Wireless Biological Electronic Sensors
Abstract
:1. Introduction
2. Biological Electronic Sensing Transducers
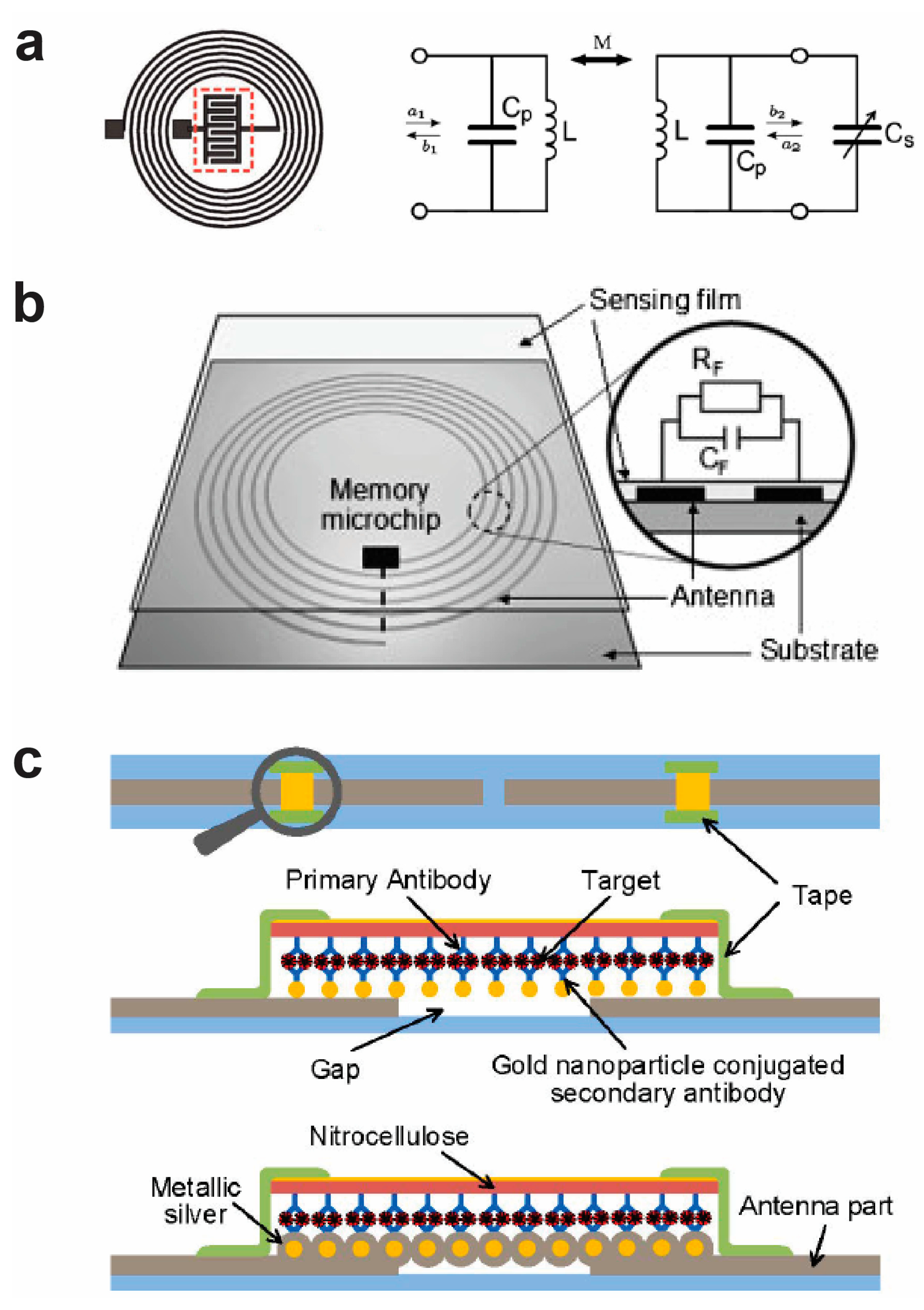
3. Wireless RFID-Based Biological Electronic Sensors
4. Wireless Acoustic Wave-Based Biological Electronic Sensors
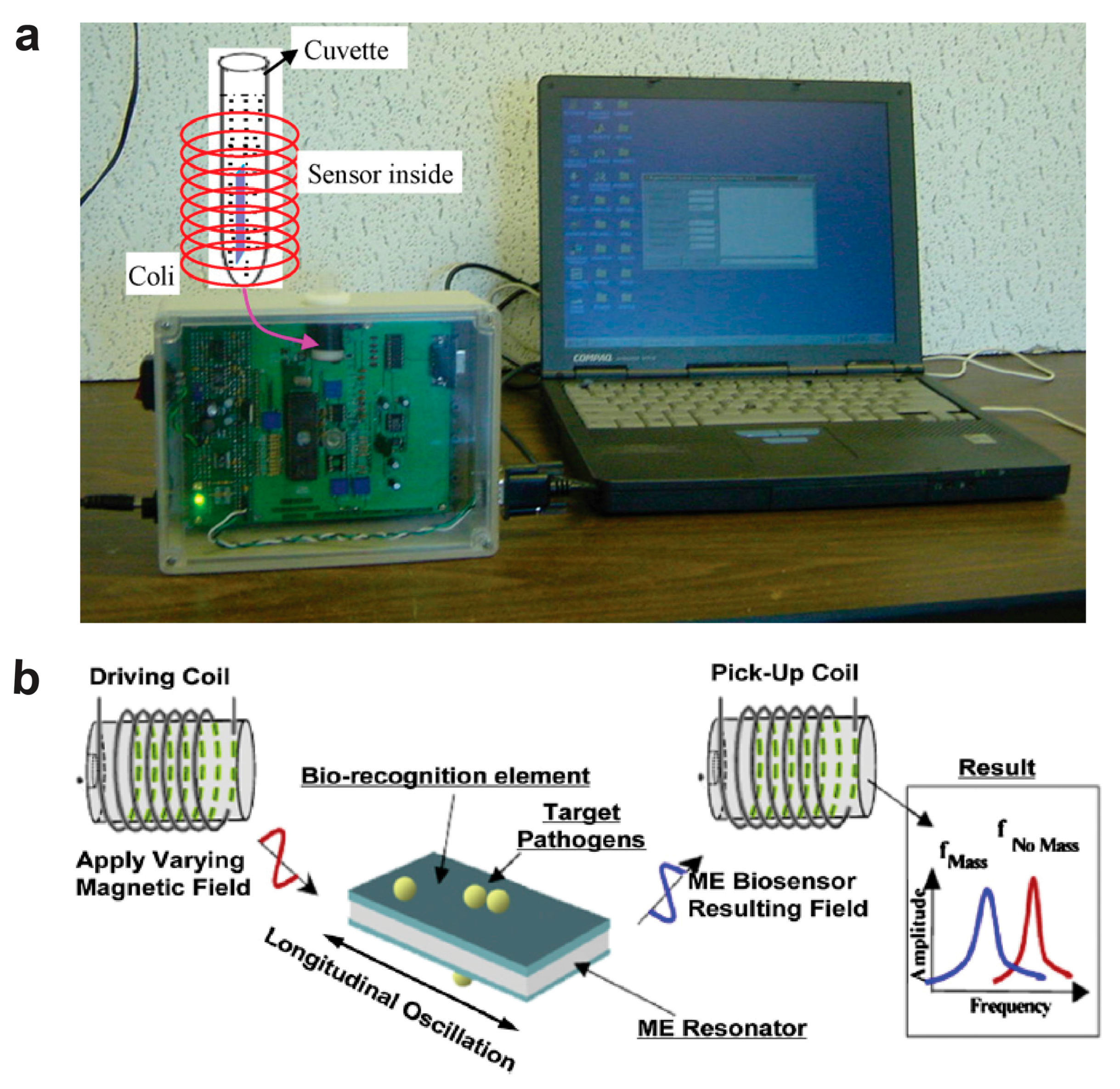
5. Wireless Magnetoelastic Biological Electronic Sensors
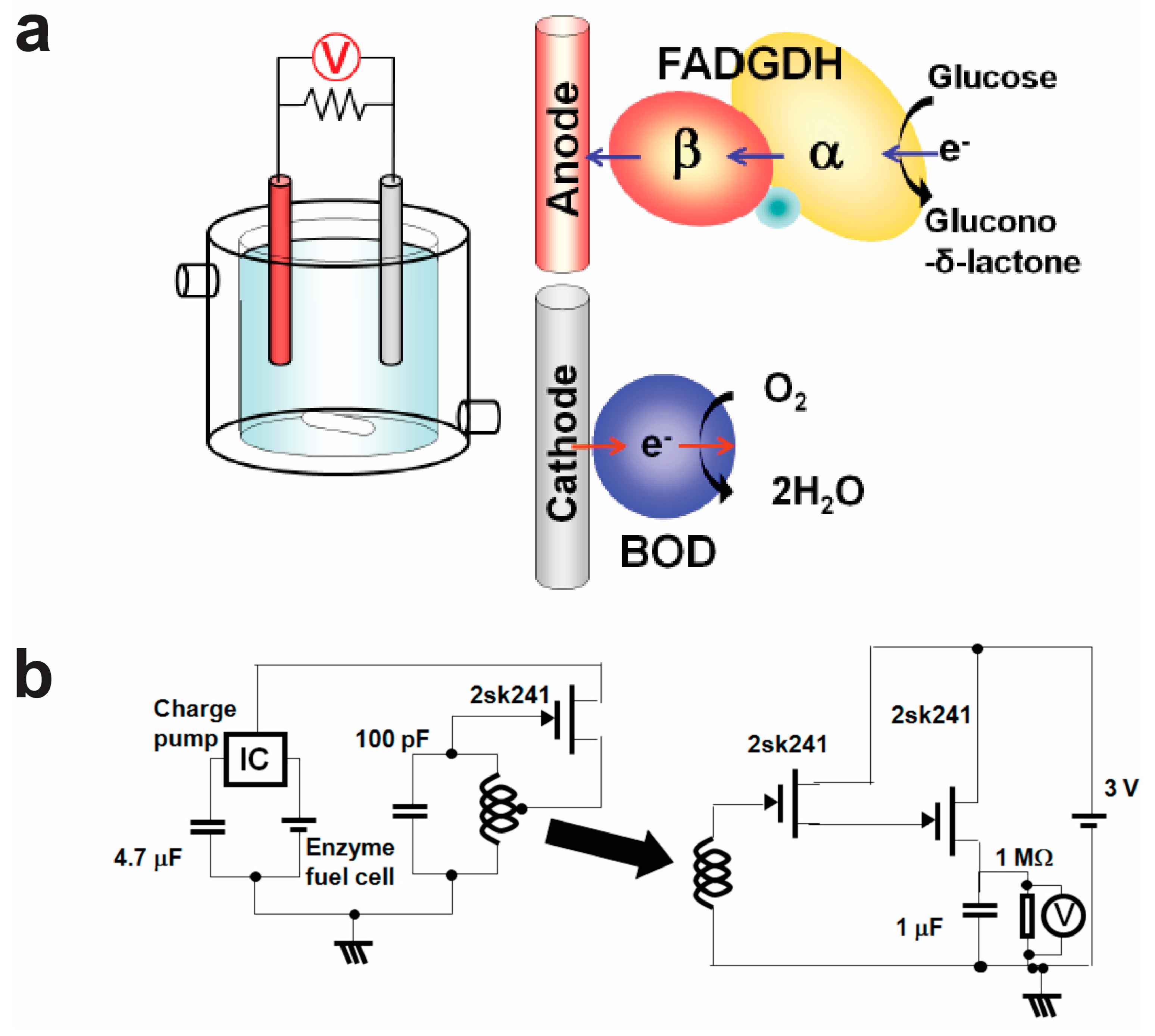
6. Wireless Self-Powered Biological Electronic Sensors
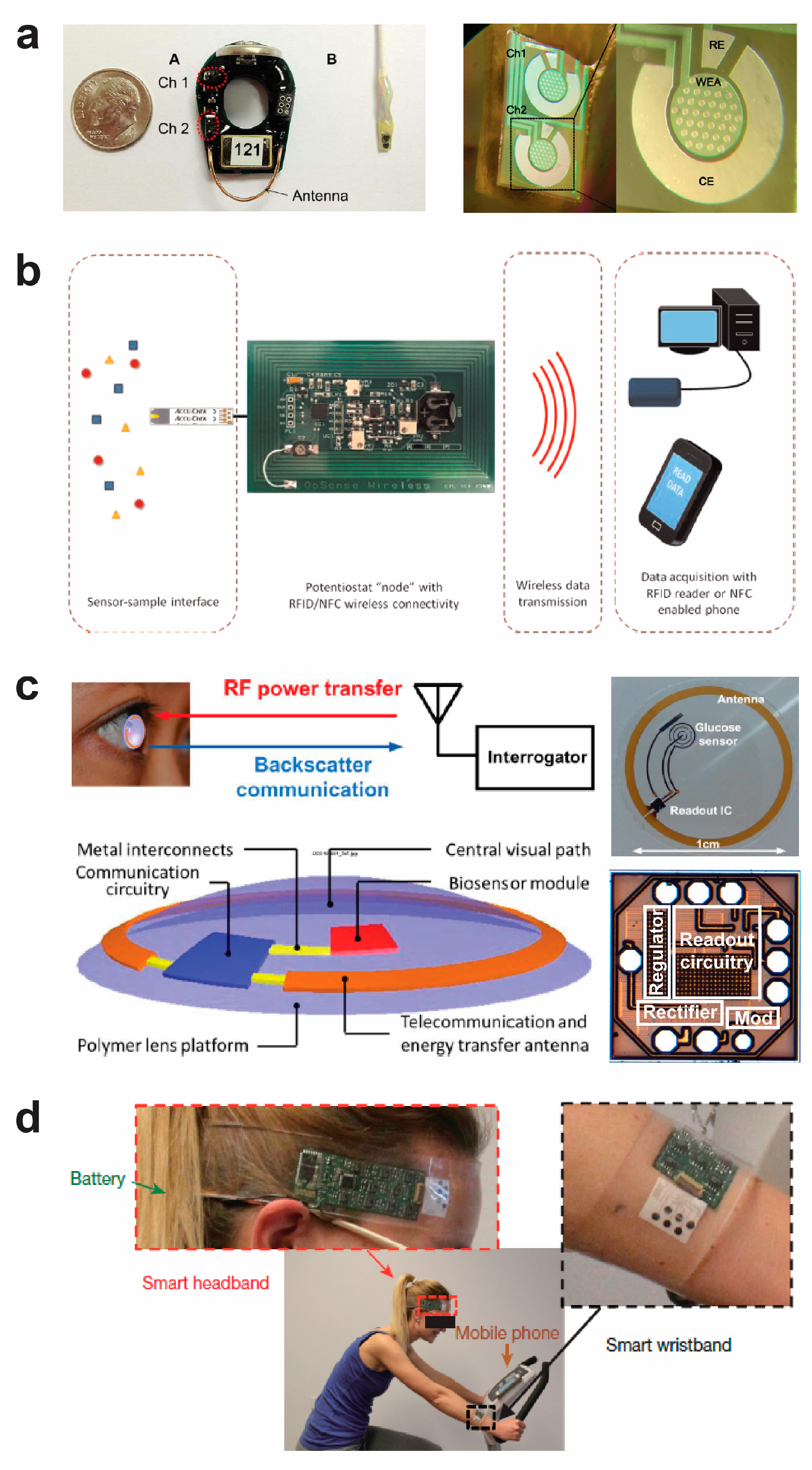
7. Wireless Potentiostat Systems for Biological Electronic Sensors
8. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Gao, N.; Gao, T.; Yang, X.; Dai, X.C.; Zhou, W.; Zhang, A.Q.; Lieber, C.M. Specific detection of biomolecules in physiological solutions using graphene transistor biosensors. Proc. Natl. Acad. Sci. USA 2016, 113, 14633–14638. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Turan, J.; Kesik, M.; Soylemez, S.; Goker, S.; Kolb, M.; Bahadir, M.; Toppare, L. Development of an amperometric biosensor based on a novel conducting copolymer for detection of anti-dementia drugs. J. Electroanal. Chem. 2014, 735, 43–50. [Google Scholar] [CrossRef]
- Koyuncu Zeybek, D.; Demir, B.; Zeybek, B.; Pekyardimci, Ş. A sensitive electrochemical DNA biosensor for antineoplastic drug 5-fluorouracil based on glassy carbon electrode modified with poly (bromocresol purple). Talanta 2015, 144, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Zhao, X.; Zazubovich, V. Detection of explosive compounds using Photosystem II-based biosensor. J. Electroanal. Chem. 2011, 657, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Komarova, N.V.; Andrianova, M.S.; Gubanova, O.V.; Kuznetsov, E.V.; Kuznetsov, A.E. Development of a novel enzymatic biosensor based on an ion-selective field effect transistor for the detection of explosives. Sens. Actuators B Chem. 2015, 221, 1017–1026. [Google Scholar] [CrossRef]
