Co3O4 as p-Type Material for CO Sensing in Humid Air
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Co3O4 Powder
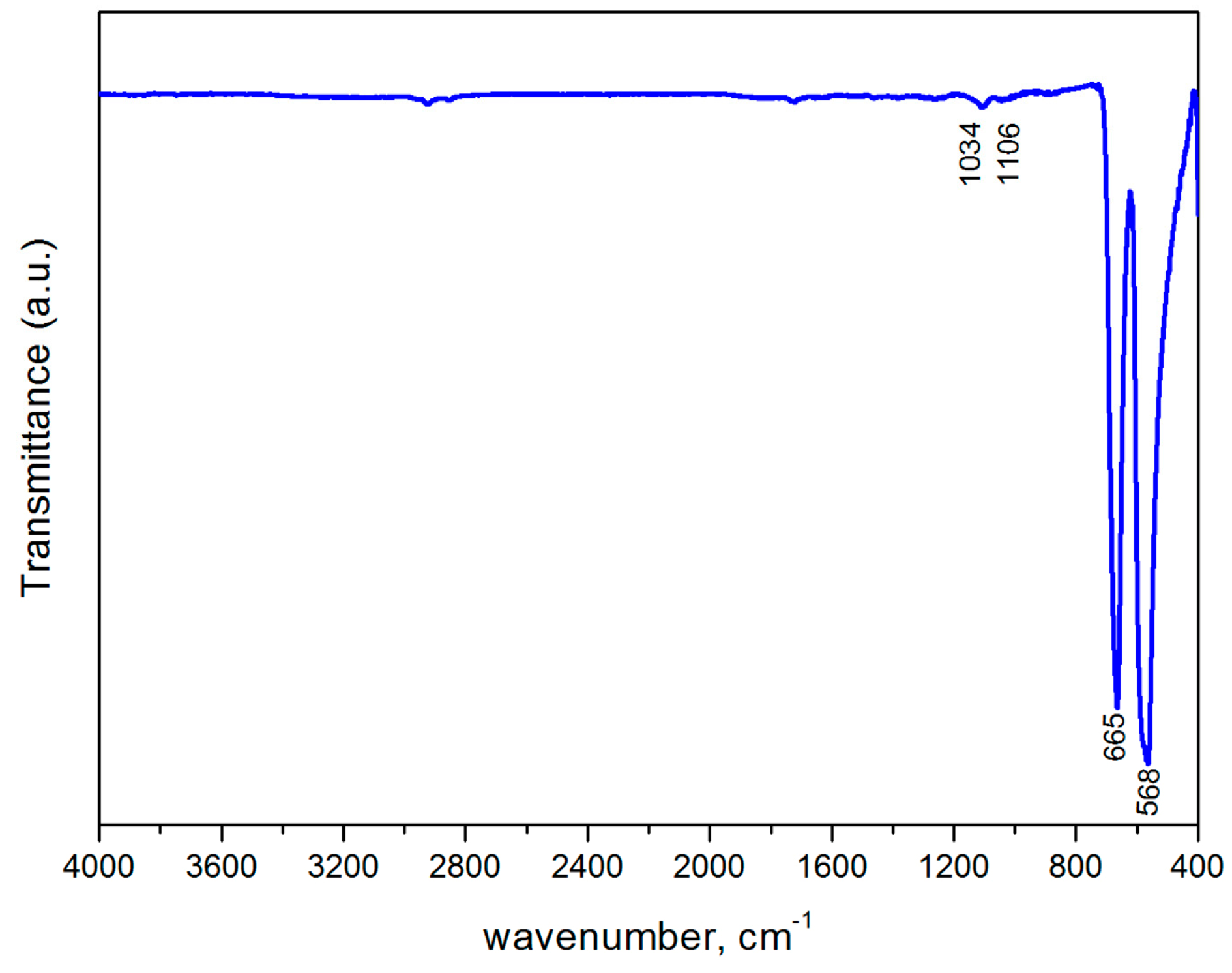
2.2. Characterization
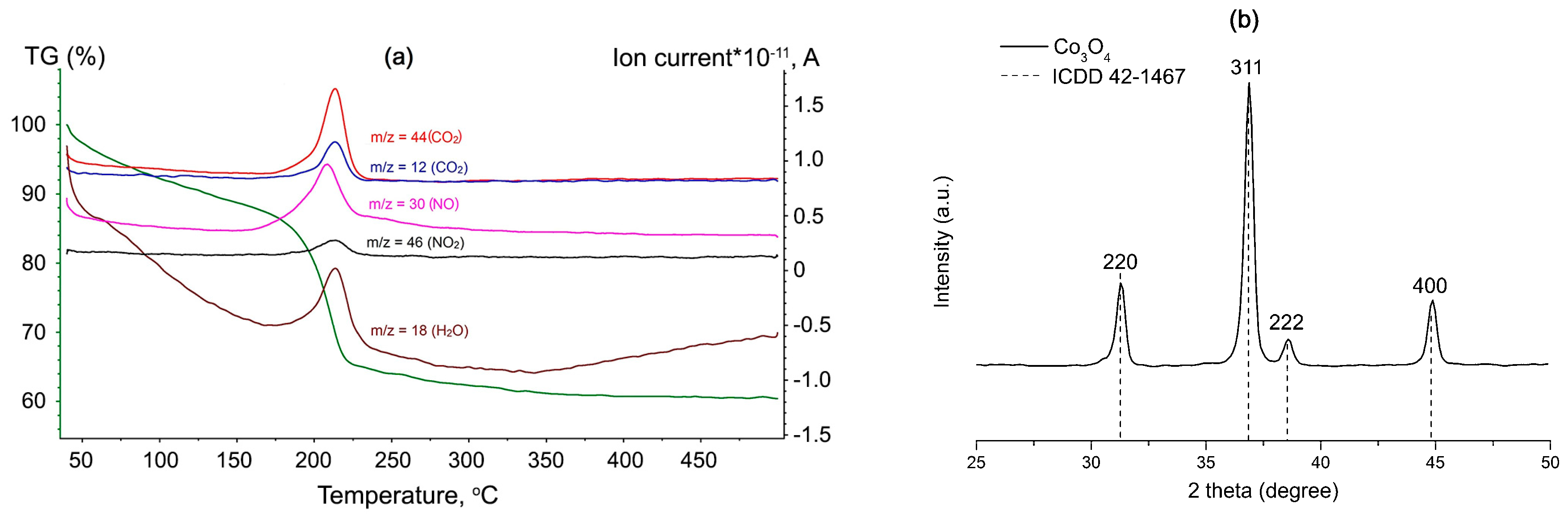
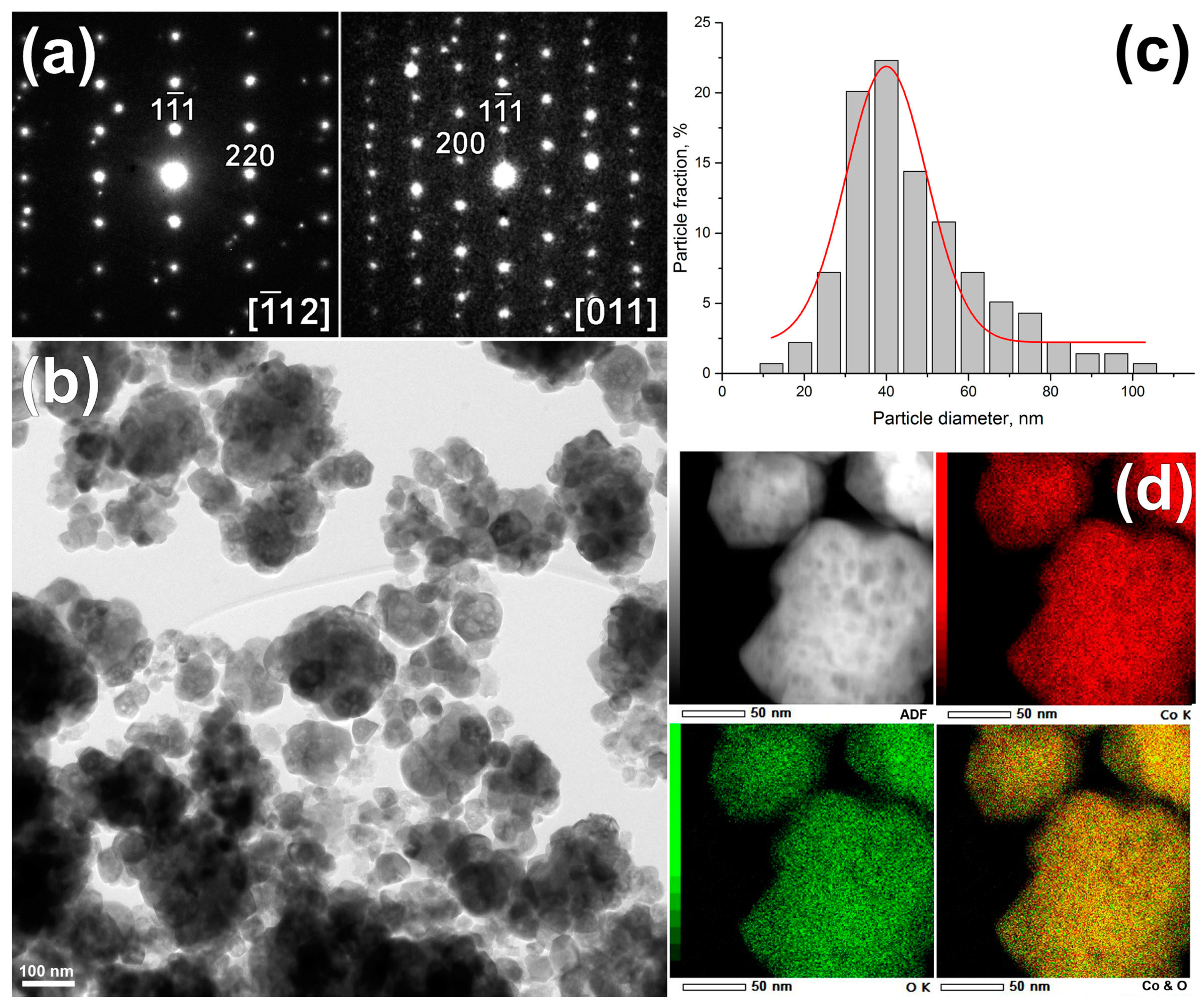
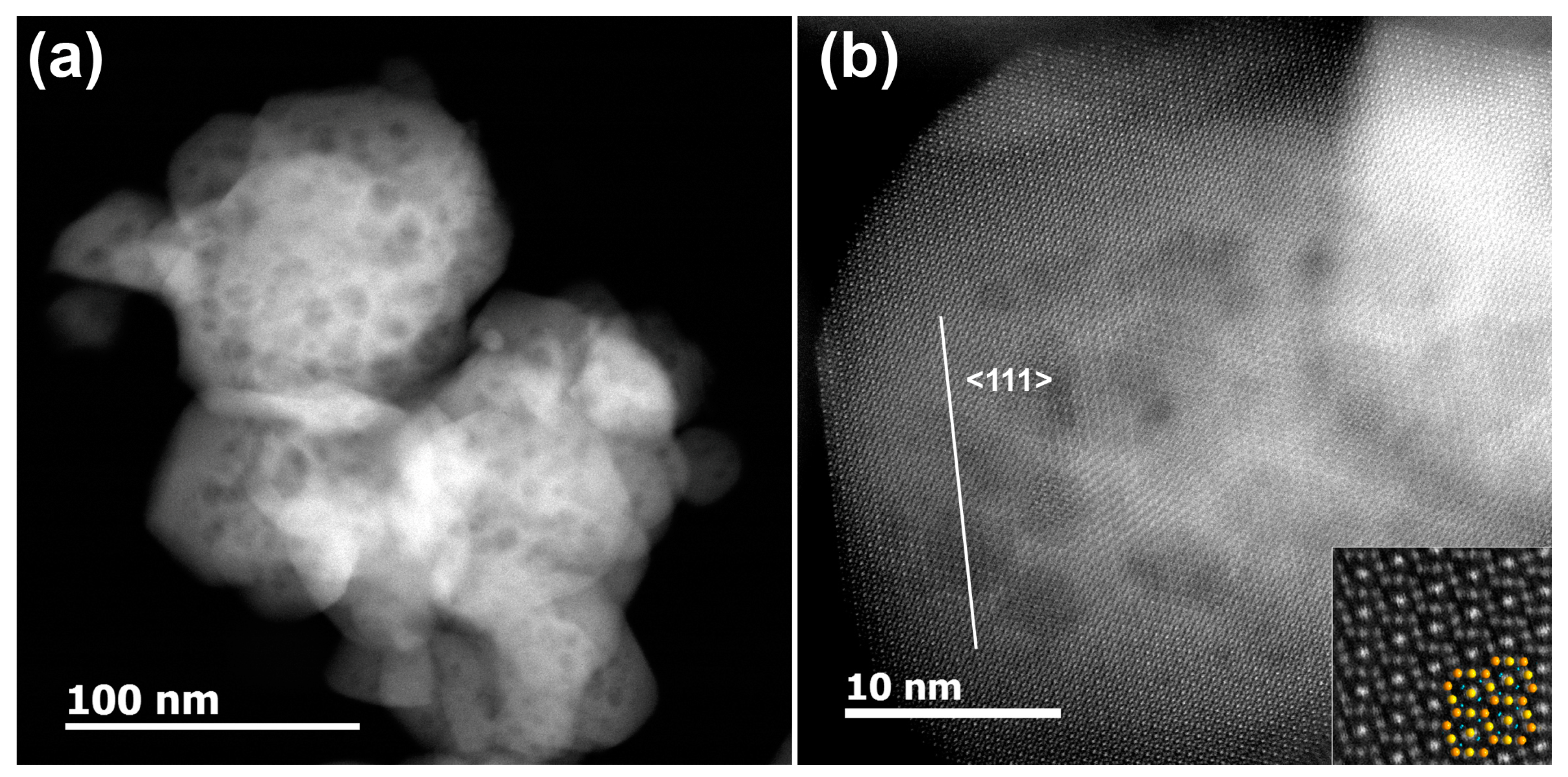
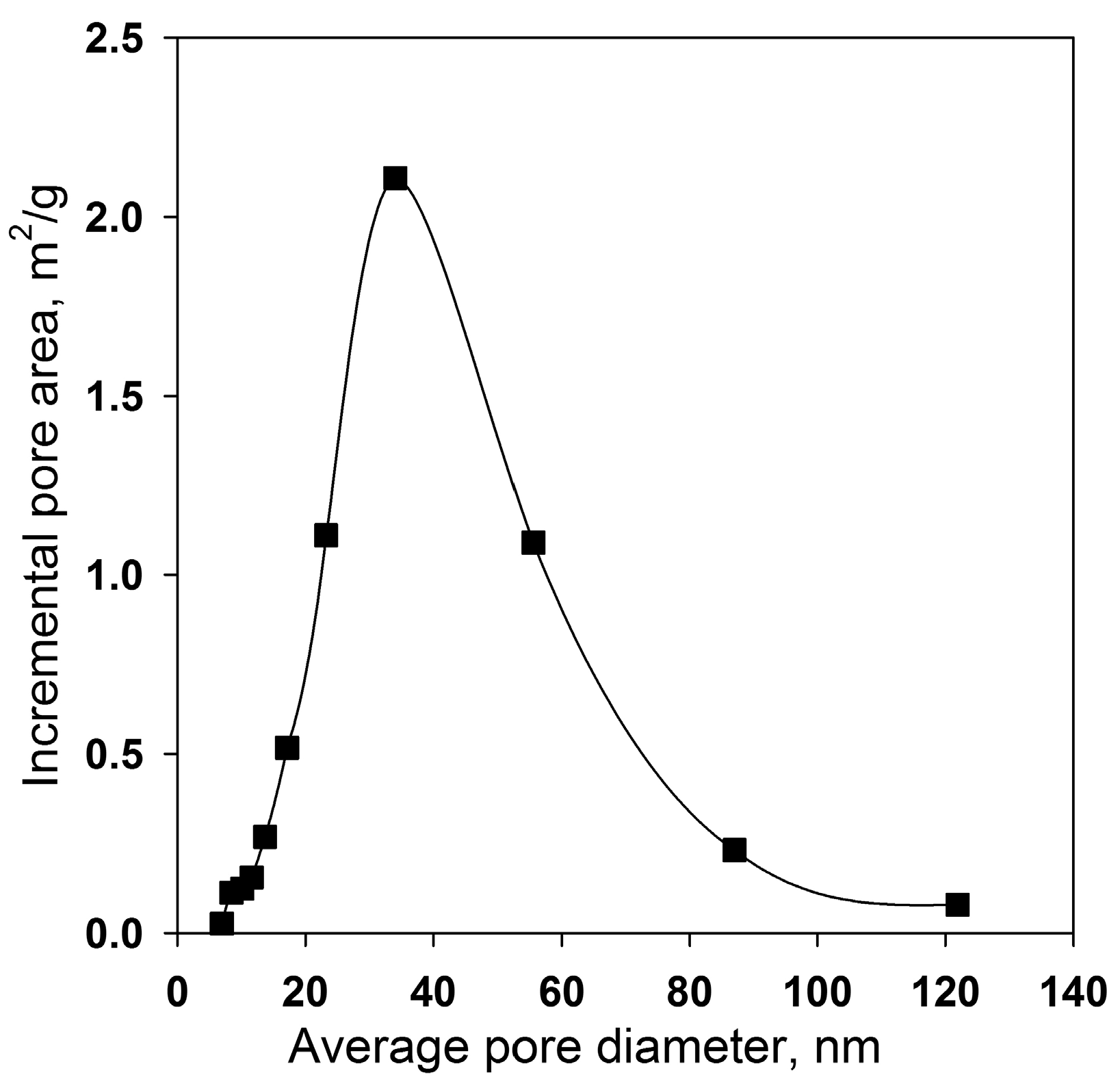
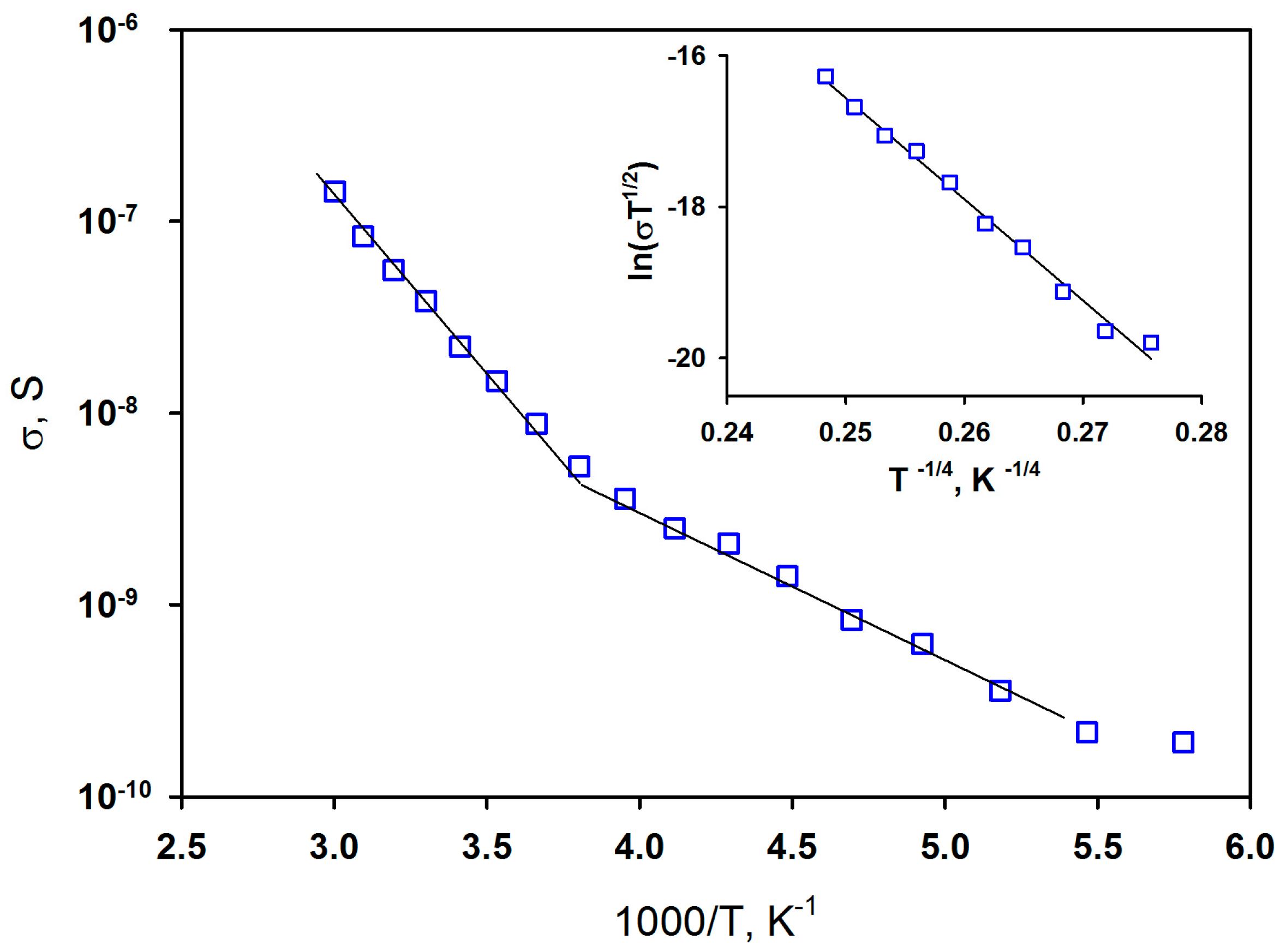
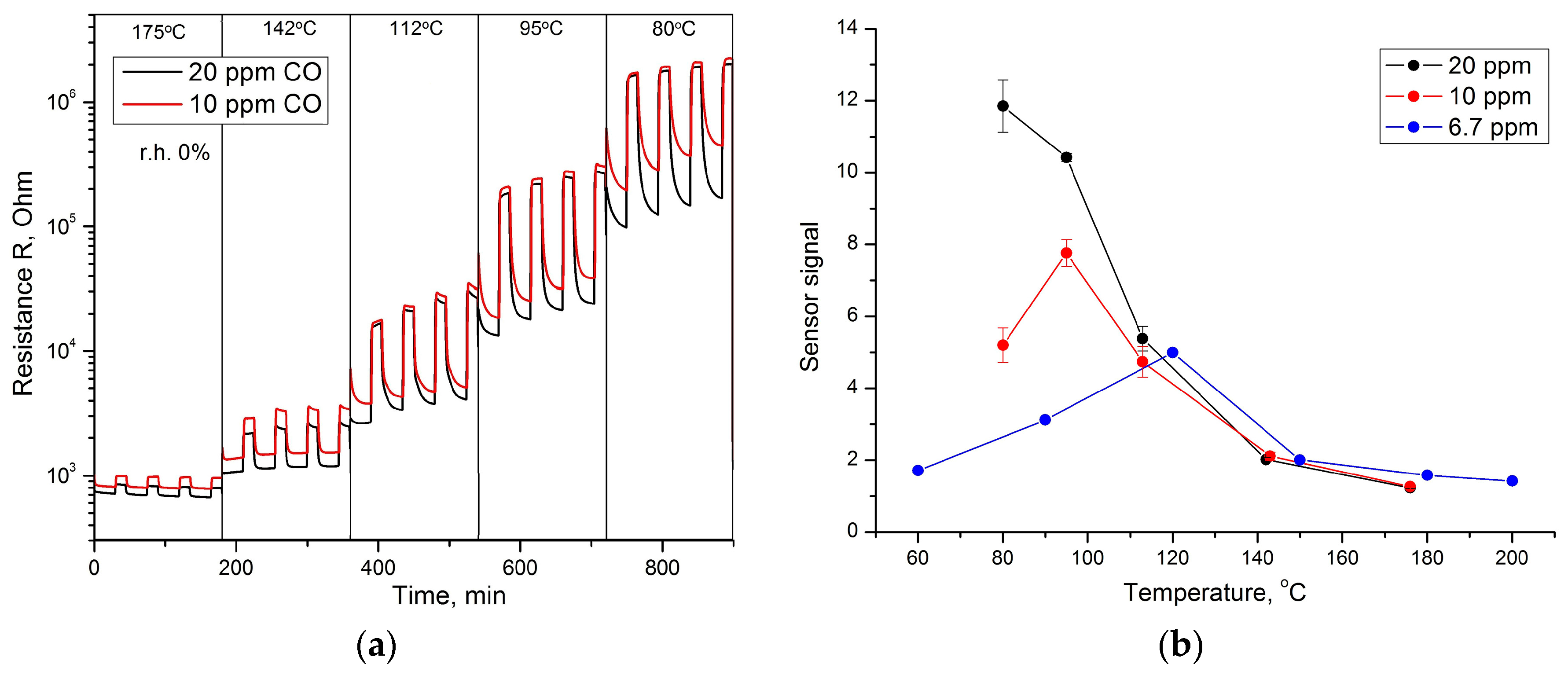
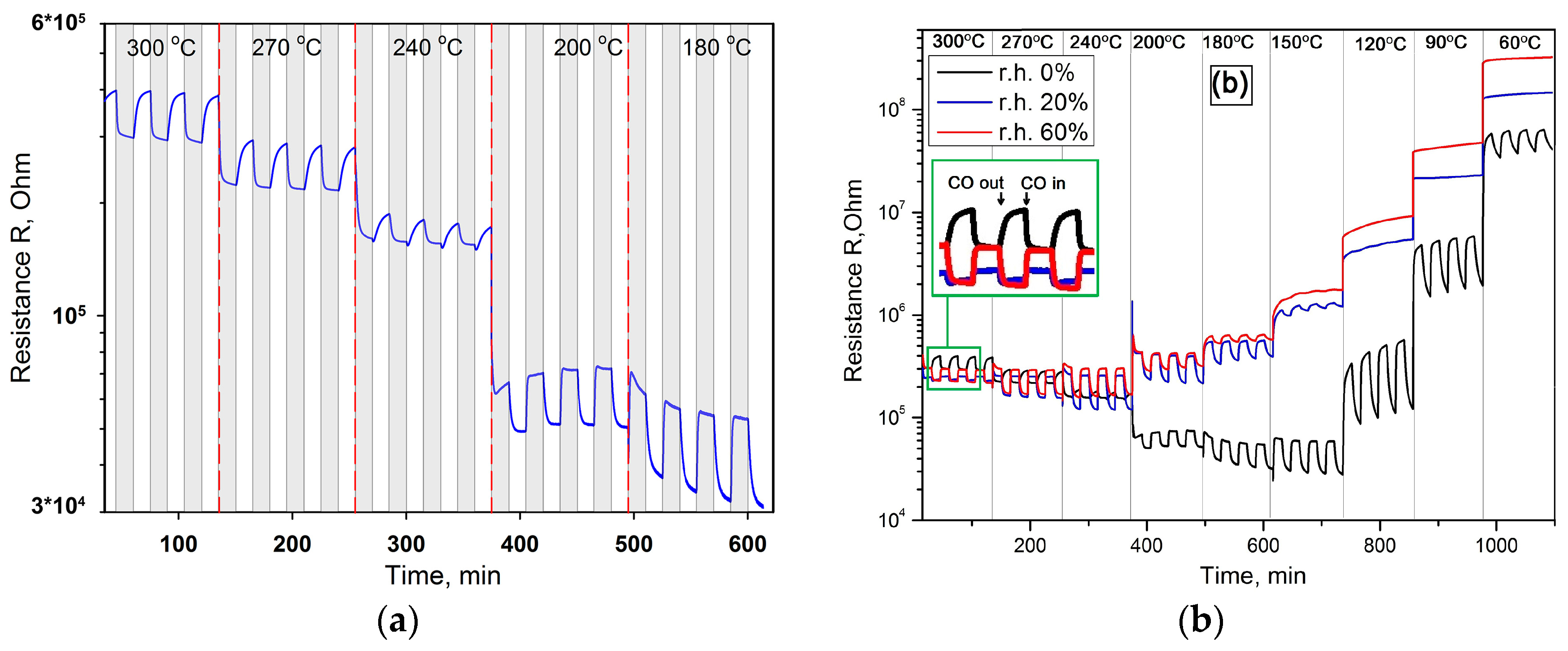
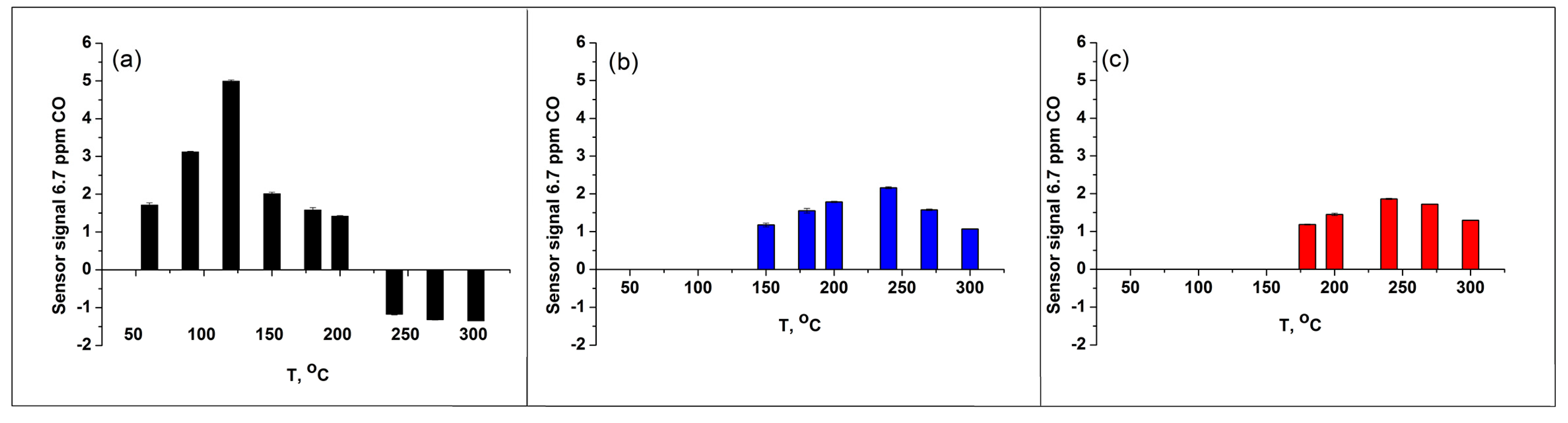
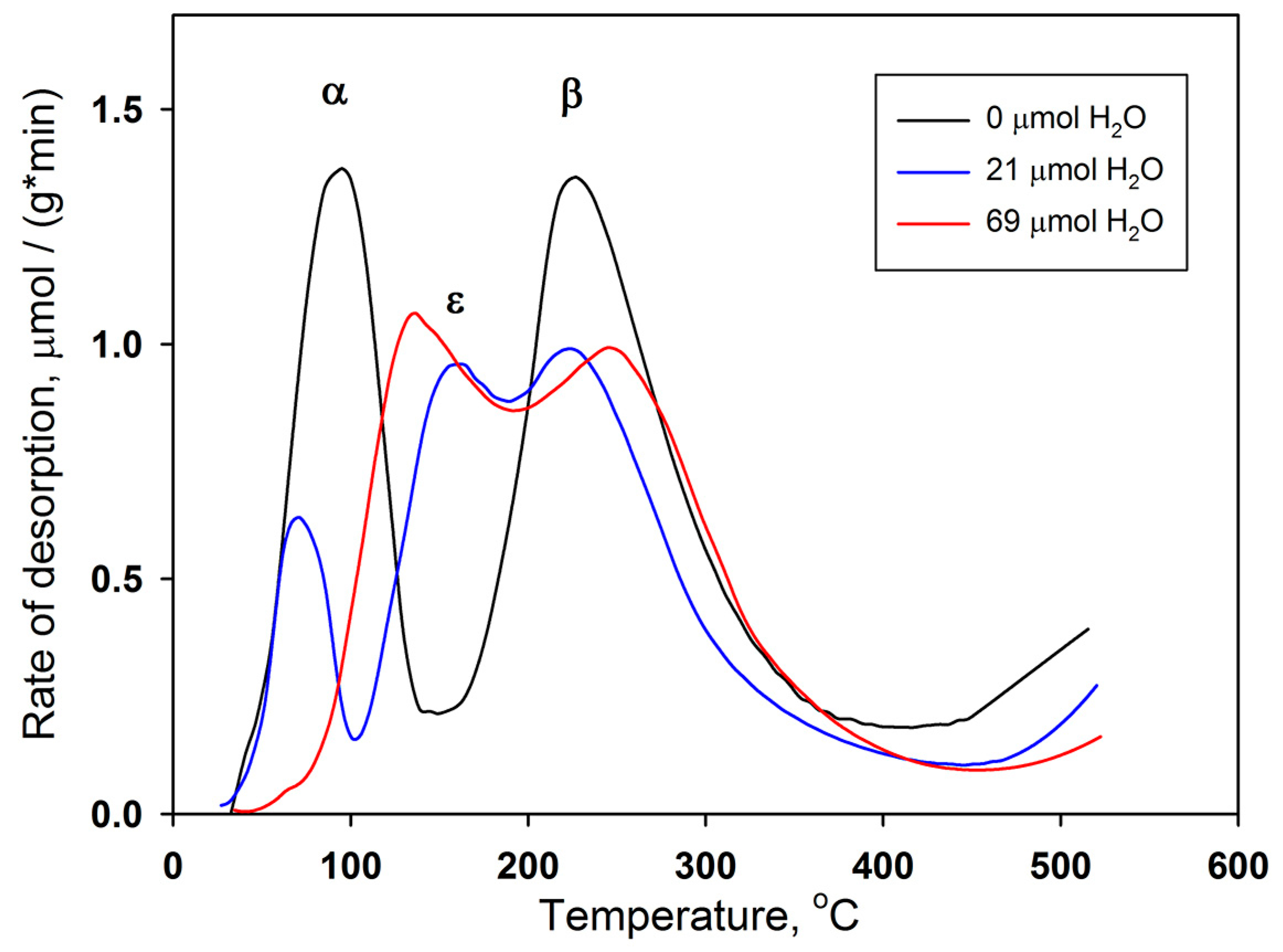
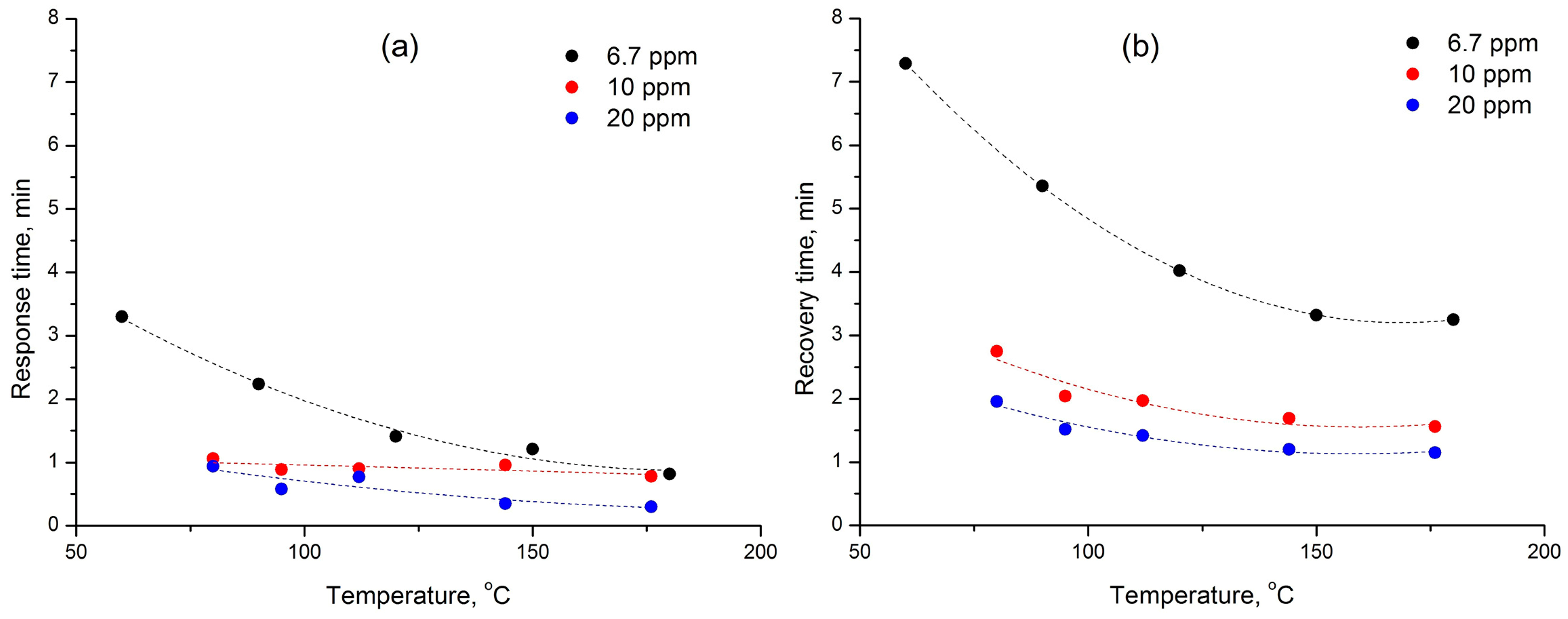
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Malkov, I.; Garshev, A.; Mordvinova, N.; Lebedev, O.I.; Dolenko, S.; Efitorov, A.; Grigoriev, T.; Rumyantseva, M.; Gaskov, A. Chemically modified nanocrystalline SnO2-based materials for nitrogen-containing gases detection using gas sensor array. J. Alloys Compd. 2017, 691, 514–523. [Google Scholar] [CrossRef]
