Experimental Study on Rebar Corrosion Using the Galvanic Sensor Combined with the Electronic Resistance Technique
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials of the Electronic Coupons and the Electrodes
2.2. The Measurement Principle of the Galvanic Sensor System
2.3. Electrochemical Measurements
2.4. Experimental Procedures
3. Experimental Results
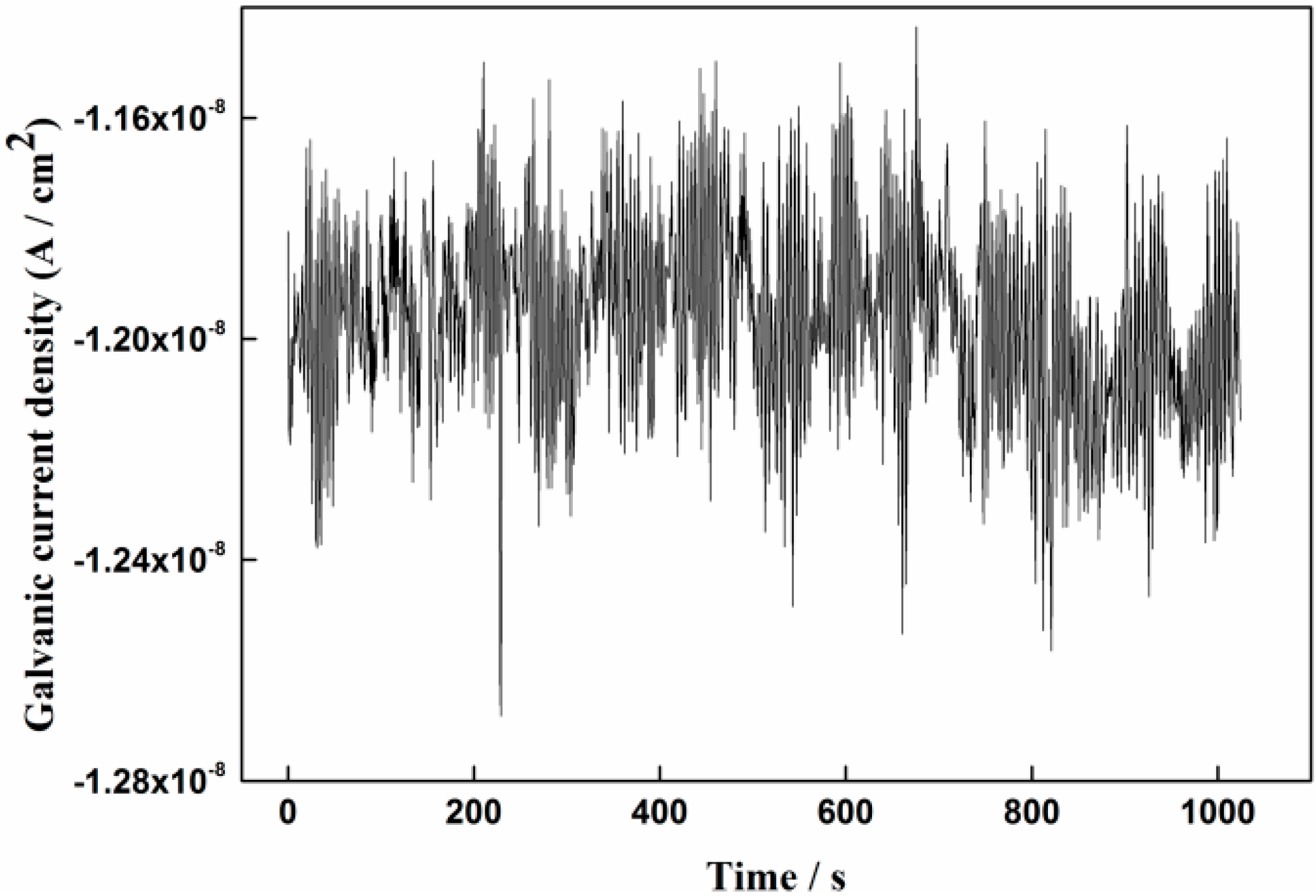
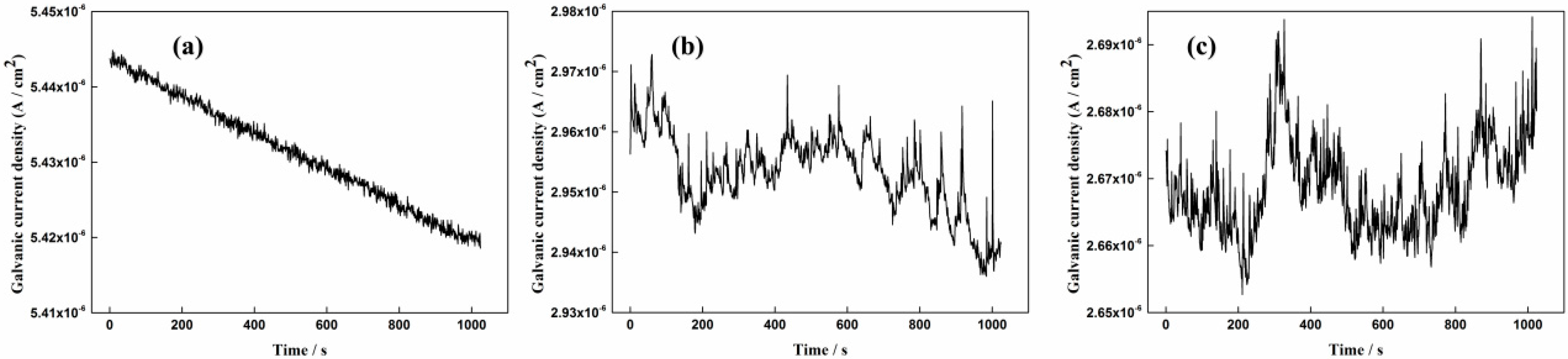
3.1. Measurement Results of the Galvanic Sensor System
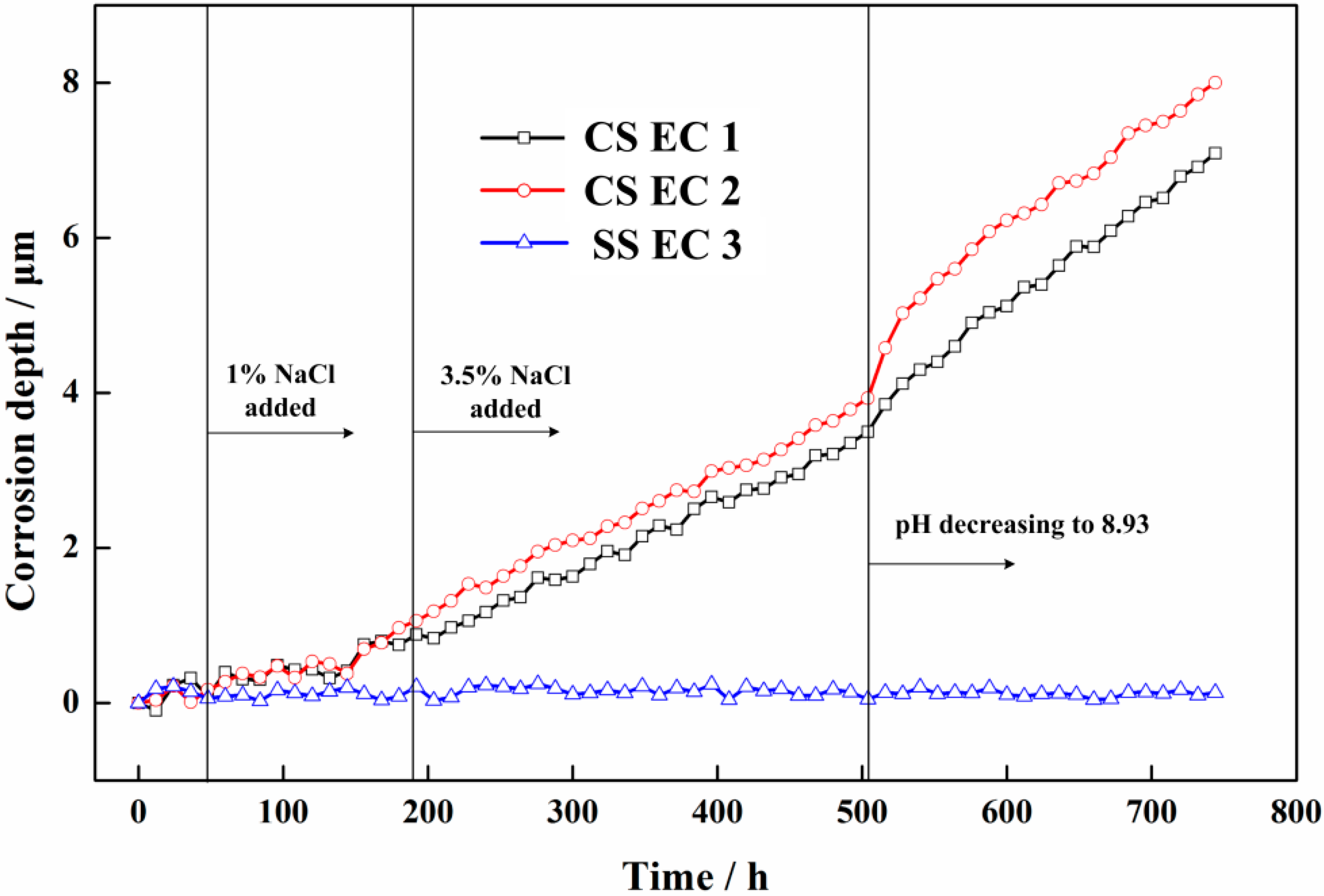