- Kochana, J.; Wapiennik, K.; Knihnicki, P.; Pollap, A.; Janus, P.; Oszajca, M.; Kustrowski, P. Mesoporous carbon-containing voltammetric biosensor for determination of tyramine in food products. Anal. Bioanal. Chem. 2016, 408, 5199–5210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz Zanini, V.; Lopez de Mishima, B.; Solis, V. An amperometric biosensor based on lactate oxidase immobilized in laponite-chitosan hydrogel on a glassy carbon electrode. Application to the analysis of l-lactate in food samples. Sens. Actuators B Chem. 2011, 155, 75–80. [Google Scholar] [CrossRef]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- Saidur, M.R.; Aziz, A.R.A.; Basirun, W.J. Recent advances in DNA-based electrochemical biosensors for heavy metal ion detection: A review. Biosens. Bioelectron. 2017, 90, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID Sensor Patch for Monitoring of Sweat Electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.J.S.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.Y.; Cheng, H.; Jeong, J.W.; Akce, A.; et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain-computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Tee, B.C.K.; Chortos, A.L.; Schwartz, G.; Tse, V.J.; Lipomi, D.; Wong, H.S.P.; McConnell, M.V.; Bao, Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Murphy, R.K.J.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdes-Ramirez, G.; Andrade, F.J.; Schoening, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Muhammad-Tahir, Z.; Alocilja, E.C. A conductometric biosensor for biosecurity. Biosens. Bioelectron. 2003, 18, 813–819. [Google Scholar] [CrossRef]
- Mohammad, R.; Ahmad, M.; Heng, L.Y. Amperometric capsaicin biosensor based on covalent immobilization of horseradish peroxidase (HRP) on acrylic microspheres for chilli hotness determination. Sens. Actuators B Chem. 2017, 241, 174–181. [Google Scholar] [CrossRef]
- Vásquez, G.; Rey, A.; Rivera, C.; Iregui, C.; Orozco, J. Amperometric biosensor based on a single antibody of dual function for rapid detection of Streptococcus agalactiae. Biosens. Bioelectron. 2017, 87, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.C.; Chen, R.T.; Liao, Y.H.; Lin, J.W.; Lin, C.Y.; Jhang, C.Y.; Chou, H.T. Fabrication of potentiometric enzymatic glucose biosensor based on graphene and magnetic beads. IEEE Sens. J. 2015, 15, 5278–5284. [Google Scholar] [CrossRef]
