Mechanical Characterization of Hybrid Vesicles Based on Linear Poly(Dimethylsiloxane-b-Ethylene Oxide) and Poly(Butadiene-b-Ethylene Oxide) Block Copolymers
Abstract
:1. Introduction
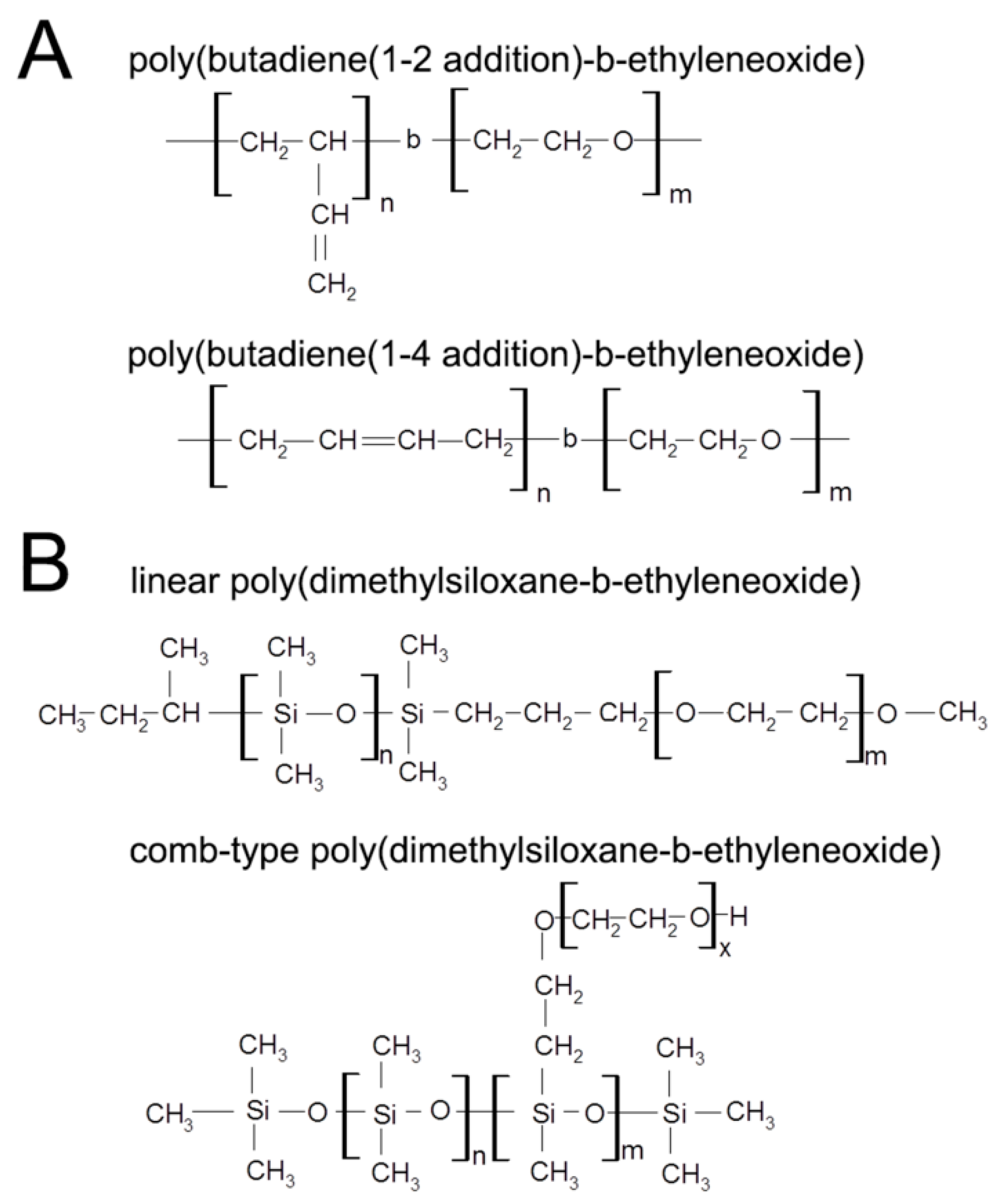
2. Materials and Methods
2.1. Formation of Polymersomes
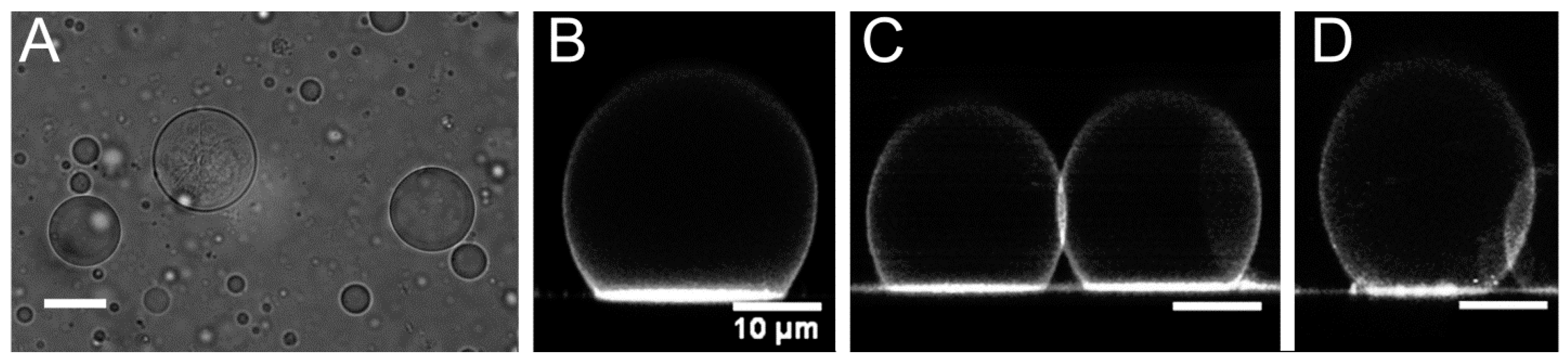
2.2. Optical Microscopy Imaging
2.3. Micropipette Aspiration Measurements
2.4. Statistical Analyses
3. Results and Discussion
3.1. Vesicle Size and Shape
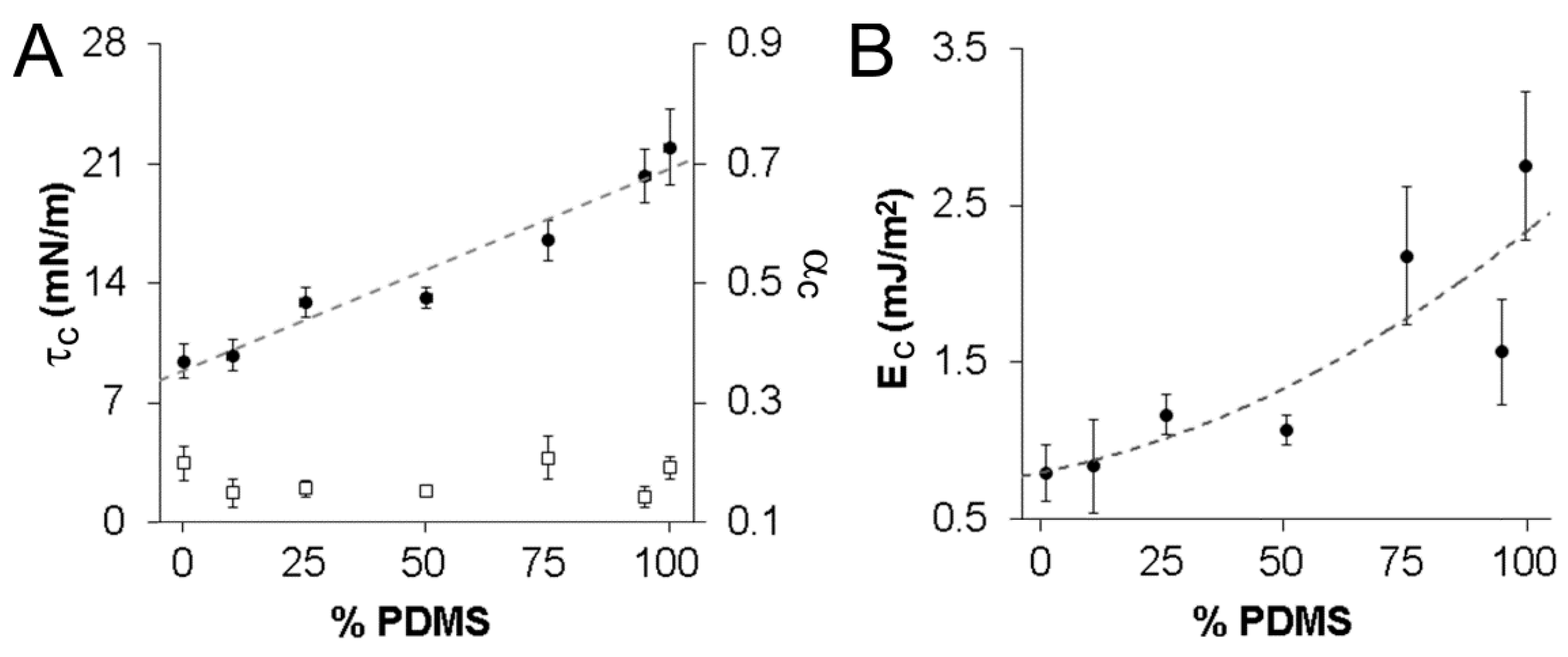
3.2. Vesicle Area Expansion Modulus
3.3. Vesicle Critical Tension, Critical Strain, and Cohesive Energy Density
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kita-Tokarczyk, K.; Grumelard, J.; Haefele, T.; Meier, W. Block copolymer vesicles—Using concepts from polymer chemistry to mimic biomembranes. Polymer 2005, 46, 3540–3563. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Le Meins, J.F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Li, M.-H.; Keller, P. Stimuli-responsive polymer vesicles. Soft Matter 2009, 5, 927–937. [Google Scholar] [CrossRef]
- Brinkhuis, R.P.; Rutjes, F.P.; van Hest, J.C. Polymeric vesicles in biomedical applications. Polym. Chem. 2011, 2, 1449–1462. [Google Scholar] [CrossRef]
- Du, J.; O’Reilly, R.K. Advances and challenges in smart and functional polymer vesicles. Soft Matter 2009, 5, 3544–3561. [Google Scholar] [CrossRef]
- Chen, D.; Santore, M.M. Hybrid copolymer-phospholipid vesicles: Phase separation resembling mixed phospholipid lamellae, but with mechanical stability and control. Soft Matter 2015, 11, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.M.; Bermudez, H.; Discher, B.M.; Sheehan, M.A.; Won, Y.Y.; Bates, F.S.; Discher, D.E. Preparation, stability, and in vitro performance of vesicles made with diblock copolymers. Biotechnol. Bioeng. 2001, 73, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, J.; Hahn, M.S.; Silas, J.A. Polymerization of hydrogels inside self-assembled block copolymer vesicles. Langmuir 2009, 25, 12878–12884. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, A.; Glaser, N.; Le Meins, J.-F.O.; Lecommandoux, S. Block copolymer vesicle permeability measured by osmotic swelling and shrinking. Langmuir 2011, 27, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, H.; Hammer, D.; Discher, D. Effect of bilayer thickness on membrane bending rigidity. Langmuir 2004, 20, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208. [Google Scholar] [CrossRef]