- Sankar Ganesha, R.; Durgadevi, E.; Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Low temperature ammonia gas sensor based on Mn- doped ZnO nanoparticle decorated microspheres. J. Alloys Compd. 2017, 721, 182–190. [Google Scholar] [CrossRef]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B Chem. 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Filatova, D.; Spiridonov, F.; Zaytsev, V.; Zaytseva, A.; Gaskov, A. Highly sensitive ZnO (Ga, In) for sub-ppm level NO2 detection: Effect of indium content. Chemosensors 2017, 5, 18. [Google Scholar] [CrossRef]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.-D.; Tiemann, M. Ordered Mesoporous In2O3: Synthesis by structure replication and application as a methane gas sensor. Adv. Funct. Mater. 2009, 19, 653–661. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Vasiliev, R.; Filatova, D.; Drozdov, K.; Krylov, I.; Marchevsky, A.; Karakulina, O.; Abakumov, A.; Gaskov, A. Visible light activation of room temperature NO2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Krško, O.; Plecenik, T.; Roch, T.; Grančič, B.; Satrapinskyy, L.; Truchlý, M.; Ďurina, P.; Gregor, M.; Kúš, P.; Plecenik, A. Flexible highly sensitive hydrogen gas sensor based on a TiO2 thin film on polyimide foil. Sens. Actuators B Chem. 2017, 240, 1058–1065. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, J.P. The advances of Co3O4 as gas sensing materials: A review. J. Alloys Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yoda, Y.; Yamazoe, N.; Seiyama, T. Study of metal oxide catalysts by temperature programmed desorption. 4. Oxygen adsorption on various metal oxides. J. Phys. Chem. 1978, 82, 2564–2570. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Vetter, S.; Haffer, S.; Wagner, T.; Tiemann, M. Nanostructured Co3O4 as a CO gas sensor: Temperature-dependent behavior. Sens. Actuators B Chem. 2015, 206, 133–138. [Google Scholar] [CrossRef]
- Boreskov, J.K. Catalytic activation of dioxygen. In Catalysis: Science and Technology, 1st ed.; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin, Germany, 1982; Volume 3, pp. 139–198. [Google Scholar]
- Patil, D.; Patil, P.; Subramanian, V.; Joy, P.A.; Potdar, H.S. Highly sensitive and fast responding CO sensor based on Co3O4 nanorods. Talanta 2010, 81, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Su, X.T.; Xiao, F.; Niu, C.G.; Wang, J.D. Synthesis of nearly monodisperse Co3O4 nanocubes via microwave-assisted solvothermal process and their gas sensing properties. Sens. Actuators B Chem. 2011, 157, 681–685. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, H.-R.; Kim, K.-M.; Liu, D.; Cao, G.; Lee, J.-H. C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction. Sens. Actuators B Chem. 2010, 146, 183–189. [Google Scholar] [CrossRef]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Tan, W.; Lin, Z.; Huang, X. Synthesis of hollow and hollowed-out Co3O4 microspheres assembled by porous ultrathin nanosheets for ethanol gas sensors: Responding and recovering in one second. Sens. Actuators B Chem. 2017, 249, 44–52. [Google Scholar] [CrossRef]
- Tan, W.; Tan, J.; Li, L.; Dun, M.; Huang, X. Nanosheets-assembled hollowed-out hierarchical Co3O4 microrods for fast response/recovery gas sensor. Sens. Actuators B Chem. 2017, 249, 66–75. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T.; Deng, J.; Wang, L. P-type Co3O4 nanomaterials-based gas sensor: Preparation and acetone sensing performance. Sens. Actuators B Chem. 2017, 242, 369–377. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Wang, N.