- Tarasov, A.; Gray, D.W.; Tsai, M.Y.; Shields, N.; Montrose, A.; Creedon, N.; Lovera, P.; O’Riordan, A.; Mooney, M.H.; Vogel, E.M. A potentiometric biosensor for rapid on-site disease diagnostics. Biosens. Bioelectron. 2016, 79, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.L.; Ng, W.N.; Shao, R.; Pereles, B.D.; Ong, K.G. A wireless, passive sensor for quantifying packaged food quality. Sensors 2007, 7, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Zeng, K.; Varghese, O.K.; Grimes, C.A. A staphylococcal enterotoxin B magnetoelastic immunosensor. Biosens. Bioelectron. 2004, 20, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Gronewold, T.M.A. Surface acoustic wave sensors in the bioanalytical field: Recent trends and challenges. Anal. Chim. Acta 2007, 603, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.; Park, J.Y.; Seo, H.; Lee, T.; Lee, W.; Kim, S.K.; Hahn, Y.K.; Jung, J.Y.; Kim, S.; et al. Sensitive and reproducible detection of cardiac troponin i in human plasma using a surface acoustic wave immunosensor. Sens. Actuators B Chem. 2013, 178, 19–25. [Google Scholar] [CrossRef]
- Ghosh, H.; RoyChaudhuri, C. Pore geometry optimization of nanocrystalline silicon oxide impedance biosensor. IEEE Sens. J. 2016, 16, 7843–7852. [Google Scholar] [CrossRef]
- Liang, G.; Man, Y.; Jin, X.; Pan, L.; Liu, X. Aptamer-based biosensor for label-free detection of ethanolamine by electrochemical impedance spectroscopy. Anal. Chim. Acta 2016, 936, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Mattiasson, B.; Hedström, M. Capacitive biosensors for ultra-sensitive assays. TrAC Trends Anal. Chem. 2016, 79, 233–238. [Google Scholar] [CrossRef]
- Basu, J.; Chaudhuri, C.R. Attomolar sensitivity of FET biosensor based on smooth and reliable graphene nanogrids. IEEE Electron Device Lett. 2016, 37, 492–495. [Google Scholar] [CrossRef]
- Lee, I.; Lee, S.W.; Lee, K.Y.; Park, C.; Kim, D.; Lee, J.S.; Yi, H.; Kim, B. A reconfigurable and portable highly sensitive biosensor platform for isfet and enzyme-based sensors. IEEE Sens. J. 2016, 16, 4443–4451. [Google Scholar] [CrossRef]