- Ghoroghchian, P.P.; Lin, J.J.; Brannan, A.K.; Frail, P.R.; Bates, F.S.; Therien, M.J.; Hammer, D.A. Quantitative membrane loading of polymer vesicles. Soft Matter 2006, 2, 973–980. [Google Scholar] [CrossRef]
- Lin, J.J.; Bates, F.S.; Hammer, D.A.; Silas, J.A. Adhesion of polymer vesicles. Phys. Rev. Lett. 2005, 95, 026101. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, R.; Mell, M.; López-Montero, I.; Netzel, J.; Hellweg, T.; Monroy, F. Polymersomes: Smart vesicles of tunable rigidity and permeability. Soft Matter 2011, 7, 1532–1542. [Google Scholar] [CrossRef]
- He, F.; Tong, Y.W. A mechanistic study on amphiphilic block co-polymer poly(butadiene-b-(ethylene oxide)) vesicles reveals the water permeation mechanism through a polymeric bilayer. RSC Adv. 2014, 4, 15304–15313. [Google Scholar] [CrossRef]
- Kickelbick, G.; Bauer, J.; Huesing, N.; Andersson, M.; Holmberg, K. Aggregation behavior of short-chain pdms-b-peo diblock copolymers in aqueous solutions. Langmuir 2003, 19, 10073–10076. [Google Scholar] [CrossRef]
- Kickelbick, G.; Bauer, J.; Hüsing, N.; Andersson, M.; Palmqvist, A. Spontaneous vesicle formation of short-chain amphiphilic polysiloxane-b-poly (ethylene oxide) block copolymers. Langmuir 2003, 19, 3198–3201. [Google Scholar] [CrossRef]
- Itel, F.; Chami, M.; Najer, A.; Lorcher, S.; Wu, D.; Dinu, L.A.; Meier, W. Molecular organization and dynamics in polymersome membranes: A lateral diffusion study. Macromolecules 2014, 47, 7588–7596. [Google Scholar] [CrossRef]
- Yan, Y.; Hoffmann, H.; Leson, A.; Mayer, C. Molecular exchange through the vesicle membrane of siloxane surfactant in water/glycerol mixed solvents. J. Phys. Chem. B 2007, 111, 6161–6166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.M.; Santore, M.; Bates, F.S.; Discher, D.E. From membranes to melts, rouse to reptation: Diffusion in polymersome versus lipid bilayers. Macromolecules 2002, 35, 323–326. [Google Scholar] [CrossRef]
