Investigation on the Mechanical and Electrical Behavior of a Tuning Fork-Shaped Ionic Polymer Metal Composite Actuator with a Continuous Water Supply Mechanism
Abstract
:1. Introduction
2. Device Design and Fabrication
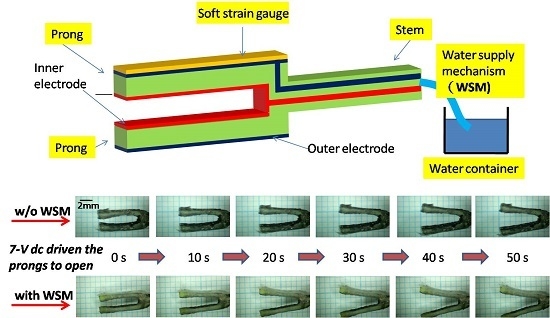
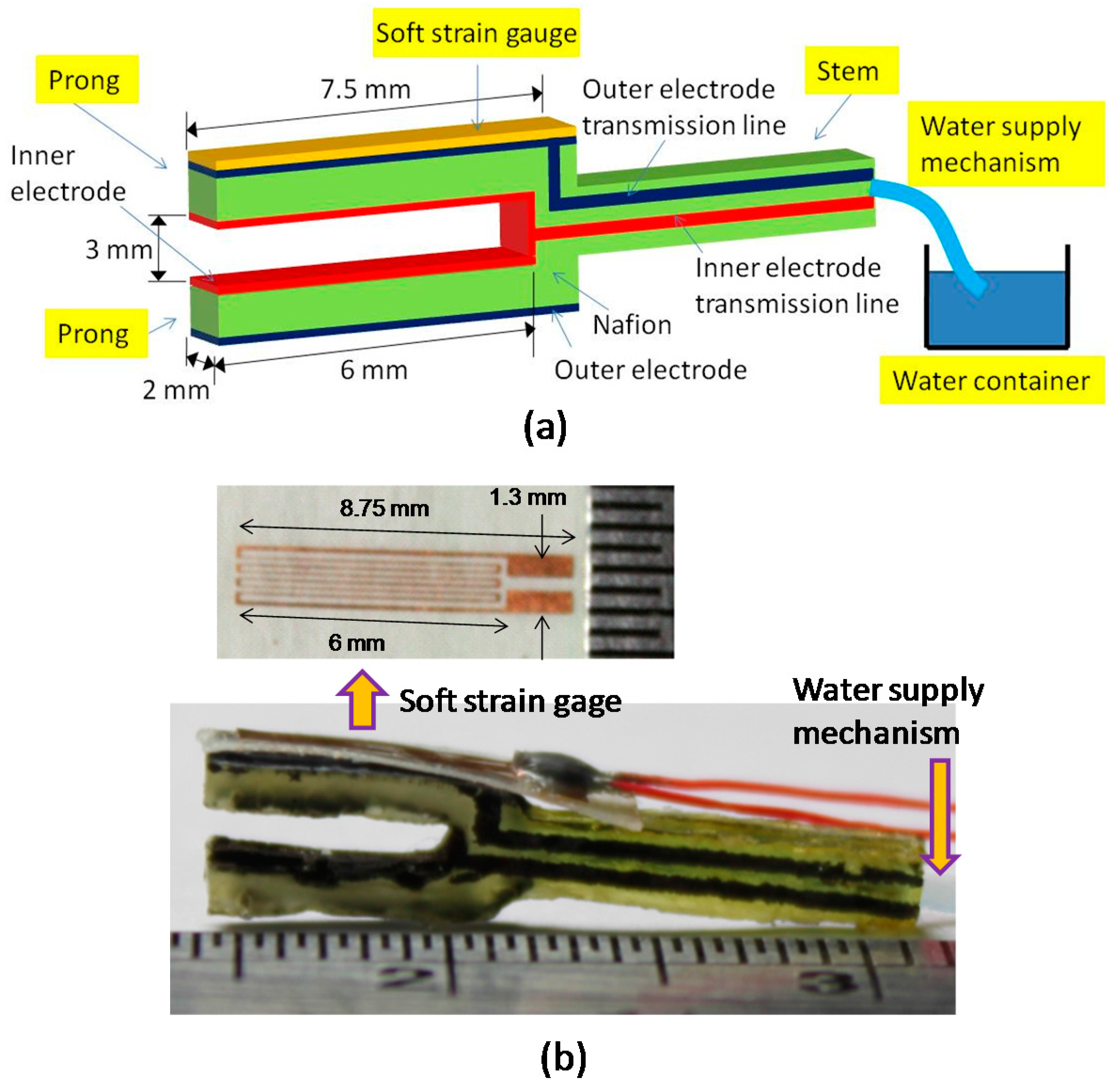
2.1. Design Concept
2.2. Fabrication
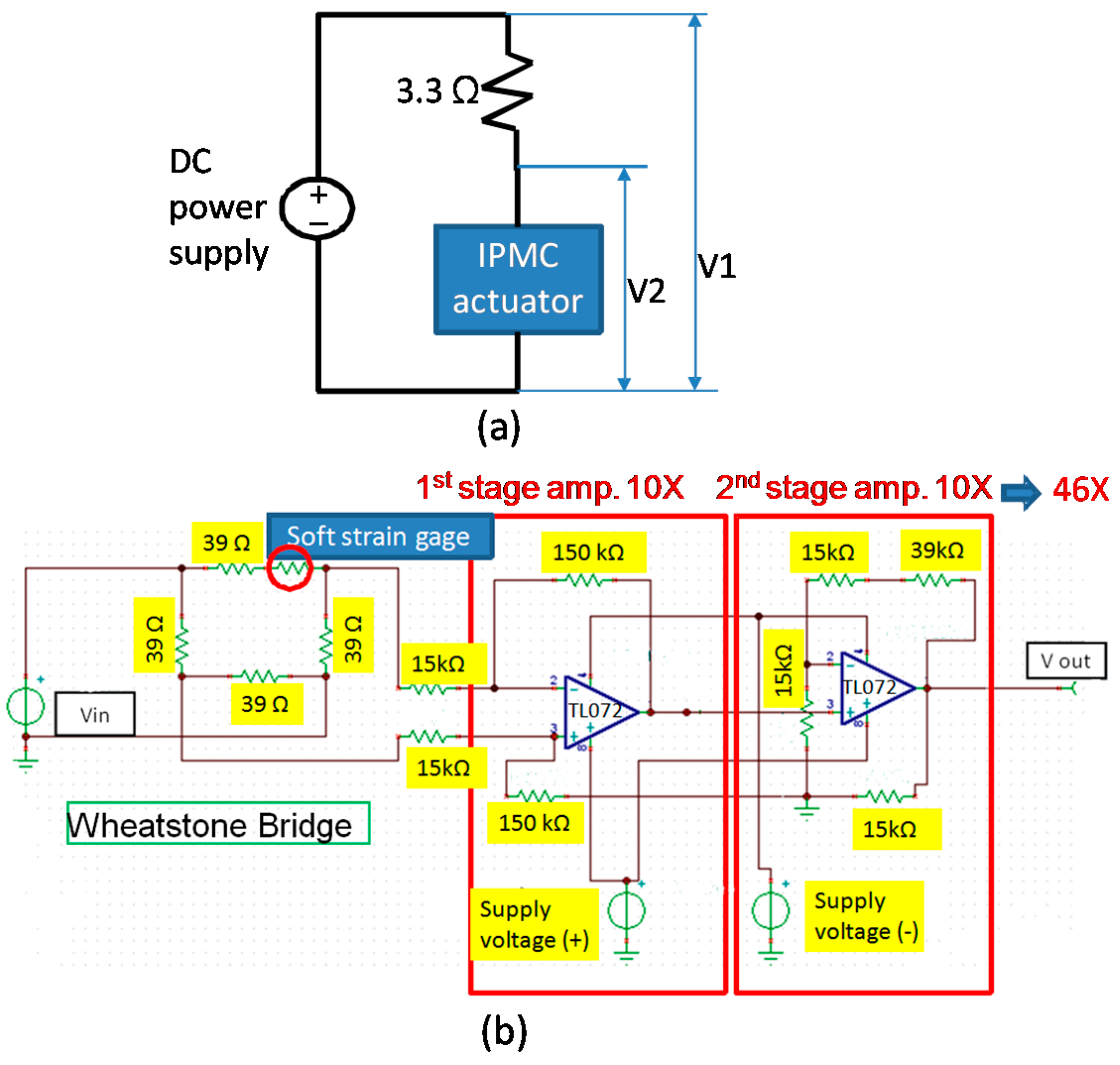
3. Experimental Setup
4. Results and Discussion
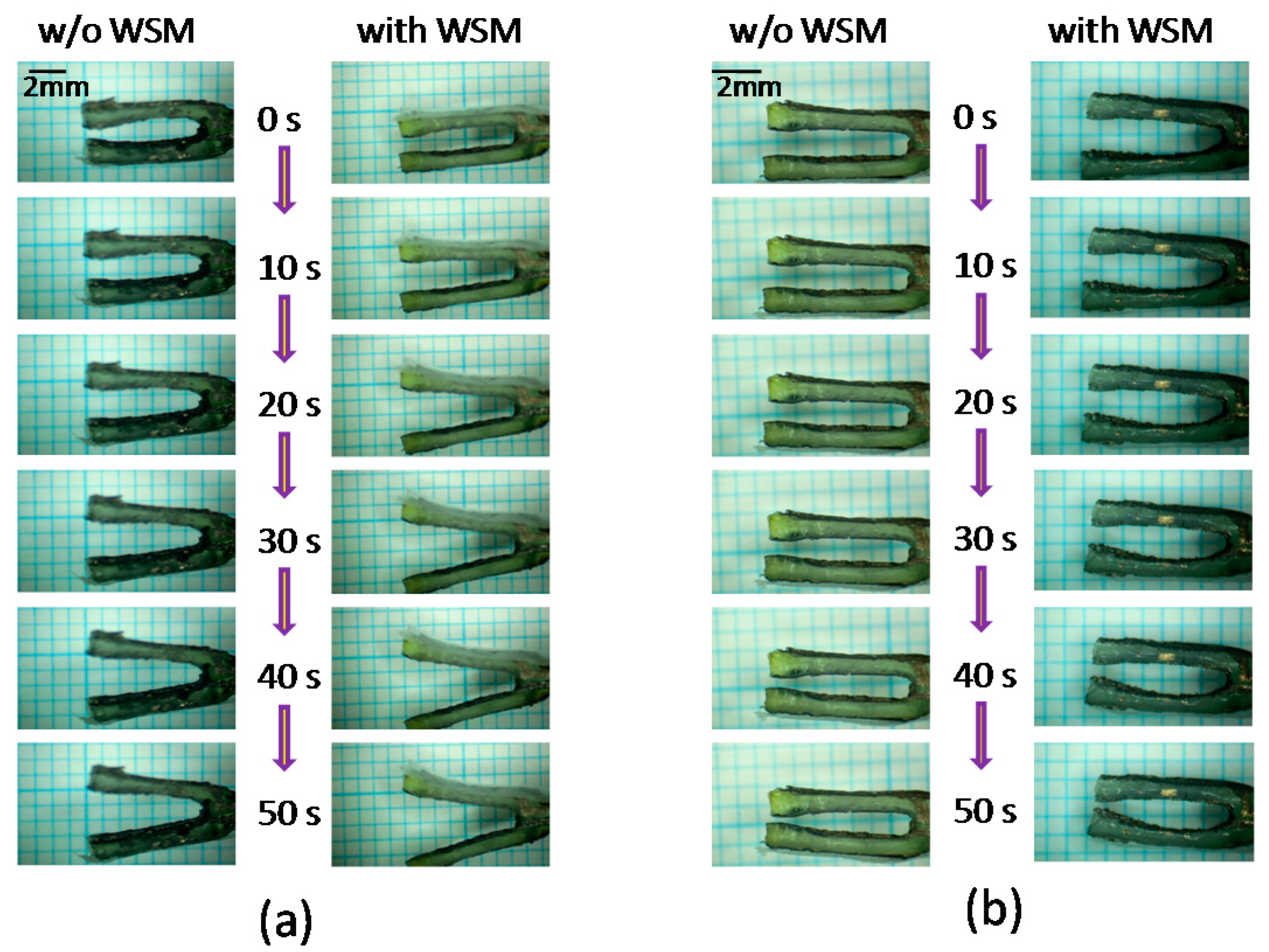
4.1. Displacement at the IPMC Actuator Prong
4.2. Current Response of the IPMC Actuator and Analysis via the Electrochemical Model
4.3. Strain Relation between the Signal of the Soft Strain Gauge and Measured Displacement
4.4. Effect on Water Content Variation of the IPMC Actuator
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaihre, B.; Alici, G.; Spinks, G.M.; Cairney, J.M. Pushing the limits for microactuators based on electroactive polymers. J. Microelectromech. Syst. 2012, 21, 574–585. [Google Scholar] [CrossRef]
- Moghadam, A.A.A.; Kouzani, A.; Torabi, K.; Kaynak, A.; Shahinpoor, M. Development of a novel soft parallel robot equipped with polymeric artificial muscles. Smart Mater. Struct. 2015, 24. [Google Scholar] [CrossRef]
- Mutlu, R.; Alici, G.; Li, W. An effective methodology to solve inverse kinematics of electroactive polymer actuators modelled as active and soft robotic structures. Mech. Mach. Theory 2013, 67, 94–110. [Google Scholar] [CrossRef]
- Kim, S.-M.; Tiwari, R.; Kim, K.J. A Novel Ionic Polymer Metal ZnO Composite (IPMZC). Sensors 2011, 11, 4674–4687. [Google Scholar] [CrossRef] [PubMed]