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. High precision NH3 sensing using network nano-sheet Co3O4 arrays based sensor at room temperature. Sens. Actuators B Chem. 2016, 235, 222–231. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. NO and NO2 Sensing Properties of WO3 and Co3O4. Sensors 2013, 13, 12467–12481. [Google Scholar] [CrossRef] [PubMed]
- Navale, S.T.; Liu, C.; Gaikar, P.; Patil, V.; Sagar, R.U.R.; Du, B.; Mane, R.S.; Stadler, F.J. Solution-processed rapid synthesis strategy of Co3O4 for the sensitive and selective detection of H2S. Sens. Actuators B Chem. 2017, 245, 524–532. [Google Scholar] [CrossRef]
- Wicker, S.; Großmann, K.; Bârsan, N.; Weimar, U. Co3O4—A systematic investigation of catalytic and gas sensing performance under variation of temperature, humidity, test gas and test gas concentration. Sens. Actuators B Chem. 2013, 185, 644–650. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, P.; Zhang, Z.; Wang, Q.; Umar, A. Perforated Co3O4 nanoneedles assembled in chrysanthemum-like Co3O4 structures for ultra-high sensitive hydrazine chemical sensor. Sens. Actuators B Chem. 2016, 235, 457–465. [Google Scholar] [CrossRef]