- Salva, R.; Le Meins, J.-F.; Sandre, O.; Brulet, A.; Schmutz, M.; Guenoun, P.; Lecommandoux, S. Polymersome shape transformation at the nanoscale. ACS Nano 2013, 7, 9298–9311. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Santore, M.M. Adhesion plaque formation dynamics between polymer vesicles in the limit of highly concentrated binding sites. Langmuir 2007, 23, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Elias, D.R.; Kamat, N.P.; Johnston, E.D.; Poloukhtine, A.; Popik, V.; Hammer, D.A.; Tsourkas, A. Improved tumor targeting of polymer-based nanovesicles using polymer-lipid blends. Bioconj. Chem. 2011, 22, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Silas, J.A.; Bermudez, H.; Milam, V.T.; Bates, F.S.; Hammer, D.A. The effect of polymer chain length and surface density on the adhesiveness of functionalized polymersomes. Langmuir 2004, 20, 5493–5500. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, K.; Rawicz, W.; Needham, D.; Evans, E. Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 2000, 79, 321–327. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.; McIntosh, T.; Needham, D.; Evans, E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef]
- Discher, B.M.; Bermudez, H.; Hammer, D.A.; Discher, D.E.; Won, Y.-Y.; Bates, F.S. Cross-linked polymersome membranes: Vesicles with broadly adjustable properties. J. Phys. Chem. B 2002, 106, 2848–2854. [Google Scholar] [CrossRef]
- Evans, E.; Rawicz, W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 1990, 64, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.M.; He, M.; Lin, Z.; Davis, H.T.; Scriven, L. Lyotropic liquid crystal phase behavior of polymeric siloxane surfactants. Langmuir 1993, 9, 2789–2798. [Google Scholar] [CrossRef]
- Christian, D.A.; Tian, A.; Ellenbroek, W.G.; Levental, I.; Rajagopal, K.; Janmey, P.A.; Liu, A.J.; Baumgart, T.; Discher, D.E. Spotted vesicles, striped micelles and janus assemblies induced by ligand binding. Nat. Mater. 2009, 8, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Fetters, L.J.; Lohse, D.J.; Colby, R.H. Chain dimensions and entanglement spacings. In Physical Properties of Polymers Handbook; Mark, J., Ed.; Springer: New York, NY, USA, 2007; pp. 447–454. [Google Scholar]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface energy and the contact of elastic solids. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Tufano, C.; Peters, G.W.M.; Meijer, H.E.H.; Anderson, P.D. Effects of partial miscibility on drop-wall and drop-drop interactions. J. Rheol. 2010, 54, 159–183. [Google Scholar] [CrossRef]