- Shahinpoor, M.; Kim, K.J. Ionic polymer-metal composites: I. Fundamentals. Smart Mater. Struct. 2001, 10, 819–833. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Bar-Cohen, Y.; Simpson, J.O.; Smith, J. Ionic polymer-metal composites (IPMCs) as biomimetic sensors, actuators and artificial muscles: A review. Smart Mater. Struct. 1998, 7, R15–R30. [Google Scholar] [CrossRef]
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer artificial muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Zhu, Z.; Chang, L.; Asaka, K.; Wang, Y.; Chen, H.; Zhao, H.; Li, D. Comparative experimental investigation on the actuation mechanisms of ionic polymer-metal composites with different backbones and water contents. J. Appl. Phys. 2014, 115. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, B.; Zheng, G.; Zheng, J.; Qiu, X.; Zhuang, M.; Sun, D. Improving the performance of IPMCs with a gradient in thickness. Smart Mater. Struct. 2013, 22. [Google Scholar] [CrossRef]
- Anand, S.V.; Arvind, K.; Bharath, P.; Mahapatra, D.R. Energy harvesting using ionic electro-active polymer thin films with Ag-based electrodes. Smart Mater. Struct. 2010, 19. [Google Scholar] [CrossRef]
- Jeon, J.H.; Yeom, S.W.; Oh, I.K. Fabrication and actuation of ionic polymer metal composites patterned by combining electroplating with electroless plating. Compos. A Appl. Sci. Manuf. 2008, 39, 588–596. [Google Scholar] [CrossRef]
- Tiwari, R.; Kim, K.J. Mechanoelectric transduction in ionic polymer-metal composite. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Feng, G.-H.; Liu, K.-M. Fabrication and Characterization of a Micromachined Swirl-Shaped Ionic Polymer Metal Composite Actuator with Electrodes Exhibiting Asymmetric Resistance. Sensors 2014, 14, 8380–8397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chang, L.; Takagi, K.; Wang, Y.; Chen, H.; Li, D. Water content criterion for relaxation deformation of Nafion based ionic polymer metal composites doped with alkali cations. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Zhao, G.; Guo, H.J.; Wang, H.J.; Yang, J.J.; Wang, Y.J.; Wang, Z.J.; Wang, C. Processing and Modification of Ionic Polymers Metal Composites (IPMC)—A Review. J. Biomim. Biomater. Biomed. Eng. 2015, 22, 13–20. [Google Scholar] [CrossRef]
- Park, I.S.; Kim, S.M.; Pugal, D.; Huang, L.; Tam-Chang, L.W.; Kim, K.J. Visualization of the cation migration in ionic polymer–metal composite under an electric field. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Cha, Y.; Cellini, F.; Porfiri, M. Electrical impedance controls mechanical sensing in ionic polymer metal composites. Phys. Rev. E 2013, 88. [Google Scholar] [CrossRef] [PubMed]
- Biswal, D.K.; Bandopadhya, D.; Dwivedy, S.K. Investigation and evaluation of effect of dehydration on vibration characteristics of silver-electroded ionic polymer-metal composite actuator. J. Intell. Mater. Syst. Struct. 2013, 24, 1197–1212. [Google Scholar] [CrossRef]
- Feng, G.-H.; Tsai, J.-W. Micromachined optical fiber enclosed 4-electrode IPMC actuator with multidirectional control ability for biomedical application. Biomedicalmicrodevices 2011, 13, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nemat-Nasser, S.; Li, J.Y. Electromechanical response of ionic polymer-metal composites. J. Appl. Phys. 2000, 87, 3321–3331. [Google Scholar] [CrossRef]
- Kanno, R.; Tadokoro, S.; Hattori, M.; Takamori, T.; Oguro, K. Modeling of ICPF (Ionic Conducting Polymer Gel Film) Actuator: 1st Report, Fundamental Characteristics and Black-Box Modeling. Trans. Jpn. Soc. Mech. Eng. C 1996, 62, 2299–2305. [Google Scholar] [CrossRef]
- Akle, B.J.; Leo, D.J. Characterization and modeling of extensional and bending actuation in ionomeric polymer transducers. Smart Mater. Struct. 2007, 16. [Google Scholar] [CrossRef]
