A Phase-Intensity Surface Plasmon Resonance Biosensor for Avian Influenza A (H5N1) Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Chemistry
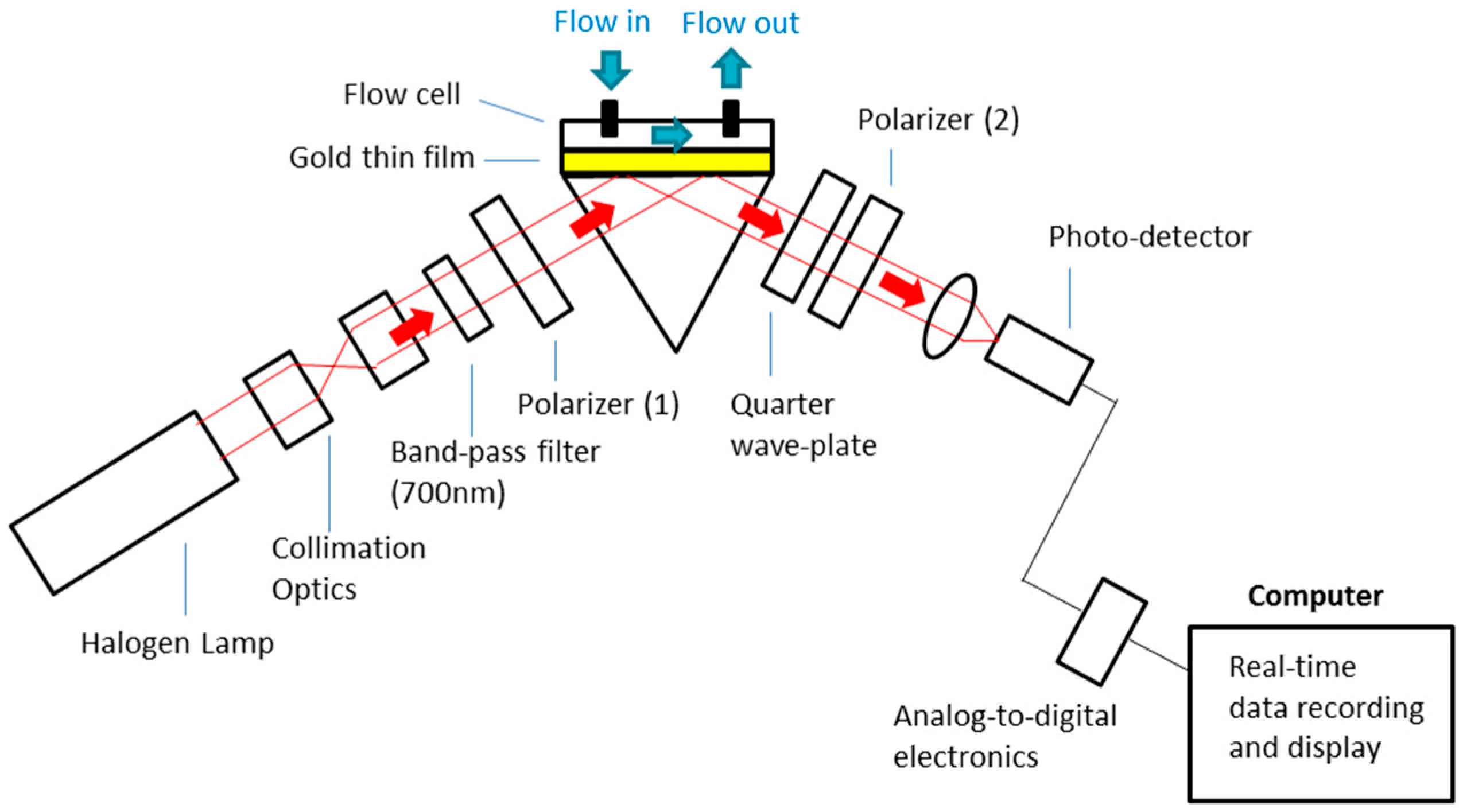
2.2. Optical Set-Up
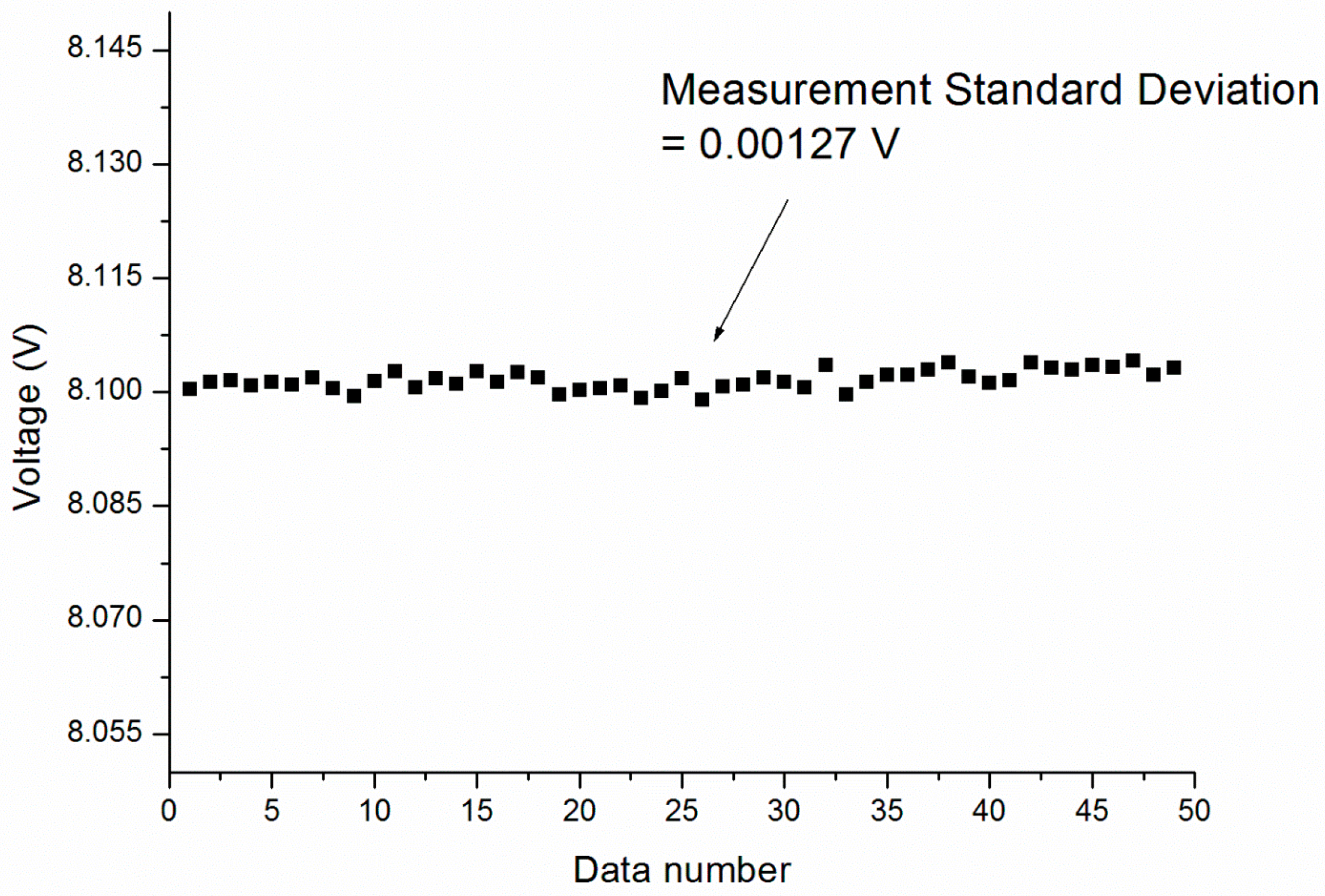
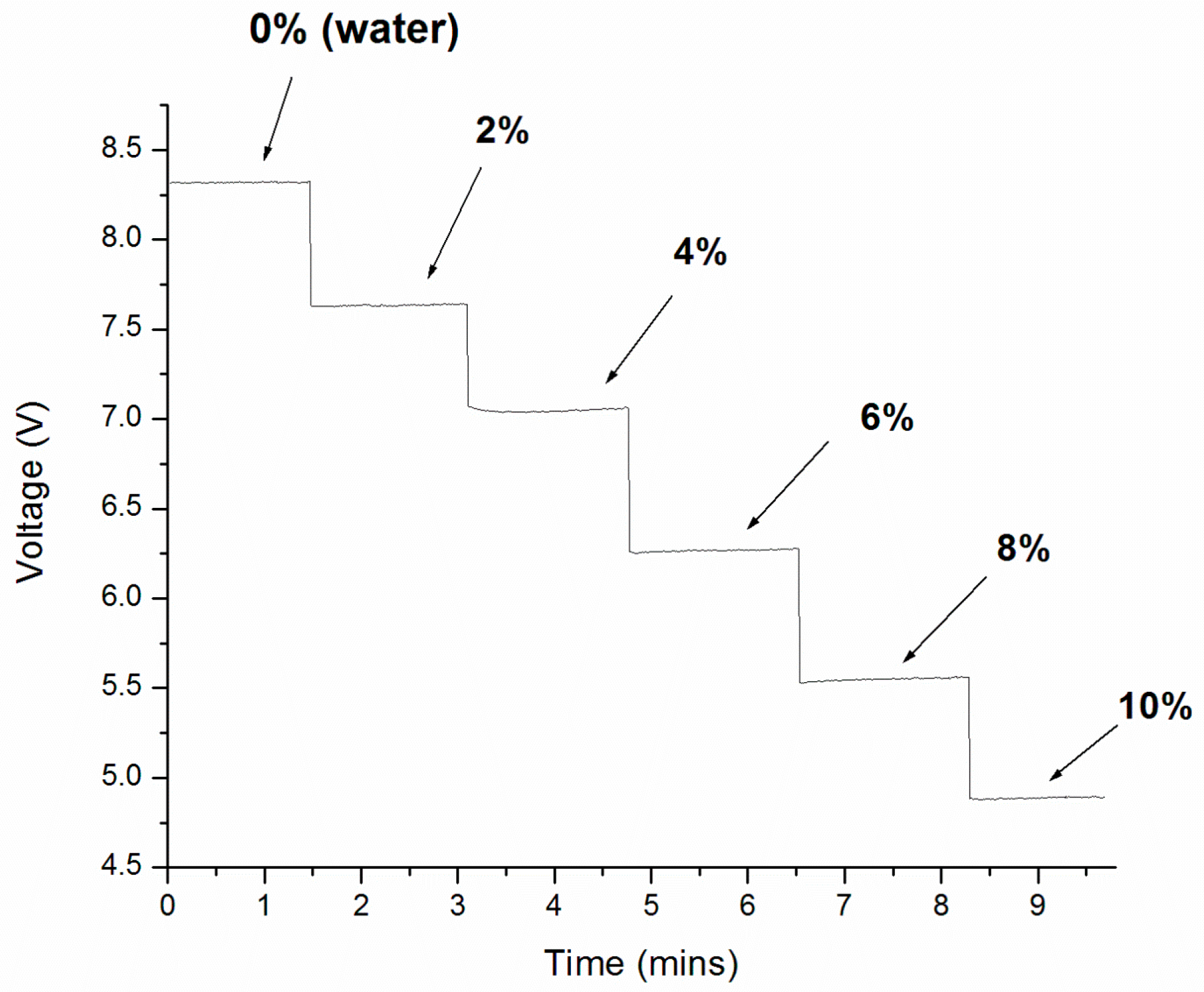
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- World Health Organization. Avian and Other Zoonotic Influenza. Available online: http://www.who.int/mediacentre/factsheets/avian_influenza/en/ (accessed on 25 September 2015).
- World Health Organization. Cumulative Number of Confirmed Human Cases of Avian Influenza A (H5N1) Reported to WHO. Available online: http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/ (accessed on 25 September 2015).
- World Health Organization. FAQs: H5N1 Influenza. Available online: http://www.who.int/influenza/human_animal_interface/avian_influenza/h5n1_research/faqs/en/ (accessed on 16 August 2016).
