Fast and Robust Real-Time Estimation of Respiratory Rate from Photoplethysmography
Abstract
:1. Introduction
2. Methods
2.1. Adaptive IIR Notch Filter
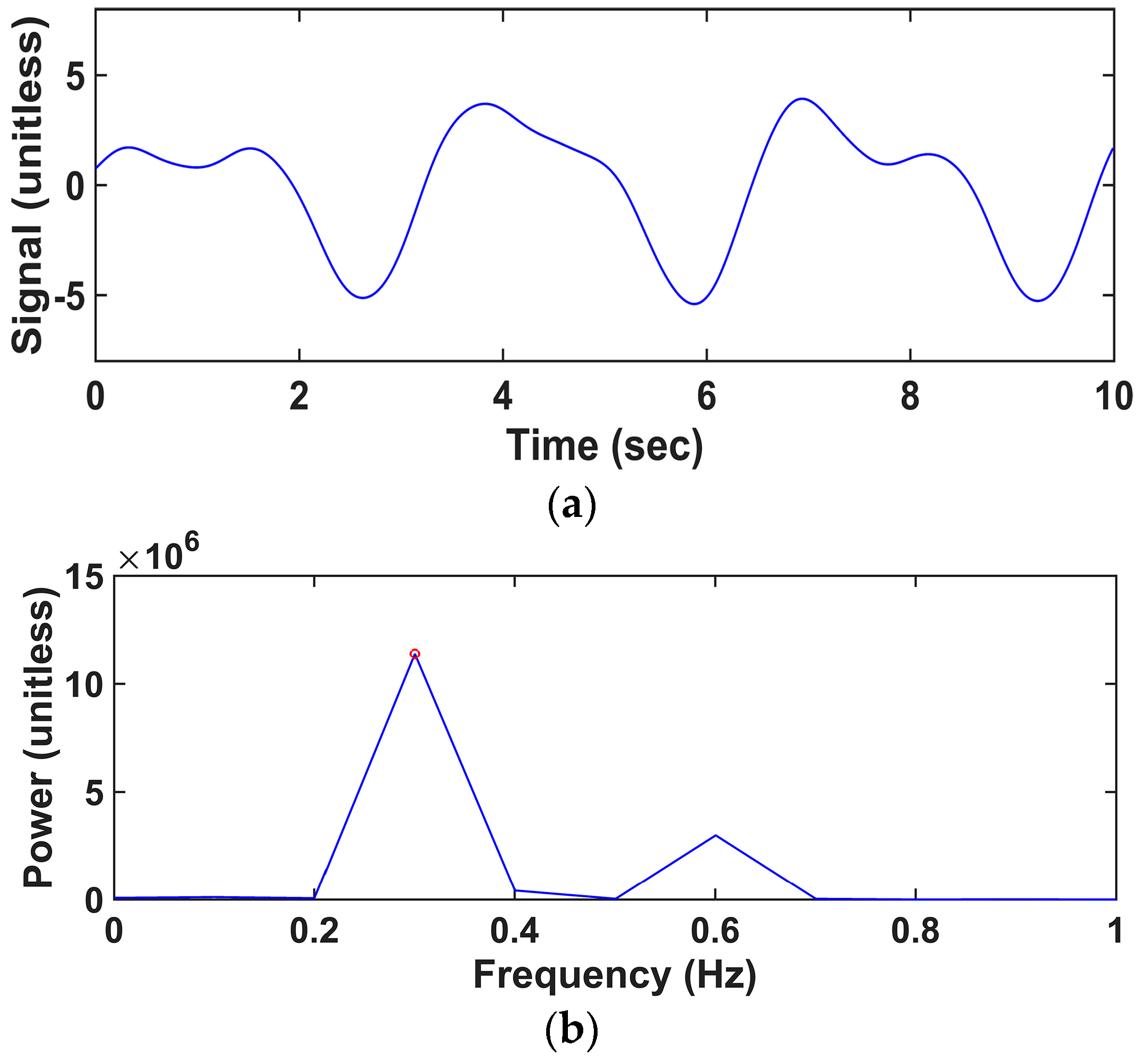
2.2. Analysis Procedure
2.3. Offline Data for Validation
2.4. Performance Evaluation
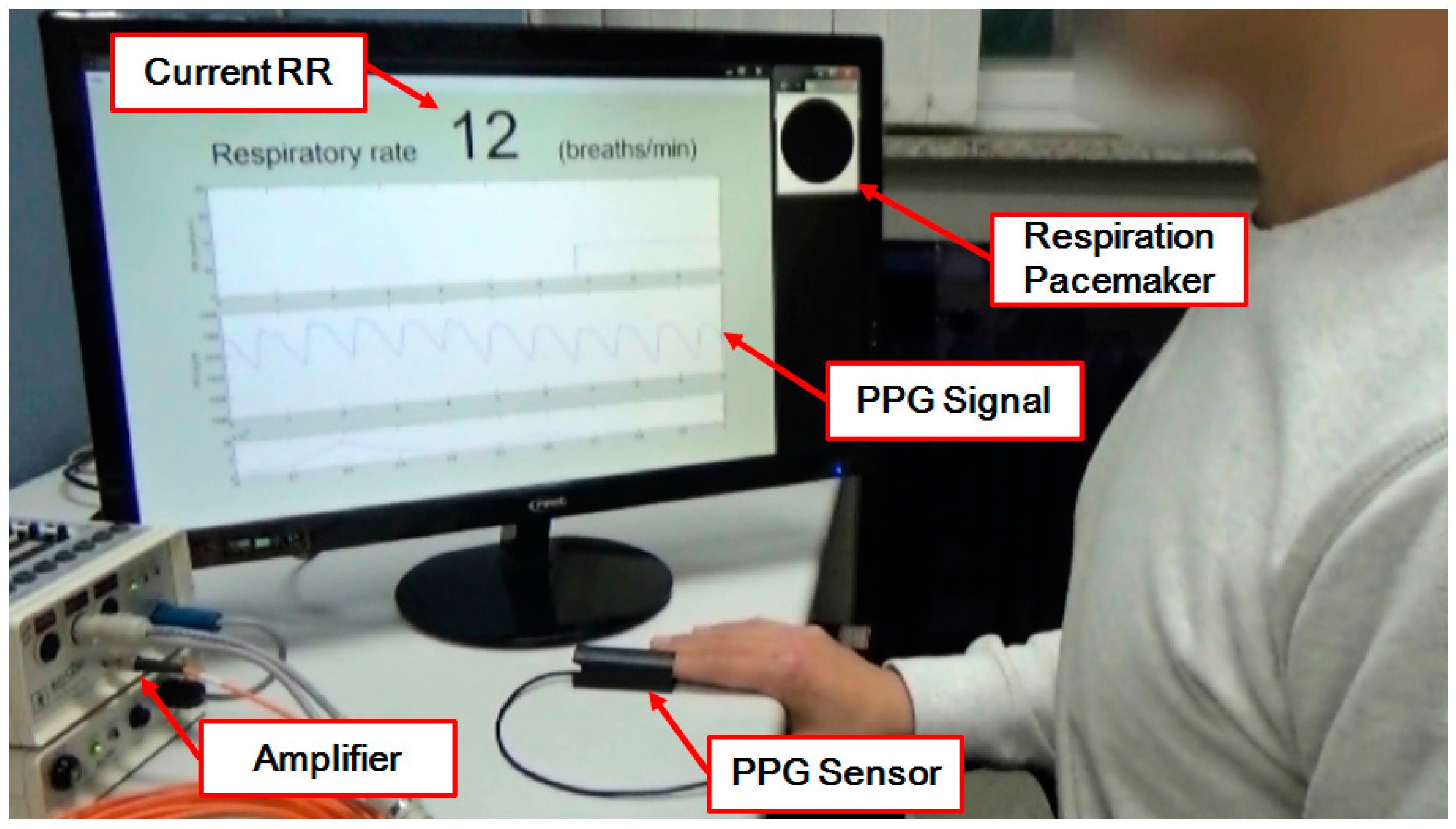
2.5. Implementation of a Real-Time RR Estimation System
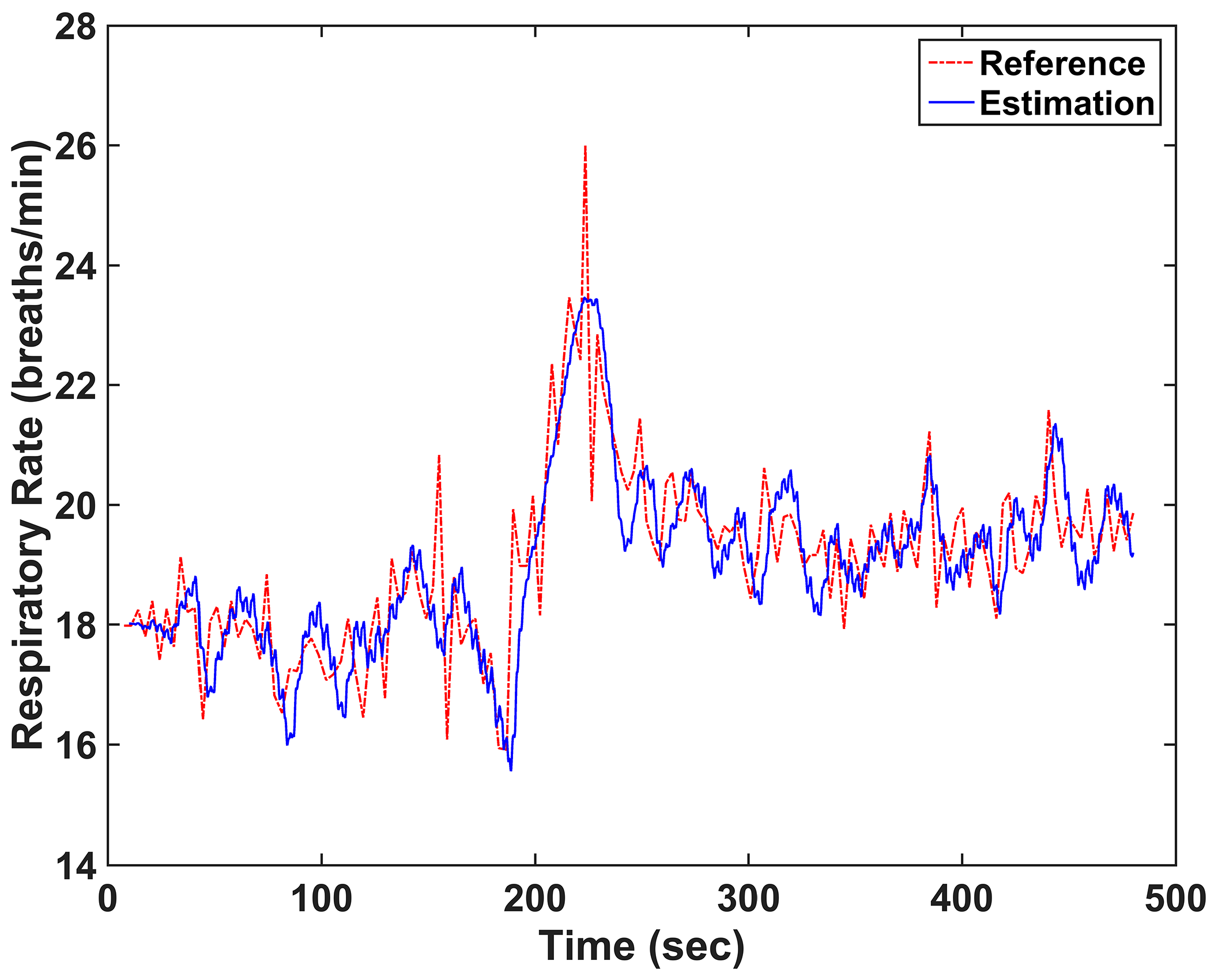
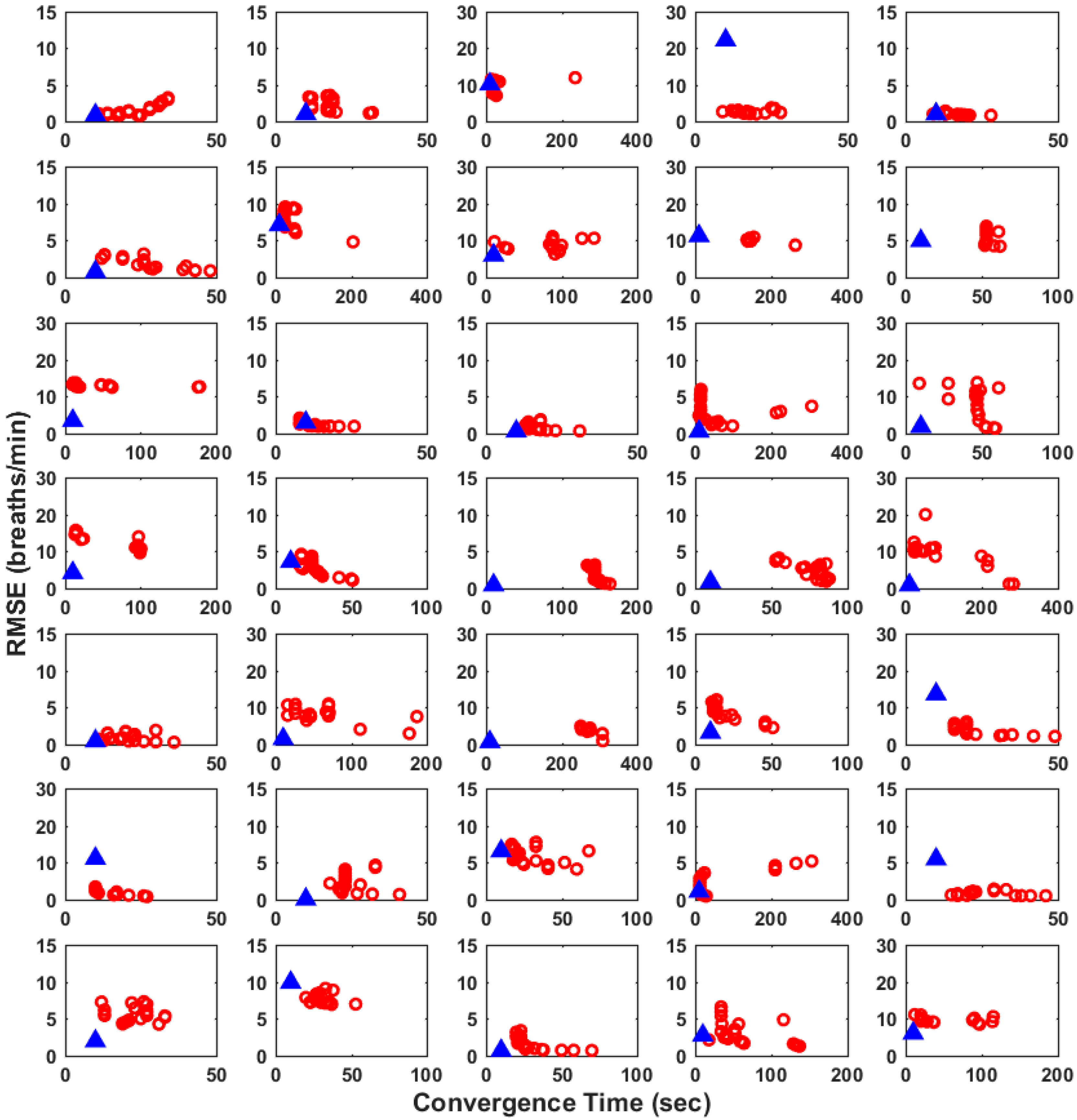
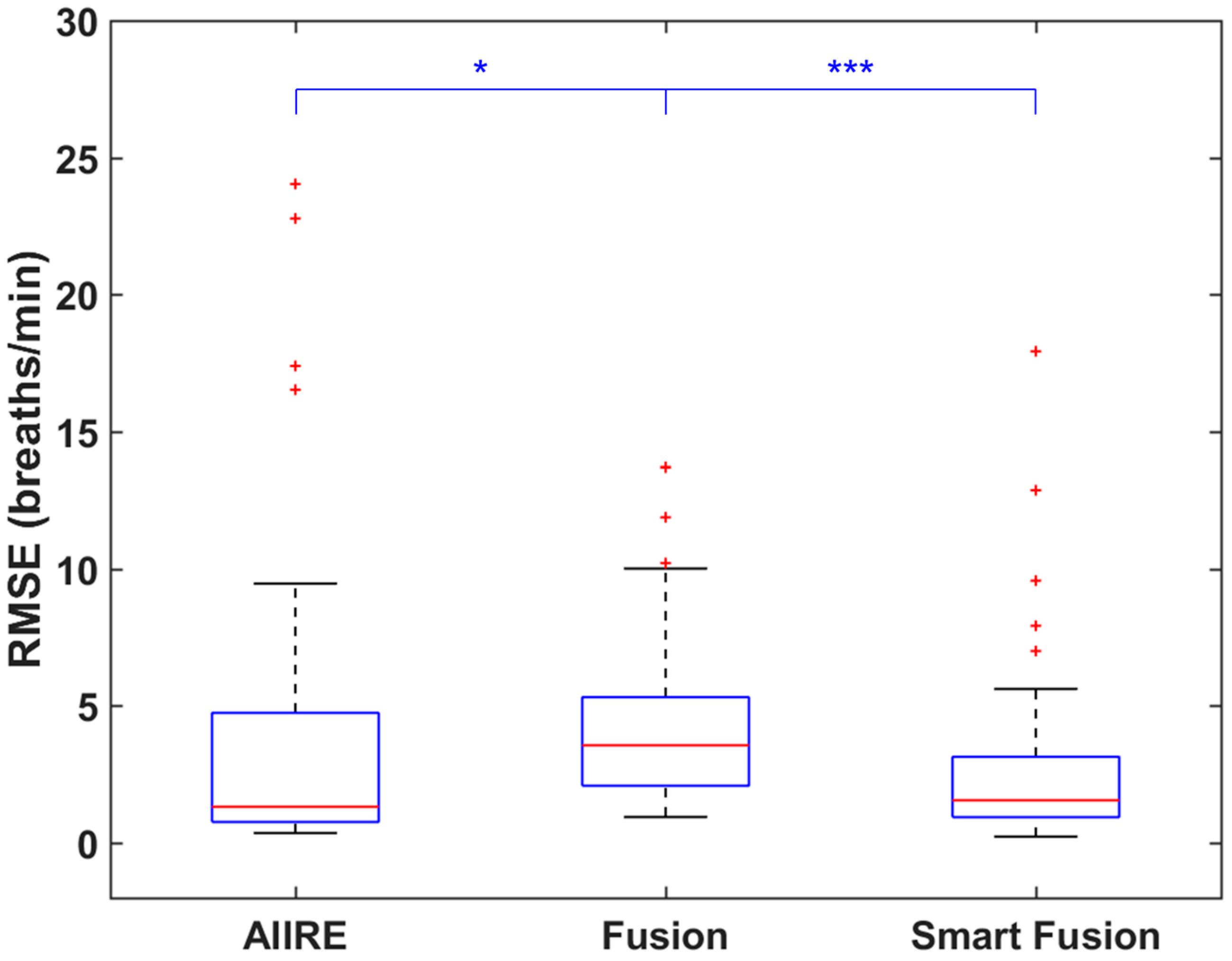
3. Results
4. Discussions and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Younes, M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J. Appl. Physiol. 2008, 105, 1389–1405. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, M.; Arne, M.; Lindahl, A.; Janson, S.; Lundbäck, B. Estimated prevalences of respiratory symptoms, asthma and chronic obstructive pulmonary disease related to detection rate in primary health care. Scand. J. Prim. Health Care 2001, 19, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.T. Slowed respiration training. Biofeedback Self Regul. 