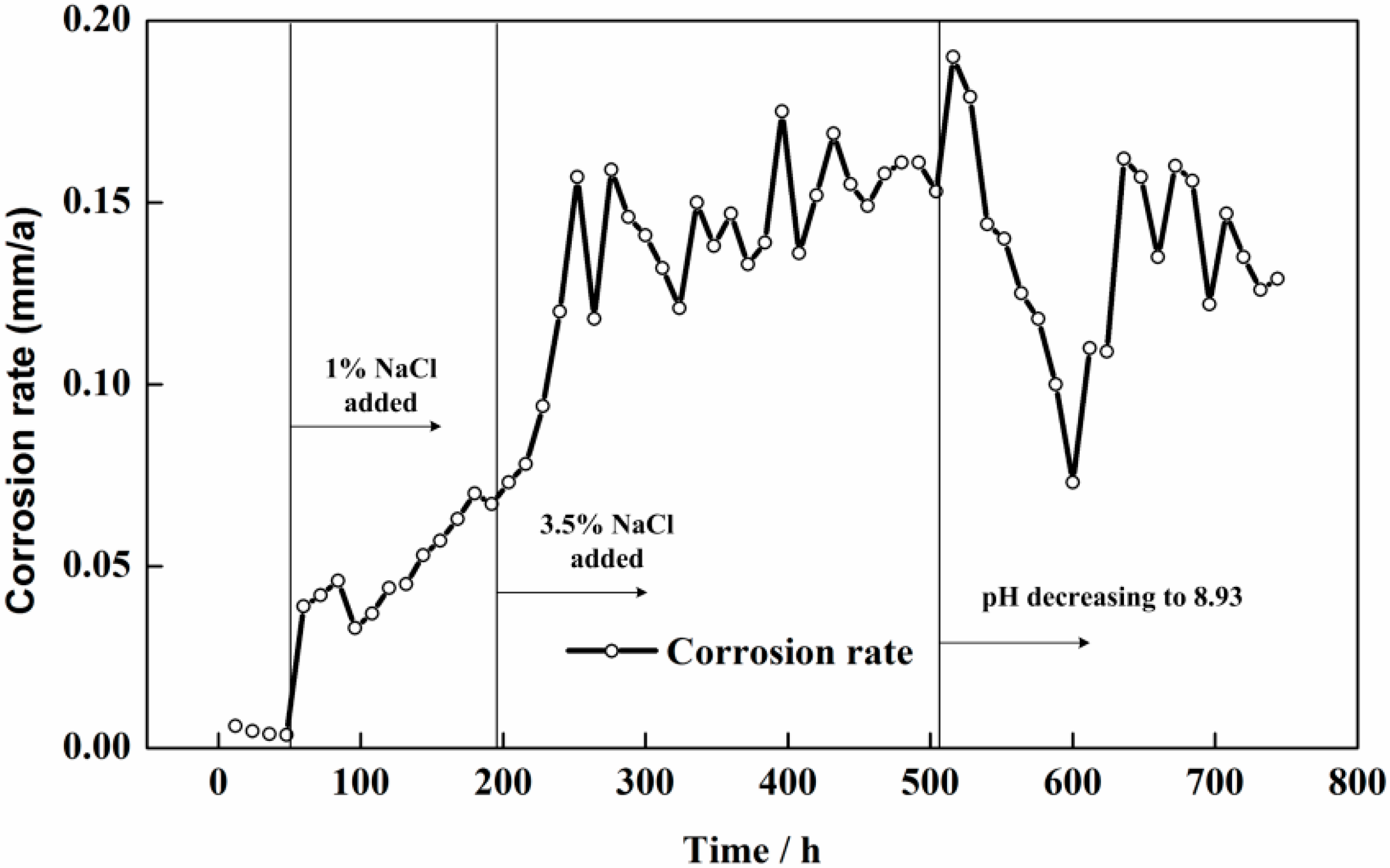
3.2. Electrochemical Measurement Results
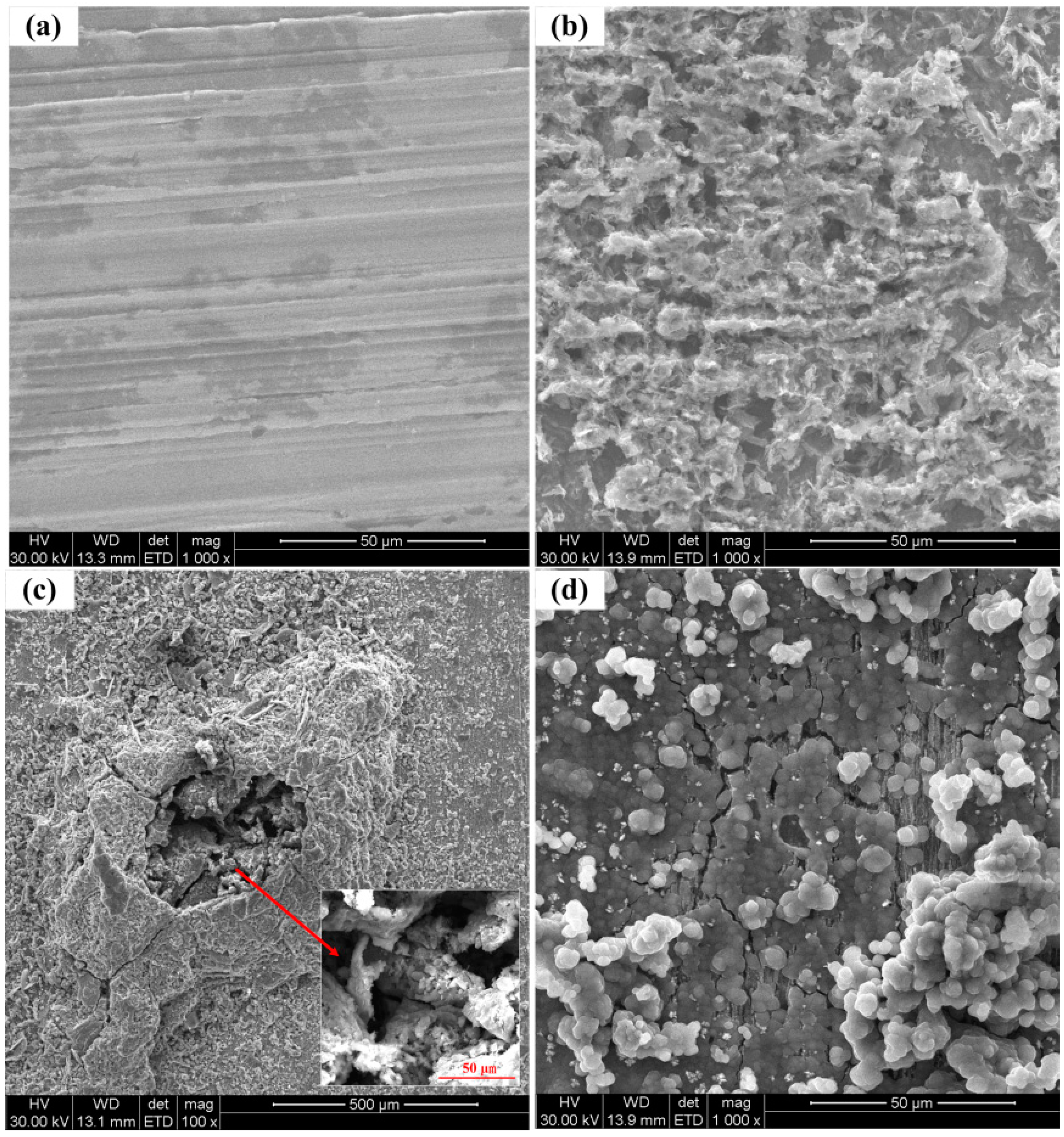
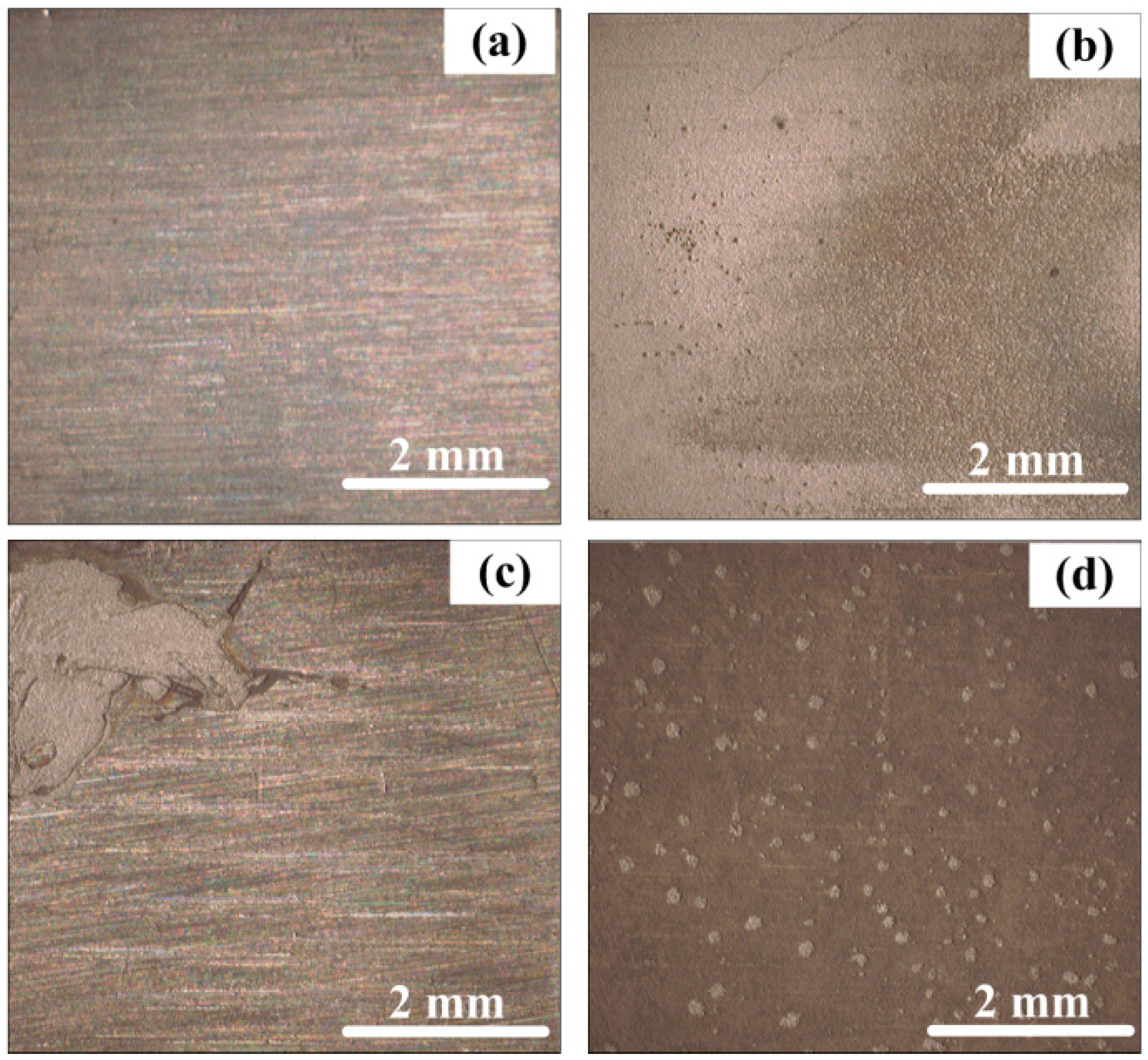
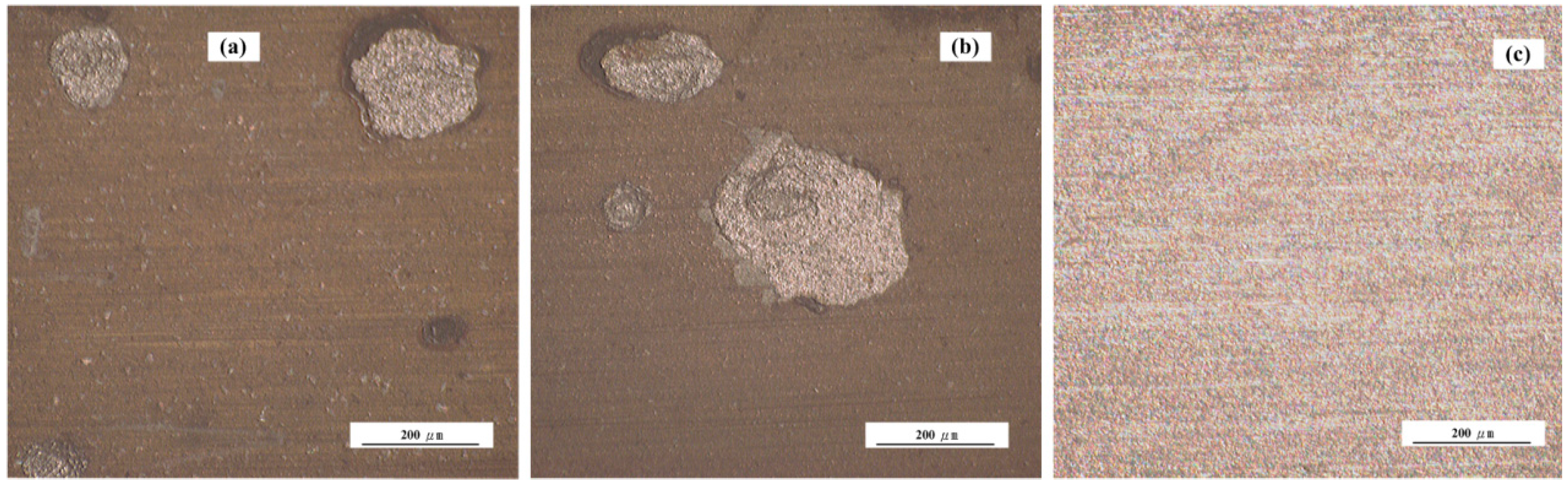
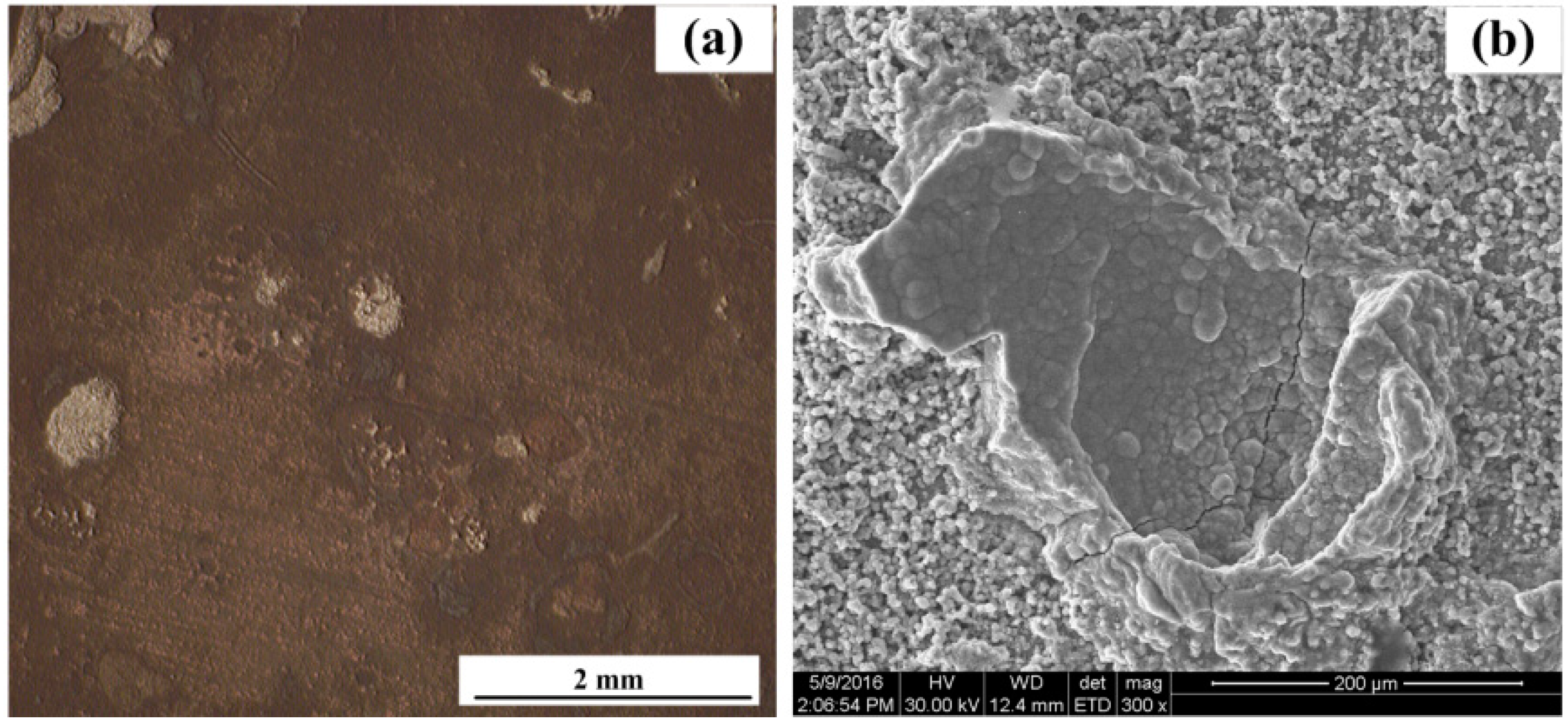
3.3. Surface Characterization
4. Discussions
4.1. The Corrosion Processes in Different Solution Conditions
4.2. The Relationship between the Galvanic Current and the Corrosion Current Measured by the LPR Method
4.3. The Corrosion Rates Measurement Differences between the Electronic Coupons and the LPR Method