- Cui, Y.; Barford, J.P.; Renneberg, R. Development of a glucose-6-phosphate biosensor based on coimmobilized p-hydroxybenzoate hydroxylase and glucose-6-phosphate dehydrogenase. Biosens. Bioelectron. 2007, 22, 2754–2758. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kim, S.N.; Naik, R.R.; McAlpine, M.C. Biomimetic peptide nanosensors. Acc. Chem. Res. 2012, 45, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Guzmán, M.; García-Carmona, L.; Molinero-Fernández, Á.; Cava, F.; López Gil, M.Á.; Escarpa, A. Bi-enzymatic biosensor for on-site, fast and reliable electrochemical detection of relevant D-amino acids in bacterial samples. Sens. Actuators B Chem. 2017, 242, 95–101. [Google Scholar] [CrossRef]
- Sharma, R.; Deacon, S.E.; Nowak, D.; George, S.E.; Szymonik, M.P.; Tang, A.A.S.; Tomlinson, D.C.; Davies, A.G.; McPherson, M.J.; Wälti, C. Label-free electrochemical impedance biosensor to detect human interleukin-8 in serum with sub-pg/ml sensitivity. Biosens. Bioelectron. 2016, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ren, R.; Pu, H.; Chang, J.; Mao, S.; Chen, J. Field-effect transistor biosensors with two-dimensional black phosphorus nanosheets. Biosens. Bioelectron. 2017, 89, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Li, Y.S.; Chiang, W.H.; Pei, Z. A high sensitivity field effect transistor biosensor for methylene blue detection utilize graphene oxide nanoribbon. Biosens. Bioelectron. 2017, 89, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Labroo, P.; Cui, Y. Flexible graphene bio-nanosensor for lactate. Biosens. Bioelectron. 2013, 41, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Niazi, J.H.; Kallempudi, S.; Gurbuz, Y. Label-free capacitive biosensor for sensitive detection of multiple biomarkers using gold interdigitated capacitor arrays. Biosens. Bioelectron. 2010, 25, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, D.; Chen, J.; Wang, G. The effect of a SiO2 layer on the performance of a ZnO-based SAW device for high sensitivity biosensor applications. Smart Mater. Struct. 2009, 18, 115021. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.B.; Kim, Y.I. Mass sensitivity calculation of the protein layer using love wave SAW biosensor. J. Nanosci. Nanotechnol. 2012, 12, 6107–6112. [Google Scholar] [CrossRef] [PubMed]
- Perng, J.K.; Hunt, W.D.; Edmonson, P.J. Development of a shear horizontal SAW RFID biosensor. In Proceedings of the 2007 IEEE Sensors, Atlanta, GA, USA, 28–31 October 2007; pp. 691–694. [Google Scholar]