- Kim, D.K.; Poudel, B. Tools to Detect Influenza Virus. Yonsei Med. J. 2013, 54, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J. Influenza. In Principles and Practice of Infectious Disease, 2nd ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2005; pp. 2000–2024. [Google Scholar]
- Heinonen, S.; Silvennoinen, H.; Lehtinen, P.; Vainionpää, R.; Heikkinen, T. Feasibility of diagnosing influenza within 24 h of symptom onset in children 1–3 years of age. Eur. J. Clin. Microbiol. Infec. Dis. 2011, 30, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Serrano-Heras, G.; Castaño, M.J.; Solera, J. Real-time PCR detection chemistry. Clin. Chim. Acta 2015, 439, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.B. Detection of respiratory viruses by molecular methods. Clin. Microbiol. Rev. 2008, 21, 716–747. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, L.; Bergeron, M.G. Diagnosing infections––Current and anticipated technologies for point-of-care diagnostics and home-based testing. Clin. Microbiol. Infect. 2010, 16, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Wong, C.L.; Olivo, M. Surface Plasmon Resonance Imaging Sensors: A Review. Plasmonics 2014, 9, 809–824. [Google Scholar] [CrossRef]
- Wong, C.L.; Dinish, U.S.; Olivo, M. Recent advances in SPR and SERS for sensitive translational medical diagnostics. Photonics Lasers Med. 2015, 4, 119–149. [Google Scholar] [CrossRef]
- Gazzola, E.; Pozzato, A.; Ruffato, G.; Sovernigo, E.; Sonato, A. High-throughput fabrication and calibration of compact high-sensitivity plasmonic lab-on-chip for biosensing. Optofluid. Microfluid. Nanofluid. 2016, 3, 13. [Google Scholar] [CrossRef]
- Yuan, W.; Ho, H.P.; Wong, C.L.; Wu, S.Y.; Suen, Y.K.; Kong, S.K.; Lin, C. Surface Plasmon Resonance Biosensor Incorporated in a Michelson Interferometer With Enhanced Sensitivity. IEEE Sens. J. 2007, 7, 70–73. [Google Scholar] [CrossRef]
- Yuan, W.; Ho, H.P.; Wong, C.L.; Wu, S.Y.; Suen, Y.K.; Kong, S.K.; Lin, C. Sensitivity enhancement based on application of multi-pass interferometry in phase-sensitive surface plasmon resonance biosensor. Opt. Commun. 2007, 275, 491–496. [Google Scholar] [CrossRef]
- Wong, C.L.; Chen, G.C.; Li, X.; Ng, B.K.K.; Shum, P.; Chen, P.; Lin, Z.; Lin, C.; Olivo, M. Colorimetric surface plasmon resonance imaging (SPRI) biosensor array based on polarization orientation. Biosens. Bioelectron. 2013, 47, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Chen, G.C.; Ng, B.K.; Agarwal, S.; Lin, Z.; Chen, P.; Ho, H.P. Multiplex spectral surface plasmon resonance imaging (SPRI) sensor based on the polarization control scheme. Opt. Express 2011, 19, 18965–18978. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Ho, H.P.; Suen, Y.K.; Kong, S.K.; Chen, Q.L.; Yuan, W.; Wu, S.Y. Real-time protein biosensor arrays based on surface plasmon resonance differential phase imaging. Biosens. Bioelectron. 2008, 24, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L. Imaging Surface Plasmon Resonance (SPR) Photonic Sensors. Ph.D. Thesis, The City University of Hong Kong, Hong Kong, China, 2007. [Google Scholar]
- Sonato, A.; Agostini, M.; Ruffato, G.; Gazzola, E.; Liuni, D.; Greco, G.; Travagliati, M.; Cecchini, M.; Romanato, F. A surface acoustic wave (SAW)-enhanced grating-coupling phase-interrogation surface plasmon resonance (SPR) microfluidic biosensor. Lab Chip 2016, 16, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Ho, H.P.; Yu, T.T.; Suen, Y.K.; Chow, W.Y.; Wu, S.Y.; Law, W.C.; Yuan, W.; Li, W.J.; Kong, S.K.; et al. Two-dimensional biosensor arrays based on surface plasmon resonance phase imaging. Appl. Opt. 2007, 46, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.P.; Wong, C.L.; Chan, K.S.; Wu, S.Y.; Lin, C. Application of 2-D spectral surface plasmon resonance to the imaging of pressure distribution in elastohydrodynamic (EHD) lubricant films. Appl. Opt. 2006, 45, 5819–5826. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Ho, H.P.; Chan, K.S.; Wong, P.L.; Wu, S.Y.; Lin, C. Optical characterization of elastohydrodynamic lubricated (EHL) contacts using surface plasmon resonance (SPR) effect. Tribol. Int. 2008, 41, 356–366. [Google Scholar] [CrossRef]
