A Rapid In Situ Colorimetric Assay for Cobalt Detection by the Naked Eye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of CG Aqueous Chemosensor and Detection of Cobalt
2.3. Optimization of Suitable Conditions for Colorimetric Detection
3. Results and Discussion
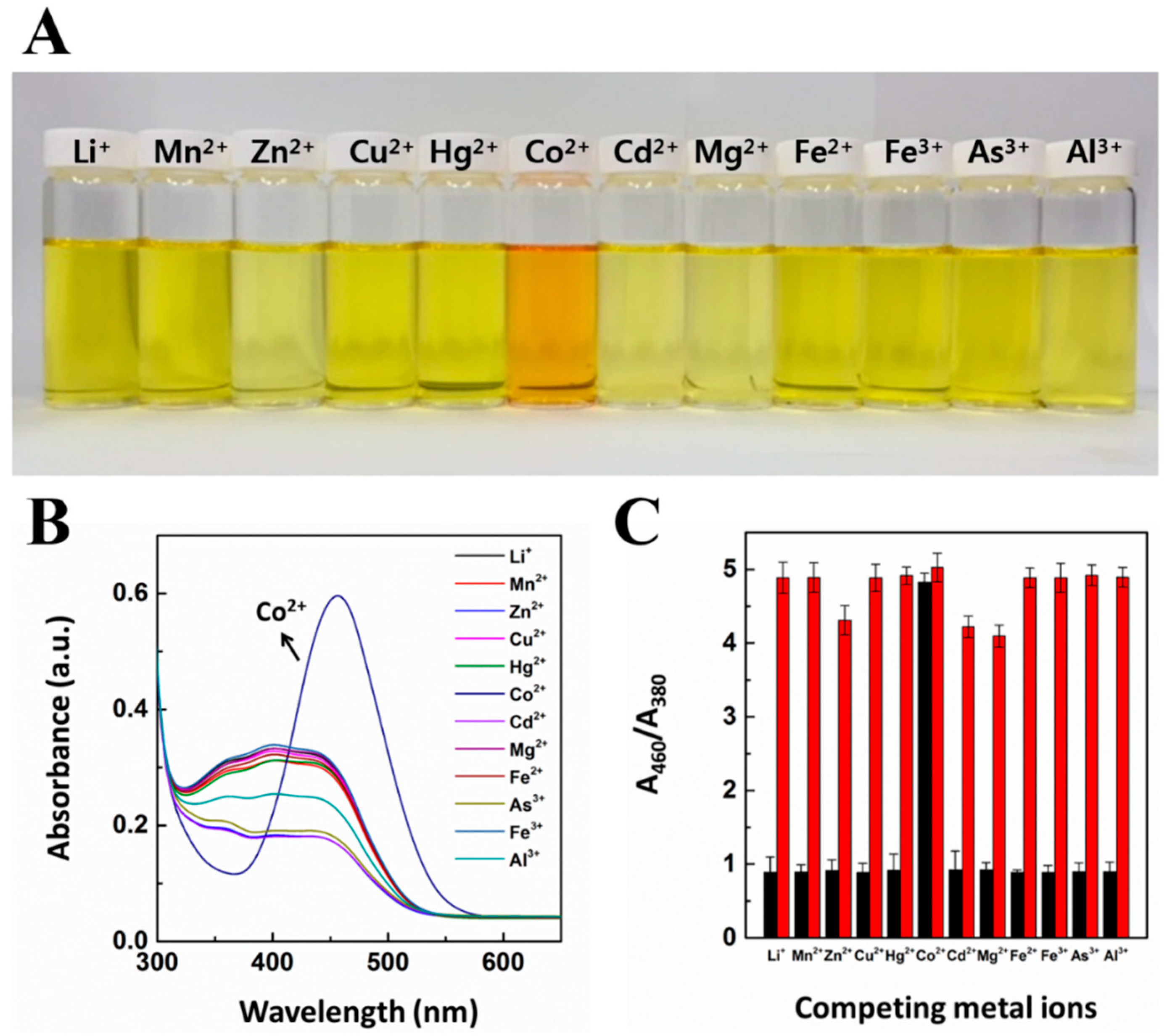
3.1. Selective Recognition Study for CG Chemosensor
3.2. The Effect of pH and CG Chemosensor Concentration
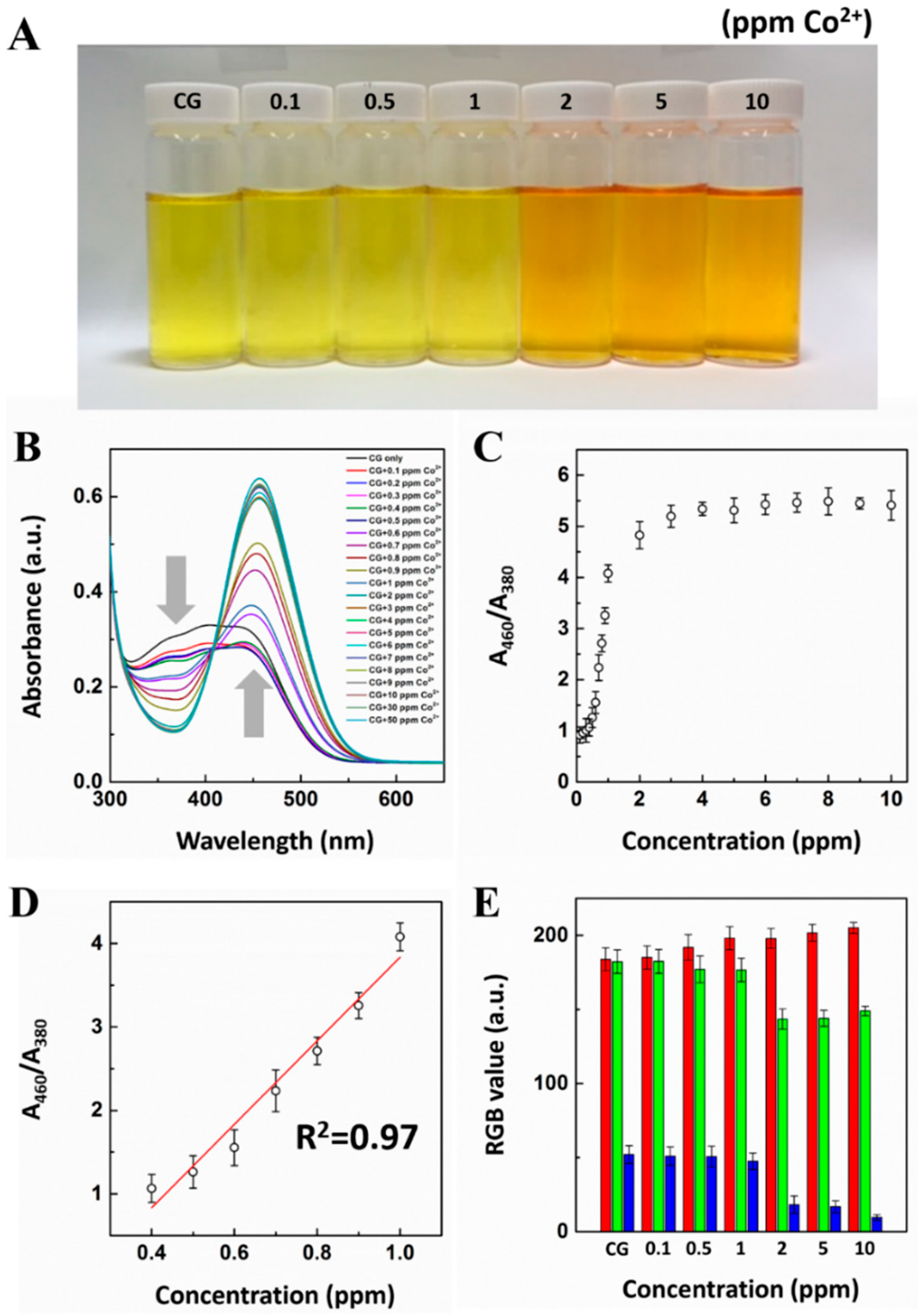
3.3. Stoichiometric Binding Study of CG-Co2+ Complex
3.4. Response Time Monitoring for CG-Co2+ Complexation
3.5. UV-Vis Titration Study for CG Chemosensor
3.6. Reversibility Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kazantzis, G. Role of cobalt, iron, lead, manganese, mercury, platinum, selenium, and titanium in carcinogenesis. Environ. Health Perspect. 1981, 40, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Siemiatycki, J.; Richardson, L.; Straif, K.; Latreille, B.; Lakhani, R.; Campbell, S.; Rousseau, M.-C.; Boffetta, P. Listing occupational carcinogens. Environ. Health Perspect. 2004, 112, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Favre, J.; Fabrègue, D.; Yamanaka, K.; Chiba, A. Modeling dynamic recrystallization of L-605 cobalt superalloy. Mater. Sci. Eng. A 2016, 653, 84–92. [Google Scholar] [CrossRef]
- Nlebedim, I.; Snyder, J.E.; Moses, A.J.; Jiles, D. Dependence of the magnetic and magnetoelastic properties of cobalt ferrite on processing parameters. J. Magn. Magn. Mater. 2010, 322, 3938–3942. [Google Scholar] [CrossRef]
- Li, J.; Guo, L.; Zhao, N.; Yang, X.; Yi, R.; Li, K.; Zeng, Q.; Li, X.; Zeng, X.; Lu, Y. Determination of cobalt in low-alloy steels using laser-induced breakdown spectroscopy combined with laser-induced fluorescence. Talanta 2016, 151, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Jonynaite, D.; Senvaitiene, J.; Beganskiene, A.; Kareiva, A. Spectroscopic analysis of blue cobalt smalt pigment. Vib. Spectrosc. 2010, 52, 158–162. [Google Scholar] [CrossRef]
- Khelifa, A.; Moulay, S.; Naceur, A. Treatment of metal finishing effluents by the electroflotation technique. Desalination 2005, 181, 27–33. [Google Scholar] [CrossRef]
- Kříbek, B.; Majer, V.; Veselovský, F.; Nyambe, I. Discrimination of lithogenic and anthropogenic sources of metals and sulphur in soils of the central-northern part of the Zambian Copperbelt Mining District: A topsoil vs. subsurface soil concept. J. Geochem. Explor. 2010, 104, 69–86. [Google Scholar] [CrossRef]