- Araya-Kleinsteuber, B.; Roque, A.C.A.; Kioupritzi, E.; Stevenson, A.C.; Lowe, C.R. Magnetic acoustic resonance immunoassay (MARIA): A multifrequency acoustic approach for the non-labelled detection of biomolecular interactions. J. Mol. Recognit. 2006, 19, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Horikawa, S.; Wikle, H.C.; Wang, Z.; Chin, B.A. Surface-scanning coil detectors for magnetoelastic biosensors: A comparison of planar-spiral and solenoid coils. Appl. Phys. Lett. 2013, 103, 173510. [Google Scholar] [CrossRef]
- Park, M.K.; Park, J.W.; Wikle Iii, H.C.; Chin, B.A. Evaluation of phage-based magnetoelastic biosensors for direct detection of Salmonella Typhimurium on spinach leaves. Sens. Actuators B Chem. 2013, 176, 1134–1140. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Xiang, Y.; Zhou, L.; Wang, T.; Zhang, Z.; Sun, K.; Yin, D.; Li, Y.; Xie, G. An electrochemical biosensor for double-stranded Wnt7B gene detection based on enzymatic isothermal amplification. Biosens. Bioelectron. 2016, 86, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Alferov, S.V.; Alferov, V.A.; Reshetilov, A.N. BOD biosensor based on the yeast Debaryomyces hansenii immobilized in poly(vinyl alcohol) modified by N-vinylpyrrolidone. Enzyme Microb. Technol. 2013, 53, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dhall, P.; Kumar, A.; Joshi, A.; Saxsena, T.K.; Manoharan, A.; Makhijani, S.D.; Kumar, R. Quick and reliable estimation of BOD load of beverage industrial wastewater by developing BOD biosensor. Sens. Actuators B Chem. 2008, 133, 478–483. [Google Scholar] [CrossRef]
- Eksi, H.; Guzel, R.; Guven, B.; Boyaci, I.H.; Solak, A.O. Fabrication of an electrochemical E. coli Biosensor in biowells using bimetallic nanoparticle-labelled antibodies. Electroanalysis 2015, 27, 343–352. [Google Scholar] [CrossRef]
- Shen, Y.C.; Yang, C.H.; Chen, S.W.; Wu, S.H.; Yang, T.L.; Huang, J.J. IGZO thin film transistor biosensors functionalized with ZnO nanorods and antibodies. Biosens. Bioelectron. 2014, 54, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, M.; Yuan, F.; Li, Z.; Zhou, W. Uniform DNA biosensors based on threshold voltage of carbon nanotube thin-film transistors. Nano 2016, 11, 1650060. [Google Scholar] [CrossRef]