- Needham, D.; Evans, E. Structure and mechanical properties of giant lipid (dmpc) vesicle bilayers from 20 °C below to 10 °C above the liquid crystal-crystalline phase transition at 24 °C. Biochemistry 1988, 27, 8261–8269. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Renormalization of the tension and area expansion modulus in fluid membranes. Biophys. J. 1997, 73, 865–869. [Google Scholar] [CrossRef]
- Holderer, O.; Frielinghaus, H.; Byelov, D.; Monkenbusch, M.; Allgaier, J.; Richter, D. Dynamic properties of microemulsions modified with homopolymers and diblock copolymers: The determination of bending moduli and renormalization effects. J. Chem. Phys. 2005, 122, 094908. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Needham, D. Attraction between lipid bilayer membranes in concentrated solutions of nonadsorbing polymers: Comparison of mean-field theory with measurements of adhesion energy. Macromolecules 1988, 21, 1822–1831. [Google Scholar] [CrossRef]
- Milner, S.T.; Safran, S. Dynamical fluctuations of droplet microemulsions and vesicles. Phys. Rev. A 1987, 36, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.A. Detailed mechanics of membrane-membrane adhesion and separation. I. Continuum of molecular cross-bridges. Biophys. J. 1985, 48, 175–183. [Google Scholar] [CrossRef]
- Evans, E. Detailed mechanics of membrane-membrane adhesion and separation. II. Discrete kinetically trapped molecular cross-bridges. Biophys. J. 1985, 48, 185–192. [Google Scholar] [CrossRef]
- Leibler, S.; Singh, R.R.; Fisher, M.E. Thermodynamic behavior of two-dimensional vesicles. Phys. Rev. Lett. 1987, 59. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, P.; Lipowsky, R.; Kierfeld, J. Persistence length of semiflexible polymers and bending rigidity renormalization. EPL 2006, 76. [Google Scholar] [CrossRef]