- Ramankutty, C.; Sugunan, S. Surface properties and catalytic activity of ferrospinels of nickel, cobalt and copper, prepared by soft chemical methods. Appl. Catal. A 2001, 218, 39–51. [Google Scholar] [CrossRef]
- Mondal, A.K.; Liu, H.; Li, Z.-F.; Wang, G. Multiwall carbon nanotube-nickel cobalt oxide hybrid structure as high performance electrodes for supercapacitors and lithium ion batteries. Electrochim. Acta 2016, 190, 346–353. [Google Scholar] [CrossRef]
- Rafighi, P.; Yaftian, M.; Noshiranzadeh, N. Solvent extraction of cobalt(II) ions; cooperation of oximes and neutral donors. Separ. Purif. Technol. 2010, 75, 32–38. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Tahmasbi, M.; Bastami, T.R.; Besharati, J.A. Rapid removal of cobalt ion from aqueous solutions by almond green hull. J. Hazard. Mater. 2009, 166, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Seldén, A.I.; Norberg, C.; Karlson-Stiber, C.; Hellström-Lindberg, E. Cobalt release from glazed earthenware: Observations in a case of lead poisoning. Environ. Toxicol. Pharmacol. 2007, 23, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Eltis, L.D. The biological occurrence and trafficking of cobalt. Metallomics 2011, 3, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Moon, S.-H. Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins. Water Res. 2002, 36, 1783–1793. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Anirudhan, T. Kinetic and equilibrium modelling of cobalt(II) adsorption onto bagasse pith based sulphurised activated carbon. Chem. Eng. J. 2008, 137, 257–264. [Google Scholar] [CrossRef]
- Lin, W.; Yuan, L.; Long, L.; Guo, C.; Feng, J. A fluorescent cobalt probe with a large ratiometric fluorescence response via modulation of energy acceptor molar absorptivity on metal ion binding. Adv. Funct. Mater. 2008, 18, 2366–2372. [Google Scholar] [CrossRef]
- El-Safty, S.A. Functionalized hexagonal mesoporous silica monoliths with hydrophobic azo-chromophore for enhanced Co(II) ion monitoring. Adsorption 2009, 15, 227–239. [Google Scholar] [CrossRef]