- Ping, J.; Vishnubhotla, R.; Vrudhula, A.; Johnson, A.T.C. Scalable Production of high-sensitivity, label-free DNA Biosensors based on back-gated graphene field effect transistors. ACS Nano 2016, 10, 8700–8704. [Google Scholar] [CrossRef] [PubMed]
- Khatayevich, D.; Page, T.; Gresswell, C.; Hayamizu, Y.; Grady, W.; Sarikaya, M. Selective detection of target proteins by peptide-enabled graphene biosensor. Small 2014, 10, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Ryu, M.Y.; Park, C.Y.; Ahn, J.; Park, H.G.; Choi, C.; Ha, S.D.; Park, T.J.; Park, J.P. High sensitive and selective electrochemical biosensor: Label-free detection of human norovirus using affinity peptide as molecular binder. Biosens. Bioelectron. 2017, 87, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Lakshmanan, R.S.; Mathison, L.C.; Petrenko, V.A.; Chin, B.A. Phage coated magnetoelastic micro-biosensors for real-time detection of Bacillus anthracis spores. Sens. Actuators B Chem. 2009, 137, 501–506. [Google Scholar] [CrossRef]
- Huang, S.; Yang, H.; Lakshmanan, R.S.; Johnson, M.L.; Chen, I.; Wan, J.; Wikle, H.C.; Petrenko, V.A.; Barbaree, J.M.; Cheng, Z.Y.; et al. The Effect of salt and phage concentrations on the binding sensitivity of magnetoelastic biosensors for Bacillus anthracis detection. Biotechnol. Bioeng. 2008, 101, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Chahal, P.; Alocilja, E.C.; Chakrabartty, S. Wireless biosensing using silver-enhancement based self-assembled antennas in passive radio frequency identification (RFID) tags. IEEE Sens. J. 2015, 15, 4442–4450. [Google Scholar] [CrossRef]
- Gao, X.; Yang, W.; Pang, P.; Liao, S.; Cai, Q.; Zeng, K.; Grimes, C.A. A wireless magnetoelastic biosensor for rapid detection of glucose concentrations in urine samples. Sens. Actuators B Chem. 2007, 128, 161–167. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Nagraj, N.; Tang, Z.; Mondello, F.J.; Surman, C.; Morris, W. Battery-free radio frequency identification (RFID) sensors for food quality and safety. J. Agric. Food Chem. 2012, 60, 8535–8543. [Google Scholar] [CrossRef] [PubMed]