- Feng, G.-H. Numerical study on dynamic characteristics of micromachined ionic polymer metal composite devices based on molecular-scale modeling. Comput. Mater. Sci. 2010, 50, 158–166. [Google Scholar] [CrossRef]
- Davidson, J.D.; Goulbourne, N.C. Boundary layer charge dynamics in ionic liquid-ionic polymer transducers. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Aureli, M.; Porfiri, M. Effect of electrode surface roughness on the electrical impedance of ionic polymer metal composites. Smart Mater. Struct. 2012, 21. [Google Scholar] [CrossRef]
- Bahramzadeh, Y.; Shahinpoor, M. Dynamic curvature sensing employing ionic-polymer metal composite sensors. Smart Mater. Struct. 2011, 20. [Google Scholar] [CrossRef]
- Chen, Z.; Hedgepeth, D.; Tan, X. A nonlinear, control-oriented model for ionic polymer-metal composite actuators. Smart Mater. Struct. 2009, 18. [Google Scholar] [CrossRef]
- Del Bufalo, G.; Placidi, L.; Porfiri, M. A mixture theory framework for modeling the mechanical actuation of ionic polymer metal composites. Smart Mater. Struct. 2008, 17. [Google Scholar] [CrossRef]
- Jo, C.; Pugal, D.; Oh, I.K.; Kim, K.J.; Asaka, K. Recent advances in ionic polymer-metal composite actuators and their modeling and applications. Prog. Polym. Sci. 2013, 38, 1037–1066. [Google Scholar] [CrossRef]
- Shen, Q.; Kim, K.J.; Wang, T. Electrode of ionic polymer-metal composite sensors: Modeling and experimental investigation. J. Appl. Phys. 2014, 115. [Google Scholar] [CrossRef]
- Caponetto, R.; DeLuca, V.; Graziani, S. A multiphysics model of IPMC actuators dependence on relative humidity. In Proceedings of the 2015 IEEE International Instrumentation and Measurement Technology Conference, Pisa, Italy, 11–14 May 2015; pp. 1482–1487.
