated Raman scattering microscopy. Opt. Express 2009, 17, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Rock, W.; Bonn, M.; Parekh, S. Near shot-noise limited hyperspectral stimulated Raman scattering spectroscopy using low energy lasers and a fast CMOS array. Opt. Express 2013, 21, 15113–15120. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, E.; Marx, B.; Klein, T.; Huber, R.; Gilch, P. A 75 MHz light source for femtosecond stimulated raman microscopy. Opt. Express 2009, 17, 18612–18620. [Google Scholar] [CrossRef] [PubMed]
- Lioe, D.X.; Mars, K.; Kawahito, S.; Yasutomi, K.; Kagawa, K.; Yamada, T.; Hashimoto, M. A Stimulated Raman Scattering CMOS Pixel Using a High-Speed Charge Modulator and Lock-in Amplifier. Sensors 2016, 16, 532. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, S.; Baek, G.; Li, Z.; Han, S.M.; Seo, M.W. CMOS Lock-in Pixel Image Sensors with Lateral Electric Field Control for Time-Resolved Imaging. In Proceedings of the International Image Sensor Workshop, Snowbird, UT, USA, 12–16 June 2013; pp. 361–364. [Google Scholar]
- Minamikawa, T.; Murakami, Y.; Matsumura, N.; Niioka, H.; Fukushima, S.; Araki, T.; Hashimoto, M. Photo-Induced Cell Damage Analysis for Single- and Multifocus Coherent Anti-Stokes Raman Scattering Microscopy. J. Spectrosc. 2017, 2017, 5725340. [Google Scholar] [CrossRef]
- Ryu, I.S.; Camp, C.H.; Jin, Y.; Cicerone, M.T.; Lee, Y.J. Beam scanning for rapid coherent Raman hyperspectral imaging. Opt. Lett. 2015, 40, 5826–5829. [Google Scholar] [CrossRef] [PubMed]
- Cahyadi, H.; Iwatsuka, J.; Minamikawa, T.; Niioka, H.; Araki, T.; Hashimoto, M. Fast spectral coherent anti-Stokes Raman scattering microscopy with high-speed tunable picosecond laser. J. Biomed. Opt. 2013, 18, 096009. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, T.; Tanimoto, N.; Hashimoto, M.; Araki, T.; Kobayashi, M.; Fujita, K.; Kawata, S. Jitter reduction of two synchronized picosecond mode-locked lasers using balanced cross-correlator with two-photon detectors. Appl. Phys. Lett. 2006, 89, 191101. [Google Scholar] [CrossRef]
- Hu, C.-R.; Slipchenko, M.N.; Wang, P.; Wang, P.; Lin, J.D.; Simpson, G.; Hu, B.; Cheng, J.-X. Stimulated Raman scattering imaging by continuous-wave laser excitation. Opt. Lett. 2013, 38, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Steinle, T.; Kumar, V.; Steinmann, A.; Marangoni, M.; Cerullo, G.; Giessen, H. Compact, low-noise, all-solid-state laser system for stimulated Raman scattering microscopy. Opt. Lett. 2015, 40, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Standard Spectra. Available online: http://www.chem.ualberta.ca/~mccreery/ramanmaterials.html (accessed on 17 March 2017).
- Parekh, S.H.; Lee, Y.J.; Aamer, K.A.; Cicerone, M.T. Label-free cellular imaging by Broadband coherent anti-stokes raman scattering microscopy. Biophys. J. 2010, 99, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.P.; Wallach, D.F.H. Raman Spectra of Some Saturated, Unsaturated and Deuterated C18 Fatty Acid in the HCH-Deformation and CH-Rtretching Regions. Biochim. Biophys. Acta 1977, 486, 217–227. [Google Scholar] [CrossRef]
- Razavi, B. Design of Analog CMOS Integrated Circuits; McGraw-Hill Inc.: New York, NY, USA, 2001; pp. 309–314. [Google Scholar]
- Zhang, D.; Wang, P.; Slipchenko, M.N.; Cheng, J.-X. Fast Vibrational Imaging of Single Cells and Tissues by Stimulated Raman Scattering Microscopy. Acc. Chem. Res. 2014, 47, 2282–2290. [Google Scholar] [CrossRef] [PubMed]





| Initial Location | Transfer Time (ns) |
|---|---|
| A | 3.825 |
| B | 0.4333.463 |
| C | 3.463 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mars, K.; Lioe, D.X.; Kawahito, S.; Yasutomi, K.; Kagawa, K.; Yamada, T.; Hashimoto, M. Label-Free Biomedical Imaging Using High-Speed Lock-In Pixel Sensor for Stimulated Raman Scattering. Sensors 2017, 17, 2581. https://doi.org/10.3390/s17112581
Mars K, Lioe DX, Kawahito S, Yasutomi K, Kagawa K, Yamada T, Hashimoto M. Label-Free Biomedical Imaging Using High-Speed Lock-In Pixel Sensor for Stimulated Raman Scattering. Sensors. 2017; 17(11):2581. https://doi.org/10.3390/s17112581
Chicago/Turabian StyleMars, Kamel, De Xing Lioe, Shoji Kawahito, Keita Yasutomi, Keiichiro Kagawa, Takahiro Yamada, and Mamoru Hashimoto. 2017. "Label-Free Biomedical Imaging Using High-Speed Lock-In Pixel Sensor for Stimulated Raman Scattering" Sensors 17, no. 11: 2581. https://doi.org/10.3390/s17112581