- Croux, D.; Vangerven, T.; Broeders, J.; Boutsen, J.; Peeters, M.; Duchateau, S.; Cleij, T.; Deferme, W.; Wagner, P.; Thoelen, R.; et al. Molecular imprinted polymer films on RFID tags: A first step towards disposable packaging sensors. Phys. Status Solidi A 2013, 210, 938–944. [Google Scholar] [CrossRef]
- Yuan, M.; Alocilja, E.C.; Chakrabartty, S. A novel biosensor based on silver-enhanced self-assembled radio-frequency antennas. IEEE Sens. J. 2014, 14, 941–942. [Google Scholar] [CrossRef]
- Potyrailol, R.A.; Morris, W.G.; Sivavec, T.; Tomlinson, H.W.; Klensmeden, S.; Lindh, K. RFID sensors based on ubiquitous passive 13.56-MHz RFID tags and complex impedance detection. Wirel. Commun. Mob. Comput. 2009, 9, 1318–1330. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.S.; Jeon, S.; Kim, M.; Kim, S.; Kim, K.; Bien, F.; Hong, S.Y.; Park, J.U. Highly transparent and stretchable field-effect transistor sensors using graphene-nanowire hybrid nanostructures. Adv. Mater. 2015, 27, 3292–3297. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.L.; Yang, Y.; Zhang, Y.H.; Zhou, C.J.; Guo, CR.; Liu, J.; Ren, T.L. A high sensitivity wireless mass-loading surface acoustic wave DNA biosensor. Mod. Phys. Lett. B 2014, 28, 1450056. [Google Scholar] [CrossRef]
- Oh, H.; Fu, C.; Kim, K.; Lee, K. Wireless and simultaneous detections of multiple bio-molecules in a single sensor using Love wave biosensor. Sensors 2014, 14, 21660–21675. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lu, Q.; Ge, S.; Cai, Q.; Grimes, C.A. Detection of pathogen Escherichia coli O157:H7 with a wireless magnetoelastic-sensing device amplified by using chitosan-modified magnetic Fe3O4 nanoparticles. Sens. Actuators B Chem. 2010, 147, 343–349. [Google Scholar] [CrossRef]
- Chai, Y.; Wikle, H.C.; Wang, Z.; Horikawa, S.; Best, S.; Cheng, Z.; Dyer, D.F.; Chin, B.A. Design of a surface-scanning coil detector for direct bacteria detection on food surfaces using a magnetoelastic biosensor. J. Appl. Phys. 2013, 114, 104504. [Google Scholar] [CrossRef]
- Li, S.; Horikawa, S.; Park, M.K.; Chai, Y.; Vodyanoy, V.J.; Chin, B.A. Amorphous metallic glass biosensors. Intermetallics 2012, 30, 80–85. [Google Scholar] [CrossRef]