- Kim, S.J.; Lee, I.T.; Lee, H.Y.; Kim, Y.H. Performance improvement of an ionic polymer–metal composite actuator by parylene thin film coating. Smart Mater. Struct. 2006, 15. [Google Scholar] [CrossRef]
- Nemat-Nasser, S.; Wu, Y. Comparative experimental study of ionic polymer-metal composites with different backbone ionomers and in various cation forms. J. Appl. Phys. 2003, 93, 5255–5267. [Google Scholar] [CrossRef]
- Shoji, E.; Hirayama, D. Effects of humidity on the performance of ionic polymer-metal composite actuators: Experimental study of the back-relaxation of actuators. J. Phys. Chem. B 2007, 111, 11915–11920. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.-H.; Huang, W.-L. Development of Tuning Fork-shaped Clamps with Nickel-electroded Ionic Polymer Metal Composites. Int. J. Autom. Smart Technol. 2012, 2, 55–62. [Google Scholar] [CrossRef]
- Lei, H.; Li, W.; Tan, X. Encapsulation of ionic polymer-metal composite (IPMC) sensors with thick parylene: Fabrication process and characterization results. Sens. Actuators A Phys. 2014, 217, 1–12. [Google Scholar] [CrossRef]
- Feng, G.-H.; Huang, W.-L. A self-strain feedback tuning-fork-shaped ionic polymer metal composite clamping actuator with soft matter elasticity-detecting capability for biomedical applications. Mater. Sci. Eng. C 2014, 45, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.-H.; Hou, S.-Y. Double-section curvature tunable functional actuator with micromachined buckle and grid wire for electricity delivery. Smart Mater. Struct. 2015, 24. [Google Scholar] [CrossRef]
- Nemat-Nasser, S. Micromechanics of actuation of ionic polymer-metal composites. J. Appl. Phys. 2002, 92, 2899–2915. [Google Scholar] [CrossRef]
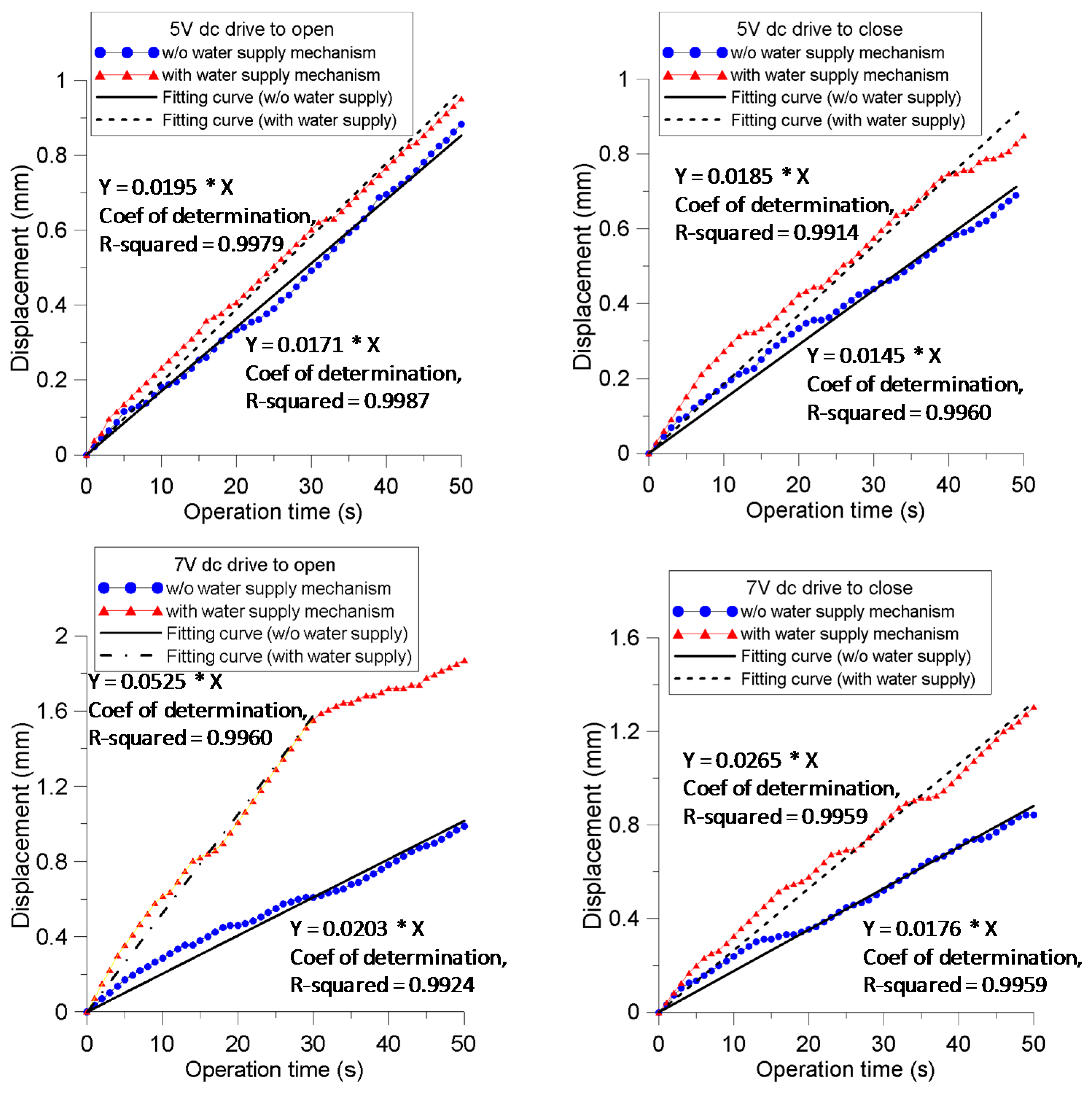

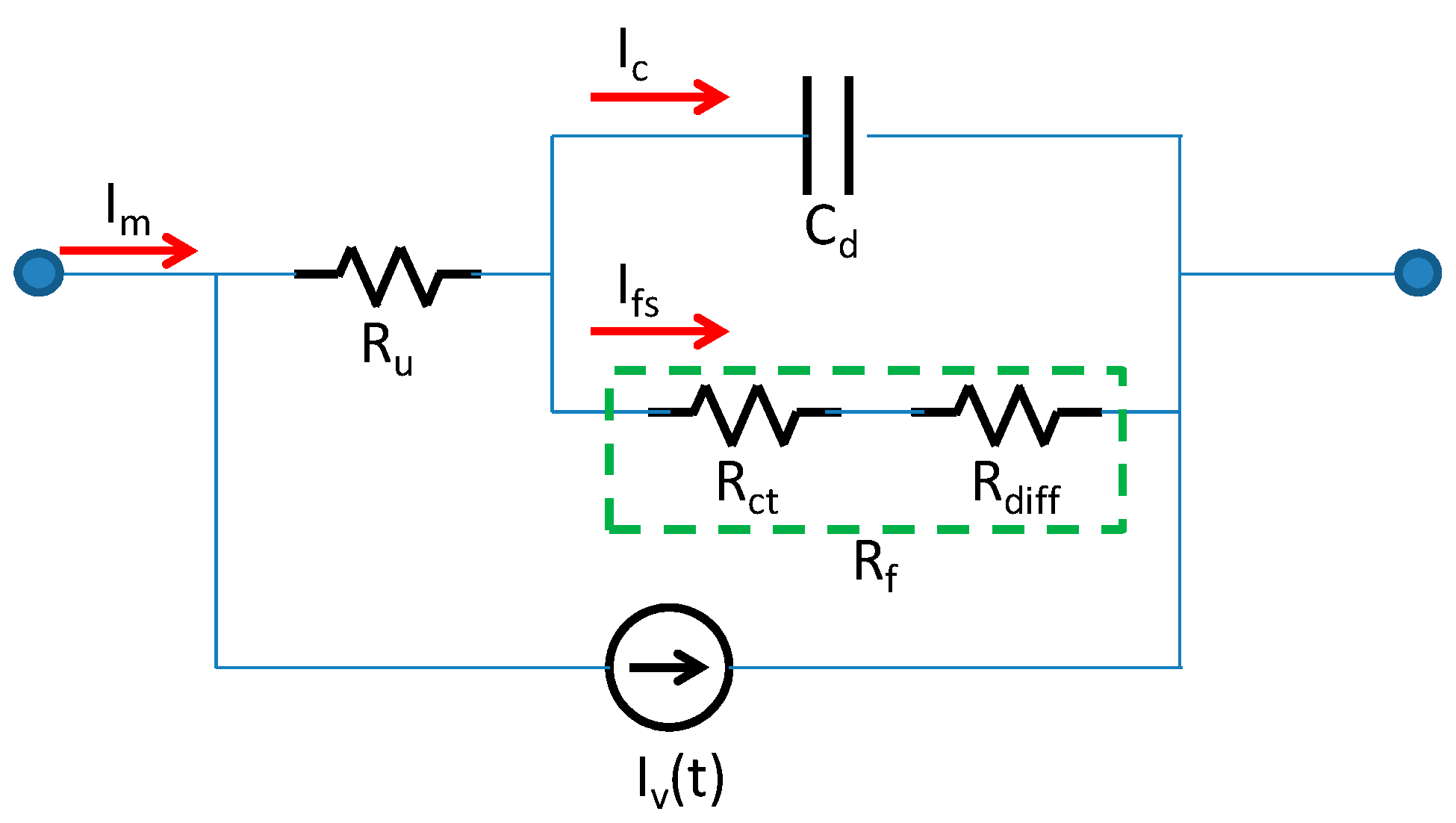