4.4. The Galvanic Corrosion Behavior between CS and SS
5. Conclusions
- Through the special designs of the electronic coupons and the measurement circuit, the ER method can be used in combination with the ZRA technique. The galvanic current between the CS coupon and the SS coupon and the corrosion depths of the electronic coupons can be monitored simultaneously. More information on the rebar corrosion can be obtained by the sensor system.
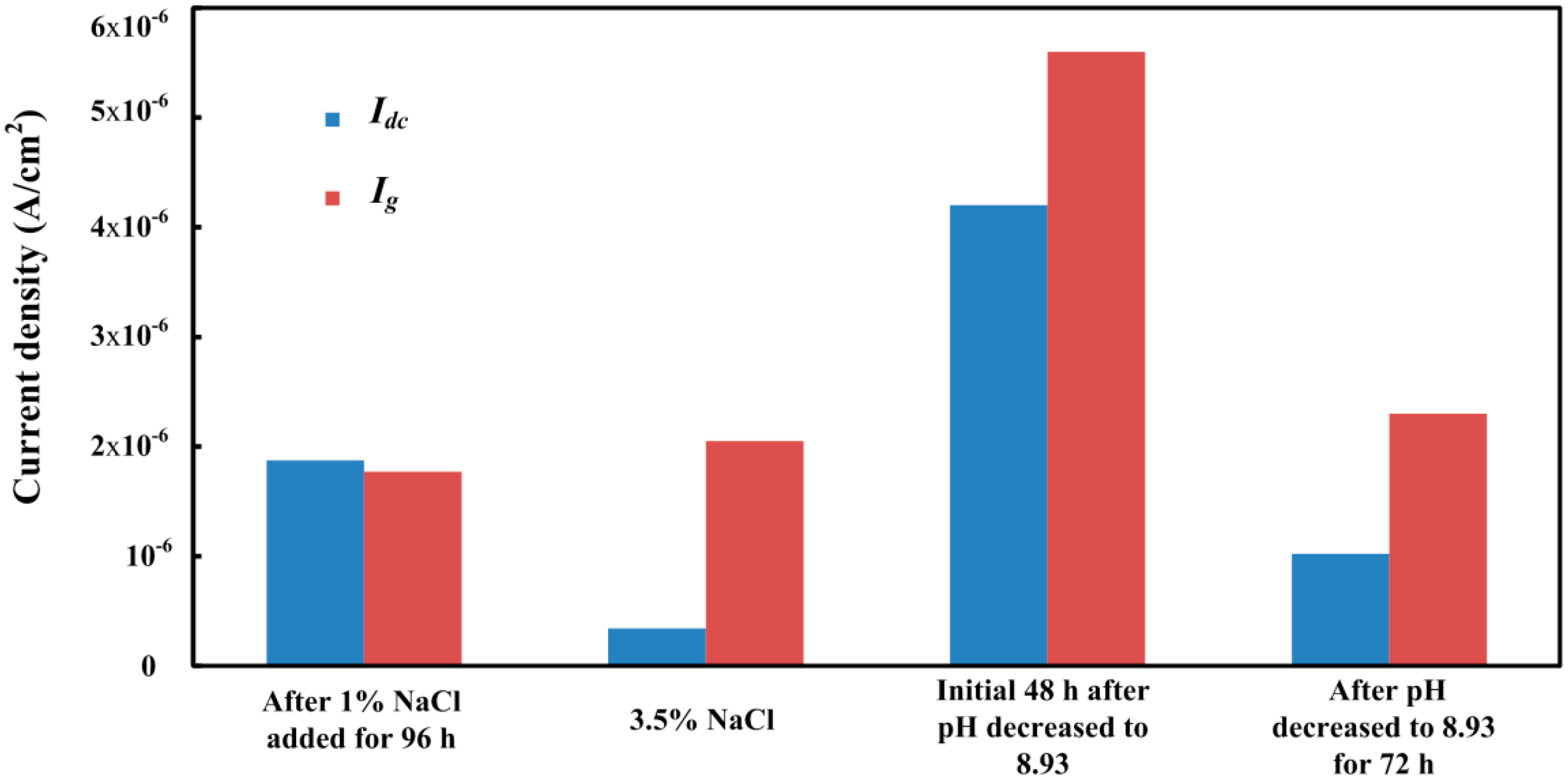
- The galvanic current noise can directly reflect the corrosion processes of the rebar in different solution conditions. The rebar trends to occur metastable pitting corrosion and localized corrosion in 1% NaCl-containing and 3.5% NaCl-containing saturated Ca(OH)2 solutions, respectively. The corrosion process of the steel will transfer to general corrosion at the initial period in chloride-containing carbonated pore solution. With the forming of the new dense product layer on the steel surface, localized corrosion will appear on the steel surface again.