- Wong, C.L.; Ho, H.P.; Chan, K.S.; Wu, S.Y.; Lin, C. Application of surface plasmon resonance sensing to studying elastohydrodynamic lubricant films. Appl. Opt. 2005, 44, 4830–4837. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.P.; Wong, C.L.; Wu, S.Y.; Law, W.C.; Lin, C.L.; Kong, S.K. Optical Sensing Devices with SPR Sensors Based on Differential Phase Interrogation and Measuring Method Using the Same. U.S. Patent 7365855, 24 November 2009. [Google Scholar]
- Wong, C.L.; Ho, H.P.; Chan, K.S.; Wu, S.Y.; Lin, C. Application of spectral surface plasmon resonance to gas pressure sensing. Opt. Eng. 2005, 44, 124403. [Google Scholar] [CrossRef]
- Lam, W.W.; Chu, L.H.; Wong, C.L.; Zhang, Y.T. A surface plasmon resonance system for the measurement of glucose in aqueous solution. Sens. Actuators B 2005, 105, 138–143. [Google Scholar] [CrossRef]
- Wong, C.L.; Yu, X.; Shum, P.; Ho, H.P. Optical Characterization of Elastohydrodynamic Lubrication Pressure with Surface Plasmon Resonance. In New Tribological Ways; Ghrib, T., Ed.; InTech: London, UK, 2011; pp. 21–46. ISBN 978-953-307-206-7. [Google Scholar]
- Wong, C.L.; Dinish, U.S.; Schmidt, M.S.; Olivo, M. Non-labeling multiplex surface enhanced Raman scattering (SERS) detection of volatile organic compounds (VOCs). Anal. Chim. Acta 2014, 844, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Dinish, U.S.; Buddharaju, K.D.; Schmidt, M.D.; Olivo, M. Surface-enhanced Raman scattering (SERS)-based volatile organic compounds (VOCs) detection using plasmonic bimetallic nanogap substrate. Appl. Phys. A 2014, 117, 687–692. [Google Scholar] [CrossRef]
- Wong, C.L.; Chen, G.C.K.; Ng, B.K.; Agarwal, S.; Fanani, N.; Lin, Z.; Vasudevan, S.; Chen, P. Photothermal imaging of nanoparticles beyond the diffraction limit. Opt. Eng. 2011, 50, 073201-1–073201-5. [Google Scholar] [CrossRef]
- Nilsson, C.E.; Abbas, S.; Bennemo, M.; Larsson, A.; Hämäläinen, M.D.; Frostell-Karlsson, A. A novel assay for influenza virus quantification using surface plasmon resonance. Vaccine 2010, 28, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Lee, S.J.; Kim, D.K.; Heo, N.S.; Park, J.Y.; Lee, S.Y. Development of label-free optical diagnosis for sensitive detection of influenza virus with genetically engineered fusion protein. Talanta 2012, 89, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tsao, Y.C.; Lee, F.J.; Tsai, W.H.; Wang, C.H.; Chuang, T.L.; Wu, M.S.; Lin, C.W. Optical fiber sensor based on surface plasmon resonance for rapid detection of avian influenza virus subtype H6: Initial studies. J. Virol. Methods 2016, 233, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon Resonance Based Sensors, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Homola, J.; Vaisocherová, H.; Dostálek, J.; Piliarik, M. Multi-analyte surface plasmon resonance biosensing. Methods 2005, 37, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.G.; Johnston, K.S.; Yee, S.S. High sensitivity surface plasmon resonance sensor based on phase detection. Sens. Actuators B 1996, 35, 187–191. [Google Scholar] [CrossRef]
- Ho, H.P.; Lam, W.W. Application of differential phase measurement technique to surface plasmon resonance sensors. Sens. Actuators B 2003, 96, 554–559. [Google Scholar] [CrossRef]
- Yu, X.L.; Wang, D.X.; Wei, X.; Ding, X.; Liao, W.; Zhao, X.S. A surface plasmon resonance imaging interferometry for protein micro-array detection. Sens. Actuators B 2005, 108, 765–771. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.C.; Tsai, W.H.; Wang, C.H.; Chuang, T.L.; Lin, C.W.; Tsao, Y.C.; Wu, M.S. Improvement of the sensitivity of the surface plasmon resonance sensors based on multi-layer modulation techniques. Opt. Commun. 2015, 335, 32–36. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.L.; Chua, M.; Mittman, H.; Choo, L.X.; Lim, H.Q.; Olivo, M. A Phase-Intensity Surface Plasmon Resonance Biosensor for Avian Influenza A (H5N1) Detection. Sensors 2017, 17, 2363. https://doi.org/10.3390/s17102363
Wong CL, Chua M, Mittman H, Choo LX, Lim HQ, Olivo M. A Phase-Intensity Surface Plasmon Resonance Biosensor for Avian Influenza A (H5N1) Detection. Sensors. 2017; 17(10):2363. https://doi.org/10.3390/s17102363
Chicago/Turabian StyleWong, Chi Lok, Marissa Chua, Heather Mittman, Li Xian Choo, Hann Qian Lim, and Malini Olivo. 2017. "A Phase-Intensity Surface Plasmon Resonance Biosensor for Avian Influenza A (H5N1) Detection" Sensors 17, no. 10: 2363. https://doi.org/10.3390/s17102363