1994, 19, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.H.; Dash, S.; Ju, K. Estimation of respiratory rate from photoplethysmogram data using time-frequency spectral estimation. IEEE Trans. Biomed. Eng. 2009, 56, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Lanatí, A.; Scilingo, E.P.; Nardini, E.; Loriga, G.; Paradiso, R.; De-Rossi, D. Comparative evaluation of susceptibility to motion artifact in different wearable systems for monitoring respiratory rate. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Wu, D.; Mei, Z.Y.; Zhu, Q.S.; Wang, L. Automatic detection of respiratory rate from electrocardiogram, respiration induced plethysmography and 3D acceleration signals. J. Cent. South Univ. 2013, 20, 2423–2431. [Google Scholar] [CrossRef]
- Dash, S.; Shelley, K.H.; Silverman, D.G.; Chon, K.H. Estimation of respiratory rate from ECG, photoplethysmogram, and piezoelectric pulse transducer signals: A comparative study of time-frequency methods. IEEE Trans. Biomed. Eng. 2010, 57, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.M. Respiration signals from photoplethysmography. Anesth. Analg. 2013, 117, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Tamura, T.; Miike, H. Monitoring of heart and respiratory rates by photoplethysmography using a digital filtering technique. Med. Eng. Phys. 1996, 18, 365–372. [Google Scholar] [CrossRef]