- Tsoutsi, D.; Guerrini, L.; Hermida-Ramon, J.M.; Giannini, V.; Liz-Marzán, L.M.; Wei, A.; Alvarez-Puebla, R.A. Simultaneous SERS detection of copper and cobalt at ultratrace levels. Nanoscale 2013, 5, 5841–5846. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lu, C.; Yan, D.; Ma, L. High selectivity sensing of cobalt in HepG2 cells based on necklace model microenvironment-modulated carbon dot-improved chemiluminescence in Fenton-like system. Biosens. Bioelectron. 2013, 45, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rajabi Khorrami, A.; Fakhari, A.R.; Shamsipur, M.; Naeimi, H. Pre-concentration of ultra trace amounts of copper, zinc, cobalt and nickel in environmental water samples using modified C18 extraction disks and determination by inductively coupled plasma-optical emission spectrometry. Int. J. Environ. Anal. Chem. 2009, 89, 319–329. [Google Scholar] [CrossRef]
- Shokoufi, N.; Shemirani, F.; Memarzadeh, F. Fiber optic-linear array detection spectrophotometry in combination with cloud point extraction for simultaneous preconcentration and determination of cobalt and nickel. Anal. Chim. Acta 2007, 601, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Ahmadi, F.; Shokrollahi, A. Simultaneous preconcentration and determination of copper, nickel, cobalt and lead ions content by flame atomic absorption spectrometry. J. Hazard. Mater. 2007, 142, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Shokrollahi, A.; Ahmadi, F.; Rajabi, H.; Soylak, M. Cloud point extraction for the determination of copper, nickel and cobalt ions in environmental samples by flame atomic absorption spectrometry. J. Hazard. Mater. 2008, 150, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.R.; Ahmadi, S.J. Development a robust ionic liquid-based dispersive liquid-liquid microextraction against high concentration of salt combined with flame atomic absorption spectrometry using microsample introduction system for preconcentration and determination of cobalt in water and saline samples. Microchim. Acta 2011, 172, 75–82. [Google Scholar]
- Badocco, D.; Pastore, P.; Favaro, G.; Maccà, C. Effect of eluent composition and pH and chemiluminescent reagent pH on ion chromatographic selectivity and luminol-based chemiluminescence detection of Co2+, Mn2+ and Fe2+ at trace levels. Talanta 2007, 72, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, Y.; Shi, M.; Huang, J.; Liu, Y.-M. Quantification of carnosine-related peptides by microchip electrophoresis with chemiluminescence detection. Anal. Biochem. 2009, 393, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jia, X.; Bian, P.; Ma, Z. A simple and novel system for colorimetric detection of cobalt ions. Analyst 2013, 139, 585–588. [Google Scholar] [CrossRef] [PubMed]