- The ER method provides excellent responses to general corrosion process. However, it has no response to the metastable corrosion process, and the corrosion rate measured by the ER method is lower than the real value during stable localized corrosion processes. The LPR method will lead to the acceleration of the local anode dissolution when localized corrosion is occurring on the steel surface. Therefore, the corrosion rate measured by the LPR method is higher than the real corrosion rate in this situation. The accurate corrosion rate is between the measurement results obtained by the ER and LPR methods when localized corrosion happens.
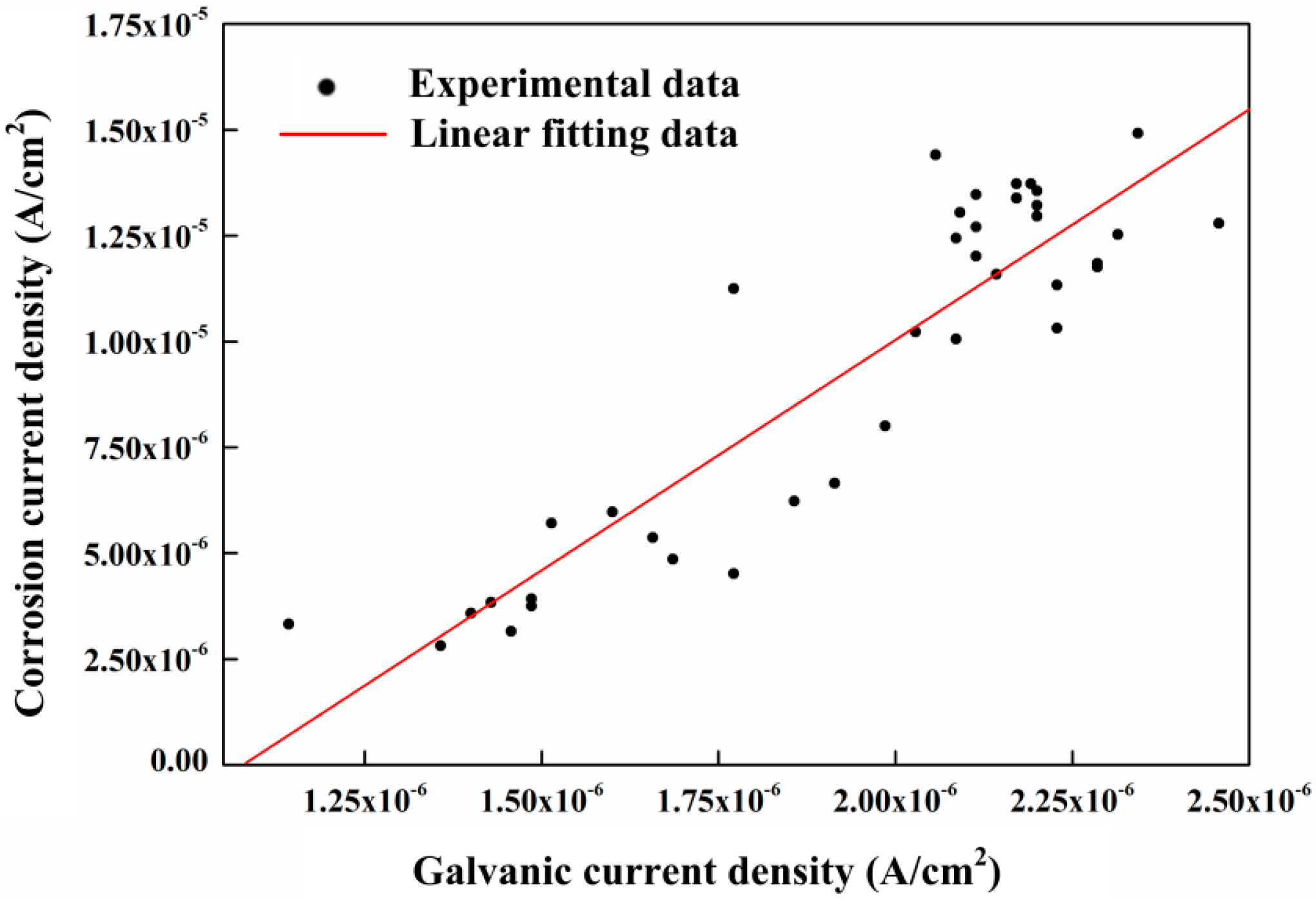
- The galvanic current density between CS and SS shows a strong linear correlation to the corrosion current density measured by the LPR method in chloride-containing saturated Ca(OH)2 solution. It indicates the galvanic current can be used as an index for the evaluation of the corrosion rate when chloride ions exist in the pore solution. However, this relationship is not suitable for the carbonated pore solution.
- Through the comparison of the corrosion current difference between CS electronic coupons and the galvanic current, the corrosion behavior can be qualitatively assessed. When the corrosion current difference occupies most part of the galvanic current, the corrosion process tends to be a general corrosion process. However, if the galvanic current is much higher than the corrosion current difference, it indicates a serious localized corrosion process is occurring on the steel surface. It also can be used as an indication for the evaluation of localized corrosion in concrete.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karthick, S.P.; Muralidharan, S.; Saraswathy, V.; Thangavel, K. Long-term relative performance of embedded sensor and surface mounted electrode for corrosion monitoring of steel in concrete structures. Sens. Actuators B Chem. 2014, 192, 303–309. [Google Scholar] [CrossRef]
- Reou, J.S.; Ann, K.Y. Electrochemical assessment on the corrosion risk of steel embedment in OPC concrete depending on the corrosion detection techniques. Mater. Chem. Phys. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Guo, X.P. Initiation and repassivation of pitting corrosion of carbon steel in carbonated concrete pore solution. Corros. Sci. 2011, 53, 1322–1330. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Guo, X.P. Localized corrosion inhibition of carbon steel in carbonated concrete pore solutions using wire beam electrode. Acta Phys. Chim. Sin. 2011, 27, 127–134. [Google Scholar]
- Huang, Y.; Yang, L.J.; Xu, Y.Z.; Cao, Y.Z.; Song, S.D. A novel system for corrosion protection of reinforced steels in the underwater zone. Corros. Eng. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Köliö, A.; Honkanen, M.; Lahdensivu, J.; Vippola, M.; Pentti, M. Corrosion products of carbonation induced corrosion in existing reinforced concrete facades. Cem. Concr. Res. 2015, 78, 200–207. [Google Scholar] [CrossRef]