- Wu, S.; Gao, X.; Cai, Q.; Grimes, C.A. A wireless magnetoelastic biosensor for convenient and sensitive detection of acid phosphatase. Sens. Actuators B Chem. 2007, 123, 856–859. [Google Scholar] [CrossRef]
- He, B.; Liao, L.; Xiao, X.; Gao, S.; Wu, Y. Monitoring of Mycoplasma genitalium growth and evaluation of antibacterial activity of antibiotics tetracycline and levofloxacin using a wireless magnetoelastic sensor. Biosens. Bioelectron. 2009, 24, 1990–1994. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.S.; Guntupalli, R.; Hu, J.; Petrenko, V.A.; Barbaree, J.M.; Chin, B.A. Detection of Salmonella typhimurium in fat free milk using a phage immobilized magnetoelastic sensor. Sens. Actuators B Chem. 2007, 126, 544–550. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Chen, H.; Horikawa, S.; Shen, W.; Simonian, A.; Chin, B.A. Direct detection of Salmonella typhimurium on fresh produce using phage-based magnetoelastic biosensors. Biosens. Bioelectron. 2010, 26, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Hanashi, T.; Yamazaki, T.; Tsugawa, W.; Ikebukuro, K.; Sode, K. BioRadioTransmitter: A self-powered wireless glucose-sensing system. J. Diabetes Sci. Techol. 2011, 5, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Kotanen, C.N.; Guiseppi-Elie, A. Characterization of a wireless potentiostat for integration with a novel implantable biotransducer. IEEE Sens. J. 2014, 14, 768–776. [Google Scholar] [CrossRef]
- Steinberg, M.D.; Kassal, P.; Kereković, I.; Steinberg, I.M. A wireless potentiostat for mobile chemical sensing and biosensing. Talanta 2015, 143, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.T.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B.P. A 3-μW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdes-Ramirez, G.; Paixao, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, ncomms11650. [Google Scholar] [CrossRef] [PubMed]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y. Wireless Biological Electronic Sensors. Sensors 2017, 17, 2289. https://doi.org/10.3390/s17102289
Cui Y. Wireless Biological Electronic Sensors. Sensors. 2017; 17(10):2289. https://doi.org/10.3390/s17102289
Chicago/Turabian StyleCui, Yue. 2017. "Wireless Biological Electronic Sensors" Sensors 17, no. 10: 2289. https://doi.org/10.3390/s17102289