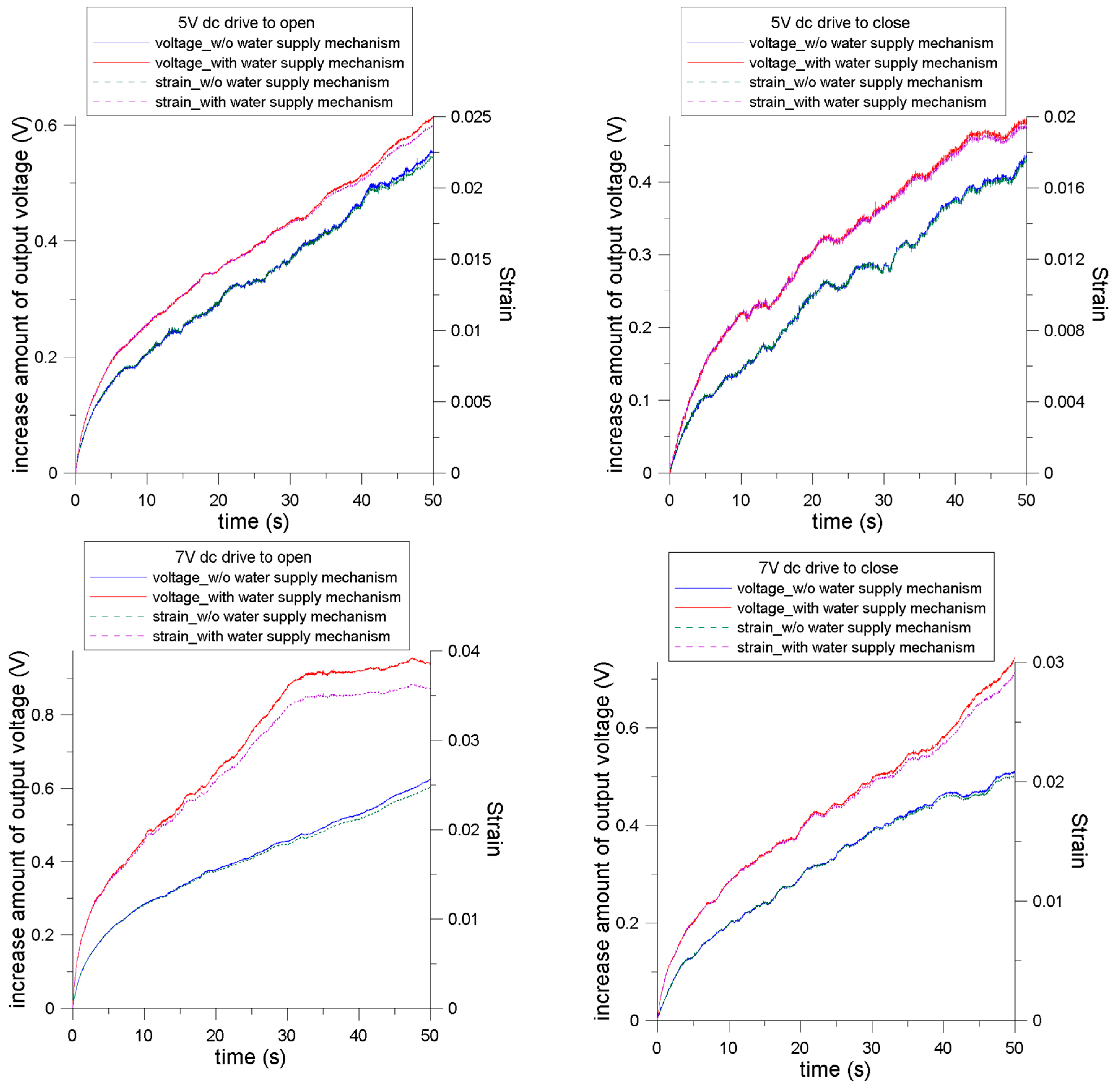
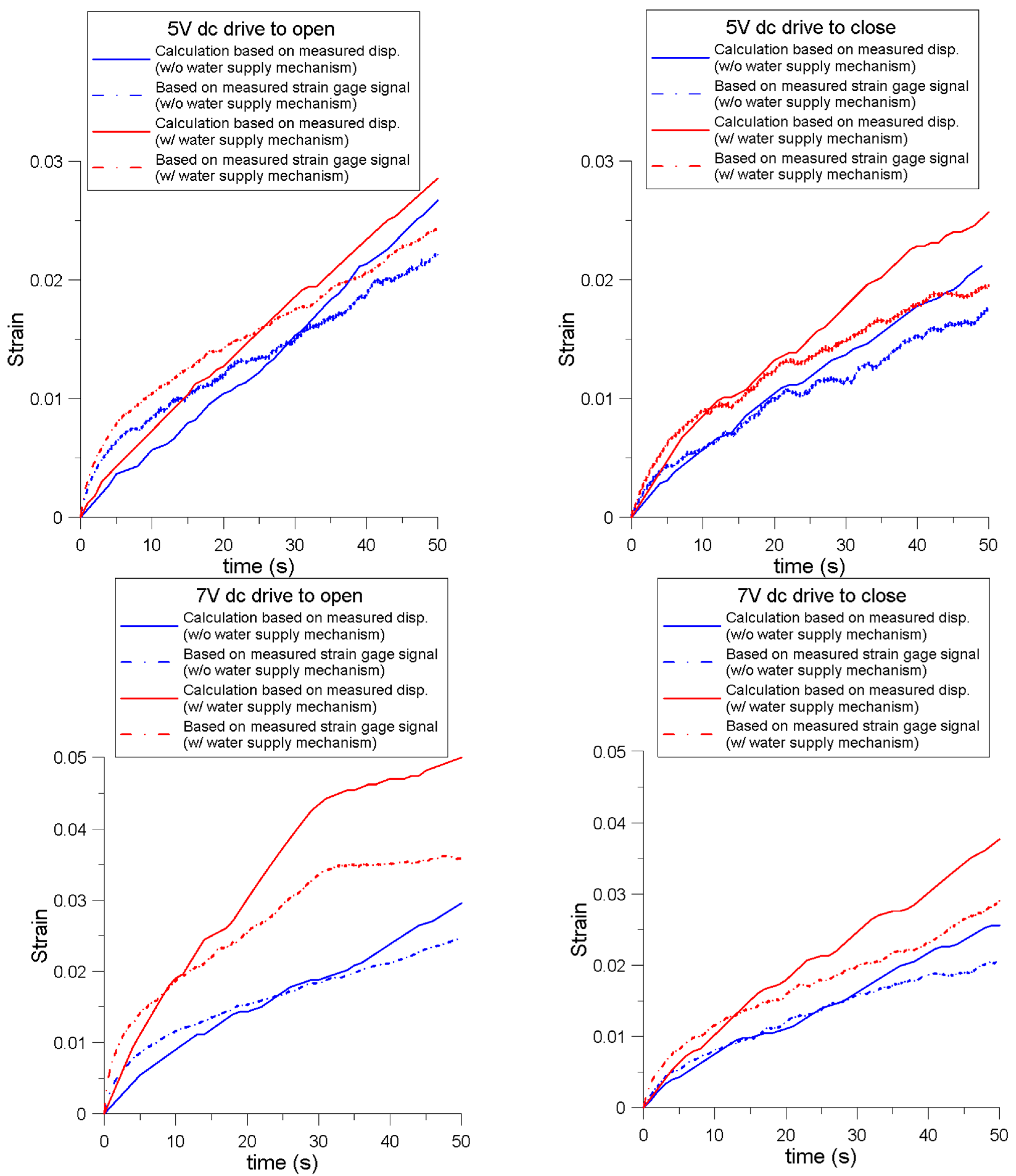
| Parameters | 5 V Open | 5 V Close | 7 V Open | 7 V Close | ||||
|---|---|---|---|---|---|---|---|---|
| w/o | w/ | w/o | w/ | w/o | w/ | w/o | w/ | |
| Cd (mF) | 17.63 | 19.1 | 9.19 | 34.9 | 16.0 | 9.11 | 5.82 | 6.38 |
| Ru(ohm) | 232.6 | 150.2 | 167.2 | 214.6 | 151.8 | 288.1 | 195.5 | 155.9 |
| Rf (ohm) | 78.0 | 183.2 | 175.3 | 132.6 | 308.7 | 128.6 | 193.4 | 216.4 |
| Ru+Rf(ohm) | 310.6 | 333.3 | 342.5 | 347.2 | 460.5 | 416.7 | 388.9 | 372.3 |
| Measured Mass (mg) | 5 V Open | 5 V Close | 7 V Open | 7 V Close | ||||
|---|---|---|---|---|---|---|---|---|
| w/o | w/ | w/o | w/ | w/o | w/ | w/o | w/ | |
| mi-mSG | 83.7 | 285.5 | 83.9 | 285.8 | 83.8 | 285.4 | 83.9 | 285.6 |
| mf-mSG | 82.3 | 285.4 | 82.4 | 285.6 | 81.5 | 285.3 | 81.8 | 285.5 |
| loss of mass (mi-mf) | 1.4 | 0.1 | 1.5 | 0.2 | 2.3 | 0.1 | 2.1 | 0.1 |
| dehydrated md | 58.2 | X | 58.7 | X | 58.6 | X | 58.9 | X |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.-H.; Huang, W.-L. Investigation on the Mechanical and Electrical Behavior of a Tuning Fork-Shaped Ionic Polymer Metal Composite Actuator with a Continuous Water Supply Mechanism. Sensors 2016, 16, 433. https://doi.org/10.3390/s16040433
Feng G-H, Huang W-L. Investigation on the Mechanical and Electrical Behavior of a Tuning Fork-Shaped Ionic Polymer Metal Composite Actuator with a Continuous Water Supply Mechanism. Sensors. 2016; 16(4):433. https://doi.org/10.3390/s16040433
Chicago/Turabian StyleFeng, Guo-Hua, and Wei-Lun Huang. 2016. "Investigation on the Mechanical and Electrical Behavior of a Tuning Fork-Shaped Ionic Polymer Metal Composite Actuator with a Continuous Water Supply Mechanism" Sensors 16, no. 4: 433. https://doi.org/10.3390/s16040433