- Legat, A.; Leban, M.; Bajt, Ž. Corrosion processes of steel in concrete characterized by means of electrochemical noise. Electrochim. Acta 2004, 49, 2741–2751. [Google Scholar] [CrossRef]
- Qian, S.; Qu, D. Theoretical and experimental study of galvanic coupling effects between carbon steel and stainless steels. J. Appl. Electrochem. 2009, 40, 247–256. [Google Scholar] [CrossRef]
- Qian, S.; Qu, D.; Coates, G. Galvanic coupling between carbon steel and stainless steel reinforcements. Can. Metall. Q. 2006, 45, 475–483. [Google Scholar] [CrossRef]
- Nóvoa, X.R.; Pérez, C.; Reyes, A.; Soage, A.; Vázquez, A. Thermogalvanic Currents in Steel Reinforced. Concr. Electrochim. Acta 2016, 202, 269–276. [Google Scholar] [CrossRef]
- McCarter, W.J.; Vennesland, Ø. Sensor systems for use in reinforced concrete structures. Constr. Build. Mater. 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Raupach, M.; Gulikers, J.; Reichling, K. Condition survey with embedded sensors regarding reinforcement corrosion. Mater. Corros. 2013, 64, 141–146. [Google Scholar] [CrossRef]
- Xu, C.; Li, Z.; Jin, W. A new corrosion sensor to determine the start and development of embedded rebar corrosion process at coastal concrete. Sensors 2013, 13, 13258–13275. [Google Scholar] [CrossRef] [PubMed]
- Yohai, L.; Schreiner, W.; Vázquez, M.; Valcarce, M.B. Phosphate ions as effective inhibitors for carbon steel in carbonated solutions contaminated with chloride ions. Electrochim. Acta 2016, 20, 2231–2242. [Google Scholar] [CrossRef]
- Tan, Y. Sensing localised corrosion by means of electrochemical noise detection and analysis. Sens. Actuators B Chem. 2009, 139, 688–698. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Wang, X.; Lin, X. Experimental study on pipeline internal corrosion based on a new kind of electrical resistance sensor. Sens. Actuators B Chem. 2016, 224, 37–47. [Google Scholar] [CrossRef]
- Prosek, T.; Taube, M.; Dubois, F.; Thierry, D. Application of automated electrical resistance sensors for measurement of corrosion rate of copper, bronze and iron in model indoor atmospheres containing short-chain volatile carboxylic acids. Corros. Sci. 2014, 87, 376–382. [Google Scholar] [CrossRef]
- Li, S.; Kim, Y.-G.; Jung, S.; Song, H.-S.; Lee, S.-M. Application of steel thin film electrical resistance sensor for in situ corrosion monitoring. Sens. Actuators B Chem. 2007, 120, 368–377. [Google Scholar] [CrossRef]
- Legat, A. Monitoring of steel corrosion in concrete by electrode arrays and electrical resistance probes. Electrochim. Acta 2007, 52, 7590–7598. [Google Scholar] [CrossRef]
- Tan, Y.-J. An experimental comparison of three wire beam electrode based methods for determining corrosion rates and patterns. Corros. Sci. 2005, 47, 1653–1665. [Google Scholar] [CrossRef]