- López, O.N.B.; Fuentes, H.C.; Perezgasga, F.V.; Casillas, H.A.M. Detection and analysis of cobalt in continuous flow using an analytical microsystem based on LTCC technology. Sens. Actuators B Chem. 2016, 227, 11–16. [Google Scholar] [CrossRef]
- Trigo-López, M.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Colorimetric detection and determination of Fe(III), Co(II), Cu(II) and Sn(II) in aqueous media by acrylic polymers with pendant terpyridine motifs. Sens. Actuators B Chem. 2016, 226, 118–126. [Google Scholar] [CrossRef]
- Na, Y.J.; Choi, Y.W.; You, G.R.; Kim, C. A novel selective colorimetric chemosensor for cobalt ions in a near perfect aqueous solution. Sens. Actuators B Chem. 2016, 223, 234–240. [Google Scholar] [CrossRef]
- Leng, Y.; Zhang, F.; Zhang, Y.; Fu, X.; Weng, Y.; Chen, L.; Wu, A. A rapid and sensitive colorimetric assay method for Co2+ based on the modified Au nanoparticles (NPs): Understanding the involved interactions from experiments and simulations. Talanta 2012, 94, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Mehtab, S.; Saxena, P. A novel potentiometric membrane sensor for determination of Co2+ based on 5-amino-3-methylisothiazole. Sens. Actuators B Chem. 2007, 120, 455–461. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Hao, Y.; He, P.; Fang, Y. Determination of Co2+ based on the cobalt(II)-catalyzed electrochemiluminescence of luminol in acidic solution. Electrochim. Acta 2005, 50, 3414–3419. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, A.; Gao, Y.; He, C.; Wu, G.; Chen, Y.; Kai, X.; Zhu, C. Functionalized CdS quantum dots-based luminescence probe for detection of heavy and transition metal ions in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yuan, L.; Cao, Z.; Feng, J.; Feng, Y. Fluorescence enhancement of coumarin–quinoline by transition metal ions: Detection of paramagnetic Ni2+ and Co2+. Dyes Pigm. 2009, 83, 14–20. [Google Scholar] [CrossRef]
- Mashraqui, S.H.; Chandiramani, M.; Betkar, R.; Poonia, K. A simple internal charge transfer probe offering dual optical detection of Co(II) via color and fluorescence modulations. Tetrahedron Lett. 2010, 51, 1306–1308. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, L.; Zhang, Y.; Wang, H.; Wu, A. A colorimetric assay method for Co2+ based on thioglycolic acid functionalized hexadecyl trimethyl ammonium bromide modified Au nanoparticles (NPs). Nanoscale 2011, 3, 2150–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Lou, T.; Pan, D.; Chen, L.; Qu, C.; Chen, Z. Label-free colorimetric sensing of cobalt(II) based on inducing aggregation of thiosulfate stabilized gold nanoparticles in the presence of ethylenediamine. Analyst 2012, 137, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Pan, D.; Chen, L. Fenton-like Reaction-Mediated Etching of Gold Nanorods for Visual Detection of Co2+. Langmuir 2015, 31, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tian, D.; Li, H. Cooperative binding of bifunctionalized and click-synthesized silver nanoparticles for colorimetric Co2+ sensing. ACS Appl. Mater. Interfaces 2010, 2, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.K.; Oh, S.Y.; Park, C.; Kim, Y. Colorimetric detection of Co2+ ion using silver nanoparticles with spherical, plate, and rod shapes. Langmuir 2013, 29, 8978–8982. [Google Scholar] [CrossRef] [PubMed]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chim. 1928, 9, 133–203. [Google Scholar]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jo, A.; Seo, B.-K.; Lee, K.-W.; Park, W.H.; Lee, T.S. Colorimetric detection of transition metal ions with azopyridine-based probing molecule in aqueous solution and in PMMA film. Fiber Polym. 2013, 14, 1993–1998. [Google Scholar] [CrossRef]
- Yoon, B.; Park, I.S.; Shin, H.; Park, H.J.; Lee, C.W.; Kim, J.M. A litmus-type colorimetric and fluorometric volatile organic compound sensor based on inkjet-printed polydiacetylenes on paper substrates. Macromol. Rapid Commun. 2013, 34, 731–735. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-M.; Jang, S.-C.; Kim, G.Y.; Lee, C.-S.; Huh, Y.S.; Roh, C. A Rapid In Situ Colorimetric Assay for Cobalt Detection by the Naked Eye. Sensors 2016, 16, 626. https://doi.org/10.3390/s16050626
Kang S-M, Jang S-C, Kim GY, Lee C-S, Huh YS, Roh C. A Rapid In Situ Colorimetric Assay for Cobalt Detection by the Naked Eye. Sensors. 2016; 16(5):626. https://doi.org/10.3390/s16050626
Chicago/Turabian StyleKang, Sung-Min, Sung-Chan Jang, Gi Yong Kim, Chang-Soo Lee, Yun Suk Huh, and Changhyun Roh. 2016. "A Rapid In Situ Colorimetric Assay for Cobalt Detection by the Naked Eye" Sensors 16, no. 5: 626. https://doi.org/10.3390/s16050626
APA StyleKang, S.-M., Jang, S.-C., Kim, G. Y., Lee, C.-S., Huh, Y. S., & Roh, C. (2016). A Rapid In Situ Colorimetric Assay for Cobalt Detection by the Naked Eye. Sensors, 16(5), 626. https://doi.org/10.3390/s16050626