- Leermakers, F.; Wijmans, C.; Fleer, G. On the structure of polymeric micelles: Self-consistent-field theory and universal properties for volume fraction profiles. Macromolecules 1995, 28, 3434–3443. [Google Scholar] [CrossRef]
- Baulin, V.A.; Zhulina, E.B.; Halperin, A. Self-consistent field theory of brushes of neutral water-soluble polymers. J. Chem. Phys. 2003, 119, 10977–10988. [Google Scholar] [CrossRef]
- Dan, N.; Tirrell, M. Effect of bimodal molecular weight distribution on the polymer brush. Macromolecules 1993, 26, 6467–6473. [Google Scholar] [CrossRef]
- Nam, J.; Beales, P.A.; Vanderlick, T.K. Giant phospholipid/block copolymer hybrid vesicles: Mixing behavior and domain formation. Langmuir 2011, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; Zhelev, D. The mechanochemistry of lipid vesicles examined by micropipet manipulationtechniques. Surf. Sci. Ser. 1996, 62, 373–444. [Google Scholar]
- Needham, D.; Nunn, R.S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 1990, 58, 997–1009. [Google Scholar] [CrossRef]
- LoPresti, C.; Massignani, M.; Fernyhough, C.; Blanazs, A.; Ryan, A.J.; Madsen, J.; Warren, N.J.; Armes, S.P.; Lewis, A.L.; Chirasatitsin, S.; et al. Controlling polymersome surface topology at the nanoscale by membrane confined polymer/polymer phase separation. ACS Nano 2011, 5, 1775–1784. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspard, J.; Casey, L.M.; Rozin, M.; Munoz-Pinto, D.J.; Silas, J.A.; Hahn, M.S. Mechanical Characterization of Hybrid Vesicles Based on Linear Poly(Dimethylsiloxane-b-Ethylene Oxide) and Poly(Butadiene-b-Ethylene Oxide) Block Copolymers. Sensors 2016, 16, 390. https://doi.org/10.3390/s16030390
Gaspard J, Casey LM, Rozin M, Munoz-Pinto DJ, Silas JA, Hahn MS. Mechanical Characterization of Hybrid Vesicles Based on Linear Poly(Dimethylsiloxane-b-Ethylene Oxide) and Poly(Butadiene-b-Ethylene Oxide) Block Copolymers. Sensors. 2016; 16(3):390. https://doi.org/10.3390/s16030390
Chicago/Turabian StyleGaspard, Jeffery, Liam M. Casey, Matt Rozin, Dany J. Munoz-Pinto, James A. Silas, and Mariah S. Hahn. 2016. "Mechanical Characterization of Hybrid Vesicles Based on Linear Poly(Dimethylsiloxane-b-Ethylene Oxide) and Poly(Butadiene-b-Ethylene Oxide) Block Copolymers" Sensors 16, no. 3: 390. https://doi.org/10.3390/s16030390