- Aung, N.N.; Tan, Y.J. A new method of studying buried steel corrosion and its inhibition using the wire beam electrode. Corros. Sci. 2004, 46, 3057–3067. [Google Scholar] [CrossRef]
- Tan, Y. Experimental methods designed for measuring corrosion in highly resistive and inhomogeneous media. Corros. Sci. 2011, 53, 1145–1155. [Google Scholar] [CrossRef]
- Huo, Y.; Tan, M.Y.; Forsyth, M. Visualising dynamic passivation and localised corrosion processes occurring on buried steel surfaces under the effect of anodic transients. Electrochem. Commun. 2016, 66, 21–24. [Google Scholar] [CrossRef]
- Shi, W.; Dong, Z.H.; Kong, D.J.; Guo, X.P. Application of wire beam electrode technique to investigate initiation and propagation of rebar corrosion. Cem. Concr. Res. 2013, 48, 25–33. [Google Scholar] [CrossRef]
- Yang, L.; Sridhar, N.; Brossia, C.S.; Dunn, D.S. Evaluation of the coupled multielectrode array sensor as a real-time corrosion monitor. Corros. Sci. 2005, 47, 1794–1809. [Google Scholar] [CrossRef]
- Andrade, C.; Garcés, P.; Martínez, I. Galvanic currents and corrosion rates of reinforcements measured in cells simulating different pitting areas caused by chloride attack in sodium hydroxide. Corros. Sci. 2008, 50, 2959–2964. [Google Scholar] [CrossRef]
- Ren, C.; Zhu, M.; Du, L.; Chen, J.; Zeng, D.; Hu, J.; Shi, T. Two-metal corrosion of casing pipe joint in CO2/H2S enviroment. Int. J. Electrochem. Sci. 2015, 10, 4029–4043. [Google Scholar]
- Park, Z.-T.; Choi, Y.-S.; Kim, J.-G.; Chung, L. Development of a galvanic sensor system for detecting the corrosion damage of the steel embedded in concrete structure. Cem. Concr. Res. 2005, 35, 1814–1819. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, D. Experimental study on seawater-pipeline internal corrosion monitoring system. Sens. Actuators B Chem. 2008, 135, 375–380. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Park, Z.-T.; Kim, J.-G.; Chung, L. Development of a galvanic sensor system for detecting the corrosion damage of the steel embedded in concrete structures: Part 1. Laboratory tests to correlate galvanic current with actual damage. Cem. Concr. Res. 2003, 33, 2057–2062. [Google Scholar] [CrossRef]
- Pereira, E.V.; Figueira, R.B.; Salta, M.M.; da Fonseca, I.T. A galvanic sensor for monitoring the corrosion condition of the concrete reinforcing steel: Relationship between the galvanic and the corrosion currents. Sensors 2009, 9, 8391–8398. [Google Scholar] [CrossRef] [PubMed]
- Omar Rihan, R. Galvanic corrosion of electric resistance welded X52 steel in CO2-containing solution. Anti-Corros. Methods Mater. 2014, 61, 431–435. [Google Scholar] [CrossRef]