- Johansson, A. Neural network for photoplethysmographic respiratory rate monitoring. Med. Biol. Eng. Comp. 2003, 41, 242–248. [Google Scholar] [CrossRef]
- Leonard, P.; Grubb, N.R.; Addison, P.S.; Clifton, D.; Watson, J.N. An algorithm for the detection of individual breaths from the pulse oximeter waveform. J. Clin. Monit. Comput. 2004, 18, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Leonard, P.A.; Douglas, J.G.; Grubb, N.R.; Clifton, D.; Addison, P.S.; Watson, J.N. A fully automated algorithm for the determination of respiratory rate from the photoplethysmogram. J. Clin. Monit. Comput. 2006, 20, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Shelley, K.H.; Awad, A.A.; Stout, R.G.; Silverman, D.G. The use of joint time frequency analysis to quantify the effect of ventilation on the pulse oximeter waveform. J. Clin. Monit. Comput. 2006, 20, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.G.; Tarassenko, L. A comparison of signal processing techniques for the extraction of breathing rate from the photoplethysmogram. Int. J. Biol. Med. Sci. 2007, 2, 232–236. [Google Scholar]
- Lee, J.; Chon, K.H. Respiratory rate extraction via an autoregressive model using the optimal parameter search criterion. Ann. Biomed. Eng. 2010, 38, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Madhav, K.V.; Ram, M.R.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. Robust extraction of respiratory activity from ppg signals using modified mspca. IEEE Trans. Instrum. Meas. 2013, 62, 1094–1106. [Google Scholar] [CrossRef]
- Ram, M.R.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Sivani, K.; Reddy, K.A. ICA-based improved DTCWT technique for MA reduction in PPG signals with restored respiratory information. IEEE Trans. Instrum. Meas. 2013, 62, 2639–2651. [Google Scholar] [CrossRef]
- Garde, A.; Karlen, W.; Dehkordi, P.; Ansermino, J.; Dumont, G. Empirical mode decomposition for respiratory and heart rate estimation from the photoplethysmogram. In Proceedings of the Computing in Cardiology Conference, Zaragoza, Spain, 22–25 September 2013; pp. 799–802.