- Makhlouf, M.T.; Abu-Zied, B.M.; Mansoure, T.H. Direct fabrication of cobalt oxide nanoparticles employing sucrose as a combustion fuel. J. Nanopart. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Y.; Zhang, X.; Yu, Z.; Zhao, Y.; Zhu, G.; Chen, G.; Ma, C.; Xu, H.; Yu, A. Co3O4 microtubules derived from a biotemplated method for improved lithium storage performance. J. Ceram. Int. 2017, 43, 9235–9240. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Appandairajan, N.K.; Viswanathan, B. Co3-xZnxO4 (0 ≤x ≤ 1) spinel oxides. Proc. Indian Acad. Sci. 1979, 88, 217–222. [Google Scholar] [CrossRef]
- Sparks, T.D. Oxide Thermoelectrics: The Role of Crystal Structure on Thermopower in Strongly Correlated Spinels. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 2012. [Google Scholar]
- Kaczmarska, A.; Grzesik, Z.; Mrowec, S. On the defect structure and transport properties of Co3O4 spinel oxide. High Temp. Proc. 2012, 31, 371–379. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Serizawa, M.; Sakata, H.; Hirayama, T. Electrical conductivity of Co3O4 films prepared by chemical vapour deposition. Mater. Chem. Phys. 1998, 53, 225–230. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in glasses containing transition metal ions. J. Non-Cryst. Solids 1968, 1, 1–17. [Google Scholar] [CrossRef]
- Takita, Y.; Tashiro, T.; Saito, Y.; Hori, F. The effects of water coadsorption on the adsorption of oxygen over metal oxides: I. Temperature-programmed desorption study of Co3O4. J. Catal. 1986, 97, 25–35. [Google Scholar] [CrossRef]
- Zasada, F.; Piskorz, W.; Janas, J.; Gryboś, J.; Indyka, P.; Sojka, Z. Reactive Oxygen Species on the (100) Facet of Cobalt Spinel Nanocatalyst and their Relevance in 16O2/18O2 Isotopic Exchange, deN2O, and deCH4 Processes—A Theoretical and Experimental Account. ACS Catal. 2015, 5, 6879–6892. [Google Scholar] [CrossRef]
- Grillo, F.; Natile, M.M.; Glisenti, A. Low temperature oxidation of carbon monoxide: the influence of water and oxygen on the reactivity of a Co3O4 powder surface. Appl. Catal. B 2004, 48, 267–274. [Google Scholar] [CrossRef]
- Lin, H.-K.; Chiu, H.-C.; Tsai, H.-C.; Chien, S.-H.; Wang, C.-B. Synthesis, characterization and catalytic oxidation of carbon monoxide over cobalt oxide. Catal. Lett. 2003, 88, 169–174. [Google Scholar] [CrossRef]
- Steinhauer, S.; Brunet, E.; Maier, T.; Mutinati, G.C.; Köck, A. Suspended CuO nanowires for ppb level sensing in dry air and humid atmosphere. Sens. Actuators B Chem. 2013, 186, 550–556. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladimirova, S.; Krivetskiy, V.; Rumyantseva, M.; Gaskov, A.; Mordvinova, N.; Lebedev, O.; Martyshov, M.; Forsh, P. Co3O4 as p-Type Material for CO Sensing in Humid Air. Sensors 2017, 17, 2216. https://doi.org/10.3390/s17102216
Vladimirova S, Krivetskiy V, Rumyantseva M, Gaskov A, Mordvinova N, Lebedev O, Martyshov M, Forsh P. Co3O4 as p-Type Material for CO Sensing in Humid Air. Sensors. 2017; 17(10):2216. https://doi.org/10.3390/s17102216
Chicago/Turabian StyleVladimirova, Svetlana, Valeriy Krivetskiy, Marina Rumyantseva, Alexander Gaskov, Natalia Mordvinova, Oleg Lebedev, Mikhail Martyshov, and Pavel Forsh. 2017. "Co3O4 as p-Type Material for CO Sensing in Humid Air" Sensors 17, no. 10: 2216. https://doi.org/10.3390/s17102216