- Kouril, M.; Prosek, T.; Scheffel, B.; Dubois, F. High sensitivity electrical resistance sensors for indoor corrosion monitoring. Corros. Eng. Sci. Technol. 2013, 48, 282–287. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. Statistical analysis of metastable pitting events on carbon steel. Br. Corros. J. 2013, 35, 125–130. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Yang, L.J.; He, L.M.; Huang, Y.; Wang, X.N. The monitoring of galvanic corrosion behaviour caused by mineral deposit in pipeline working conditions using ring form electronic resistance sensor system. Corros. Eng. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y.Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Broomfield, J.P. Techniques to assess the corrosion acitivity of steel reinforced concrete structures. ASTM STP 1996, 1276, 91–106. [Google Scholar]
- Law, D.W.; Cairns, J.; Milard, S.G.; Bungey, J.H. Measurement of loss of steel from reinforcing bars in concrete using linear polarisation resistance measurements. NDT E Int. 2004, 37, 381–388. [Google Scholar] [CrossRef]
- Shi, J.J.; Sun, W. Effects of phosphate on the chloride-induced corrosion behavior of reinforcing steel in mortars. Cem. Concr. Res. 2014, 45, 166–175. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Ying, L.; Yang, F.; Li, B.; Wang, X. Corrosion behavior of pipeline steel under deposit corrosion and the inhibition performance of organic phosphine inhibitor. Acta Metall. Sin. 2016, 52, 320–330. [Google Scholar]
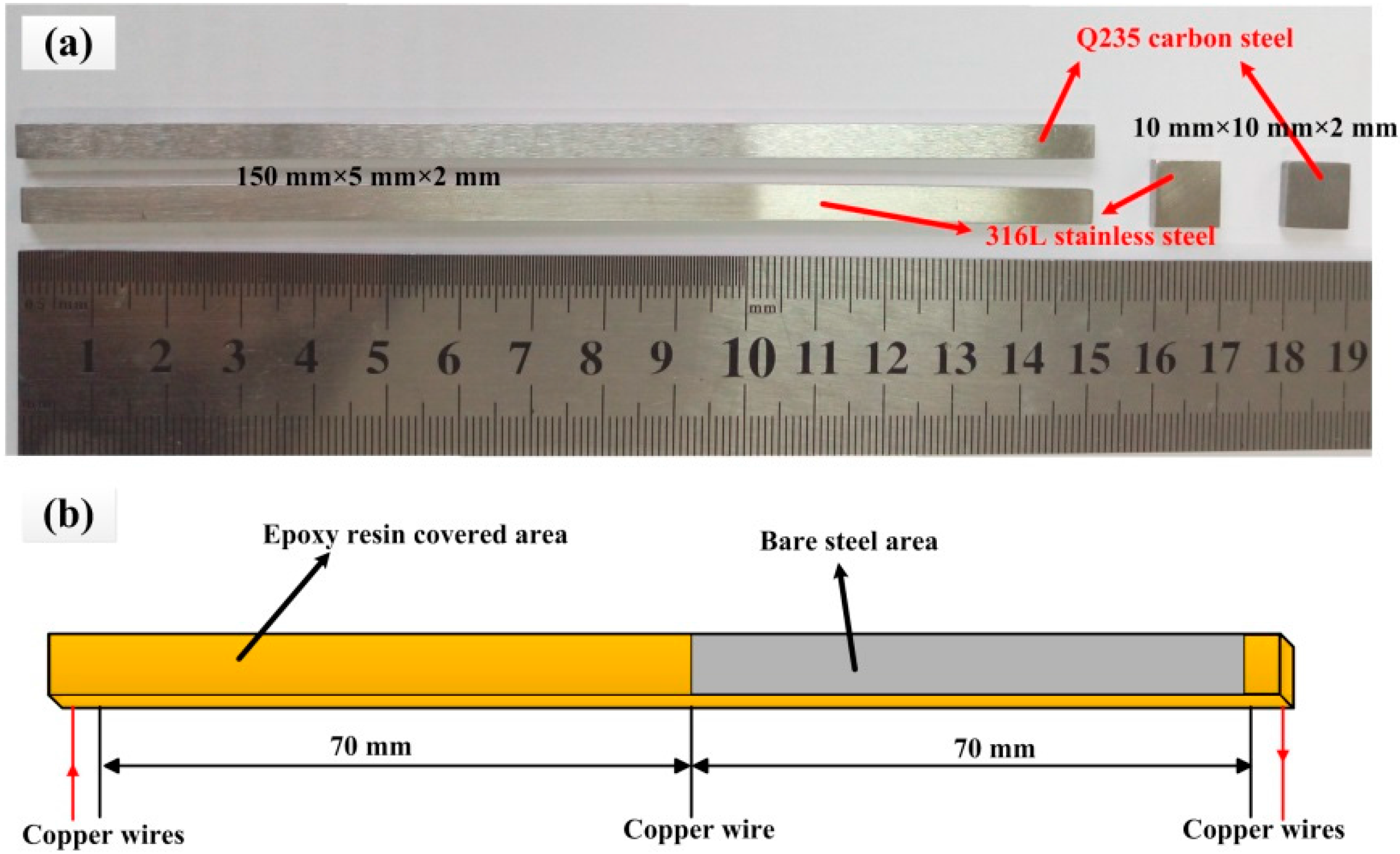
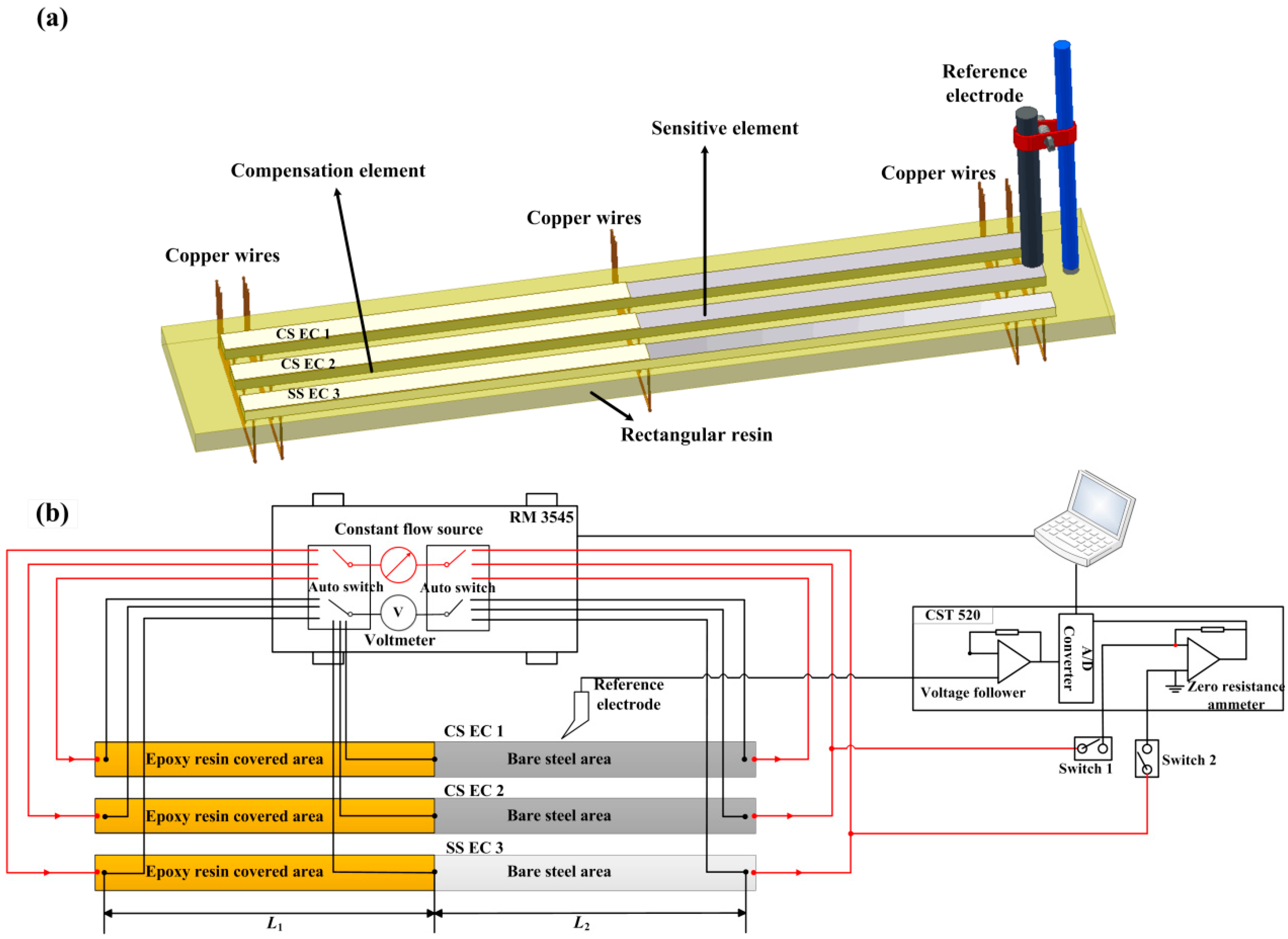

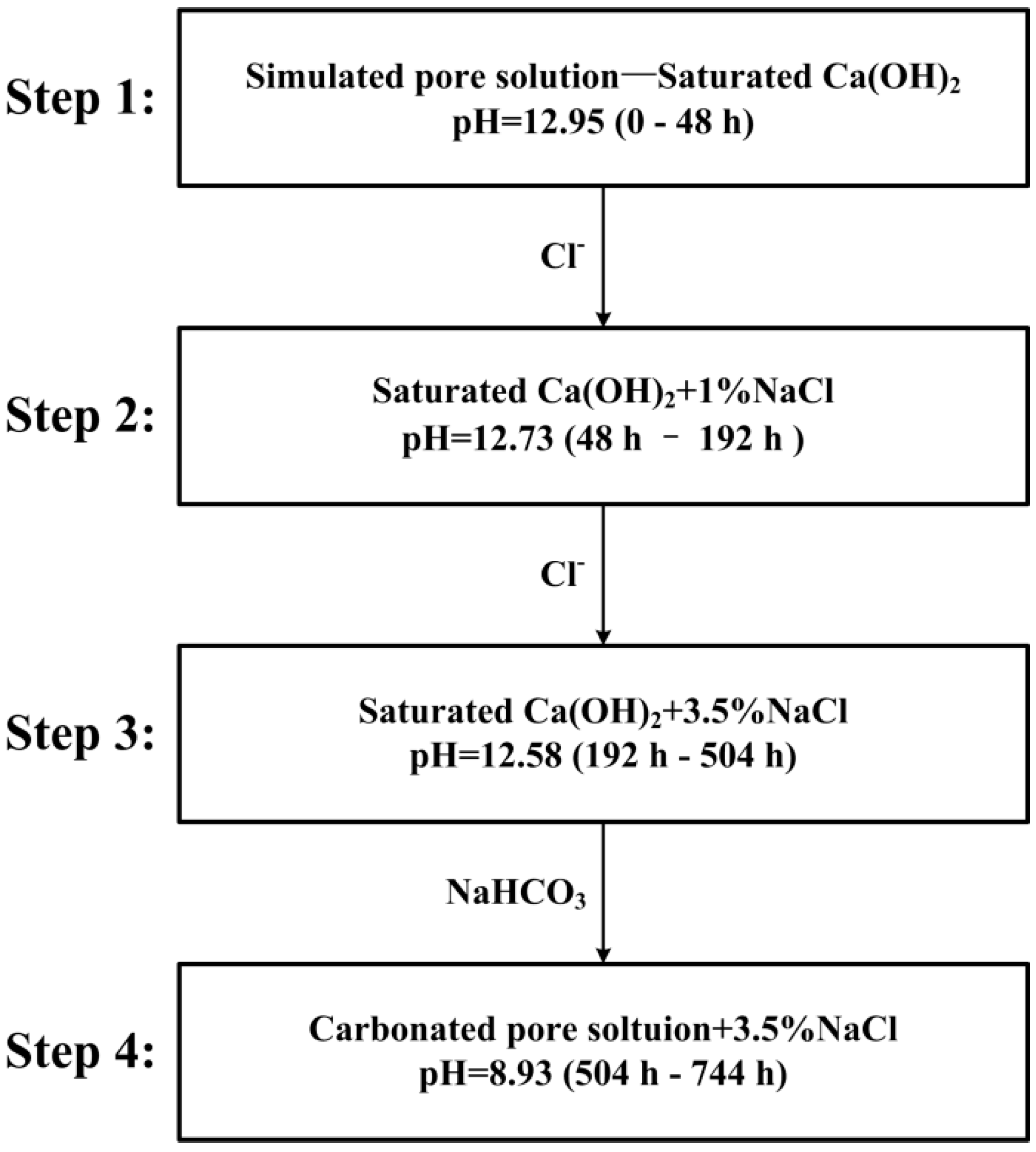
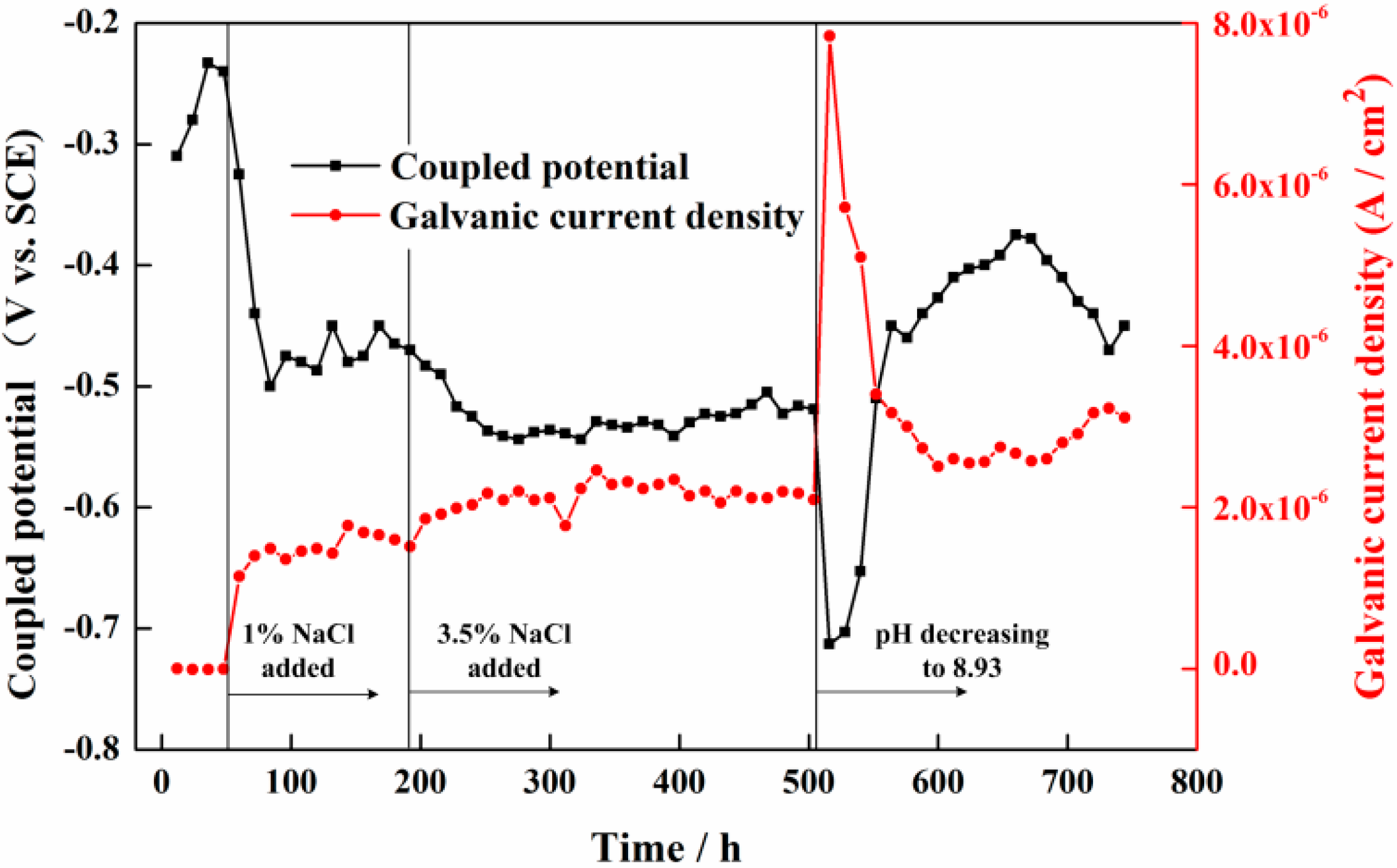


| C | Si | Mn | P | S | Mo | Cr | Ni | Fe | |
|---|---|---|---|---|---|---|---|---|---|
| Q235 | 0.22% | 0.30% | 0.40% | 0.045% | 0.04% | / | 0.01% | 0.01% | Bal. |
| 316L | 0.03% | 1.00% | 2.00% | 0.035% | 0.03% | 2.50% | 17.00% | 12.60% | Bal. |
| Solution Conditions | Steel Kinds | Ecorr/VSCE | Corrosion Rate/(mm/a) | Intersections of CS and SS | |
|---|---|---|---|---|---|
| Potential (VSCE) | Current Density (A/cm2) | ||||
| Pure Ca(OH)2 | CS | −0.18 | 0.0091 | −0.21 | 4.3 × 10−7 |
| SS | −0.27 | 0.0022 | |||
| With 1% NaCl added | CS | −0.64 | 0.14 | −0.51 | 6.3 × 10−6 |
| SS | −0.31 | 0.0041 | |||
| With 3.5% NaCl added | CS | −0.71 | 0.17 | −0.63 | 1.7 × 10−5 |
| SS | −0.37 | 0.0071 | |||
| With pH decreasing to 8.93 | CS | −0.66 | 0.18 | −0.57 | 2.2 × 10−5 |
| SS | −0.26 | 0.019 | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, K.; Liu, L.; Yang, L.; Wang, X.; Huang, Y. Experimental Study on Rebar Corrosion Using the Galvanic Sensor Combined with the Electronic Resistance Technique. Sensors 2016, 16, 1451. https://doi.org/10.3390/s16091451
Xu Y, Li K, Liu L, Yang L, Wang X, Huang Y. Experimental Study on Rebar Corrosion Using the Galvanic Sensor Combined with the Electronic Resistance Technique. Sensors. 2016; 16(9):1451. https://doi.org/10.3390/s16091451
Chicago/Turabian StyleXu, Yunze, Kaiqiang Li, Liang Liu, Lujia Yang, Xiaona Wang, and Yi Huang. 2016. "Experimental Study on Rebar Corrosion Using the Galvanic Sensor Combined with the Electronic Resistance Technique" Sensors 16, no. 9: 1451. https://doi.org/10.3390/s16091451