- Karlen, W.; Raman, S.; Ansermino, J.M.; Dumont, G.A. Multiparameter respiratory rate estimation from the photoplethysmogram. IEEE Trans. Biomed. Eng. 2013, 60, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, B. Real-time estimation of respiratory rate from a photoplethysmogram using an adaptive lattice notch filter. Biomed. Eng. Online 2014, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Ballal, T.; Shouldice, R.B.; Heneghan, C.; Zhu, A. Breathing rate estimation from a non-contact biosensor using an adaptive IIR notch filter. In Proceedings of the 2012 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), Santa Clara, CA, USA, 15–18 January 2012; pp. 5–8.
- Tan, L.; Jiang, J. Novel adaptive IIR filter for frequency estimation and tracking [dsp tips&tricks]. IEEE Signal Process. Mag. 2009, 26, 186–189. [Google Scholar]
- Karlen, W.; Turner, M.; Cooke, E.; Dumont, G.; Ansermino, J. Capnobase: Signal database and tools to collect, share and annotate respiratory signals. In Proceedings of the Annual Meeting of the Society for Technology in Anesthesia (STA), West Palm Beach, FL, USA, 13–16 January 2010; p. 25.
- CapnoBase Homepage. Available online: http://www.capnobase.org (accessed on 13 September 2016).
- Lilliefors, H.W. On the kolmogorov-smirnov test for normality with mean and variance unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Available online: https://youtu.be/mo3tNh-5okE (accessed on 13 September 2016).
- Diniz, P.S.R. Adaptive Filtering: Algorithms and Practical Implementation; Springer Science & Business Media: New York, NY, USA, 2008. [Google Scholar]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Cyrill, D.; McNames, J.; Aboy, M. Adaptive comb filters for quasiperiodic physiologic signals. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Cancun, Mexico, 17–21 September 2003; pp. 2439–2442.


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, J.-Y.; Im, C.-H. Fast and Robust Real-Time Estimation of Respiratory Rate from Photoplethysmography. Sensors 2016, 16, 1494. https://doi.org/10.3390/s16091494
Kim H, Kim J-Y, Im C-H. Fast and Robust Real-Time Estimation of Respiratory Rate from Photoplethysmography. Sensors. 2016; 16(9):1494. https://doi.org/10.3390/s16091494
Chicago/Turabian StyleKim, Hodam, Jeong-Youn Kim, and Chang-Hwan Im. 2016. "Fast and Robust Real-Time Estimation of Respiratory Rate from Photoplethysmography" Sensors 16, no. 9: 1494. https://doi.org/10.3390/s16091494






