Improving Pulse Rate Measurements during Random Motion Using a Wearable Multichannel Reflectance Photoplethysmograph
Abstract
:1. Introduction
2. Experimental Section
2.1. Device Description and Experimental Setup
2.1.1. Sensor Description
2.1.2. Data Collection
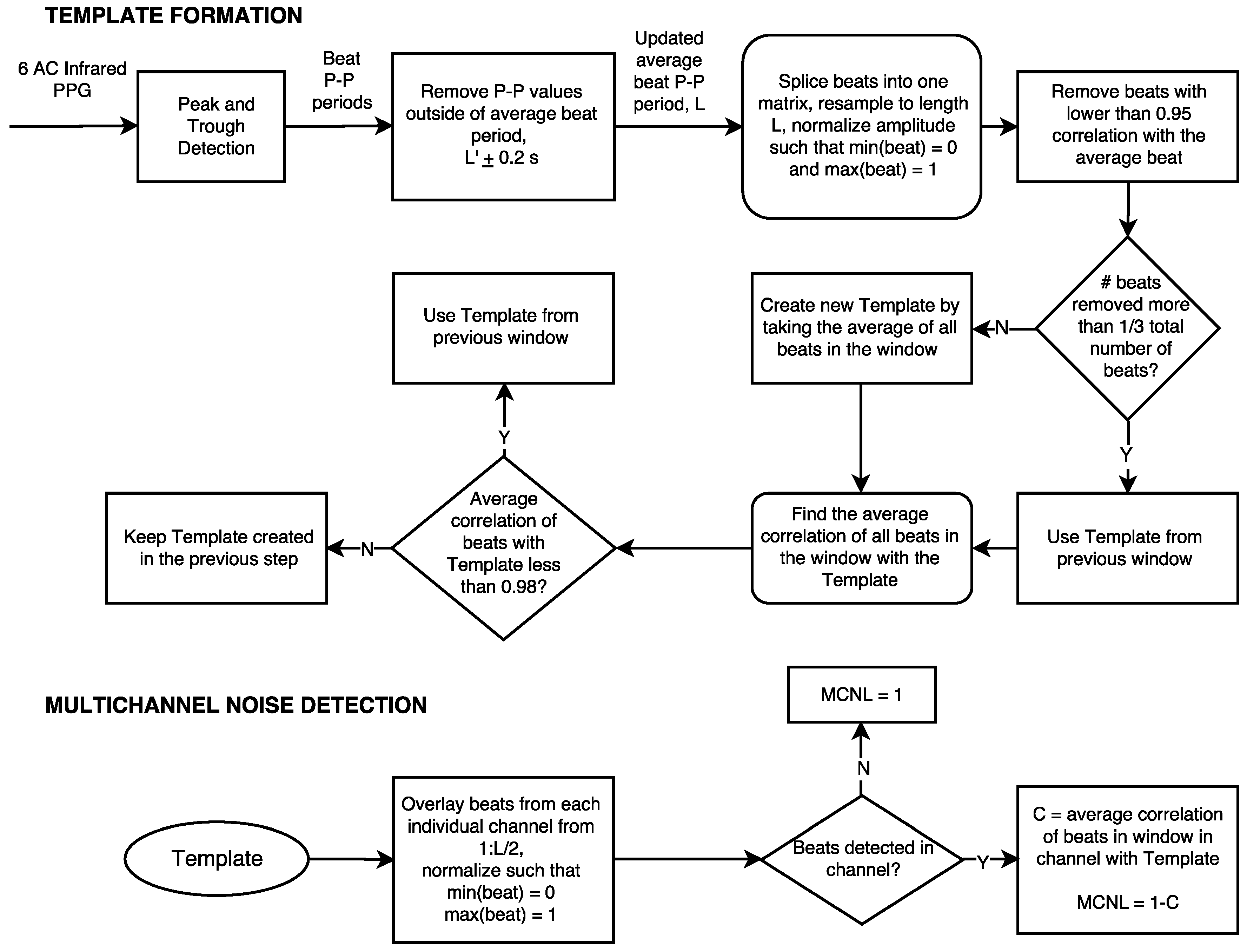
2.2. Methodology
2.2.1. Motion Quantification
Multichannel Template Matching
Accelerometer Amplitude
Motion Frequency Differences
2.2.2. Pulse Rate Performance Metrics
3. Results
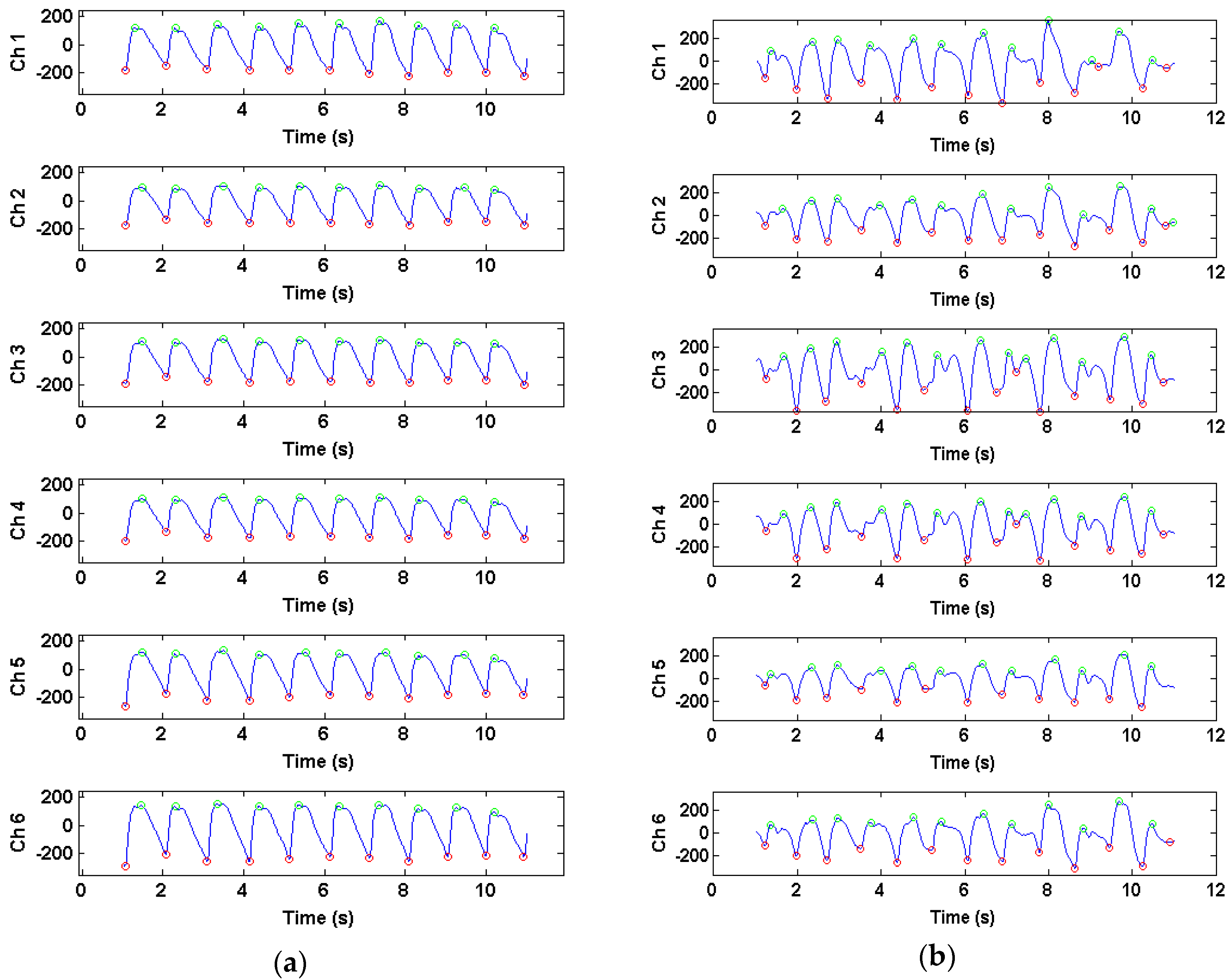
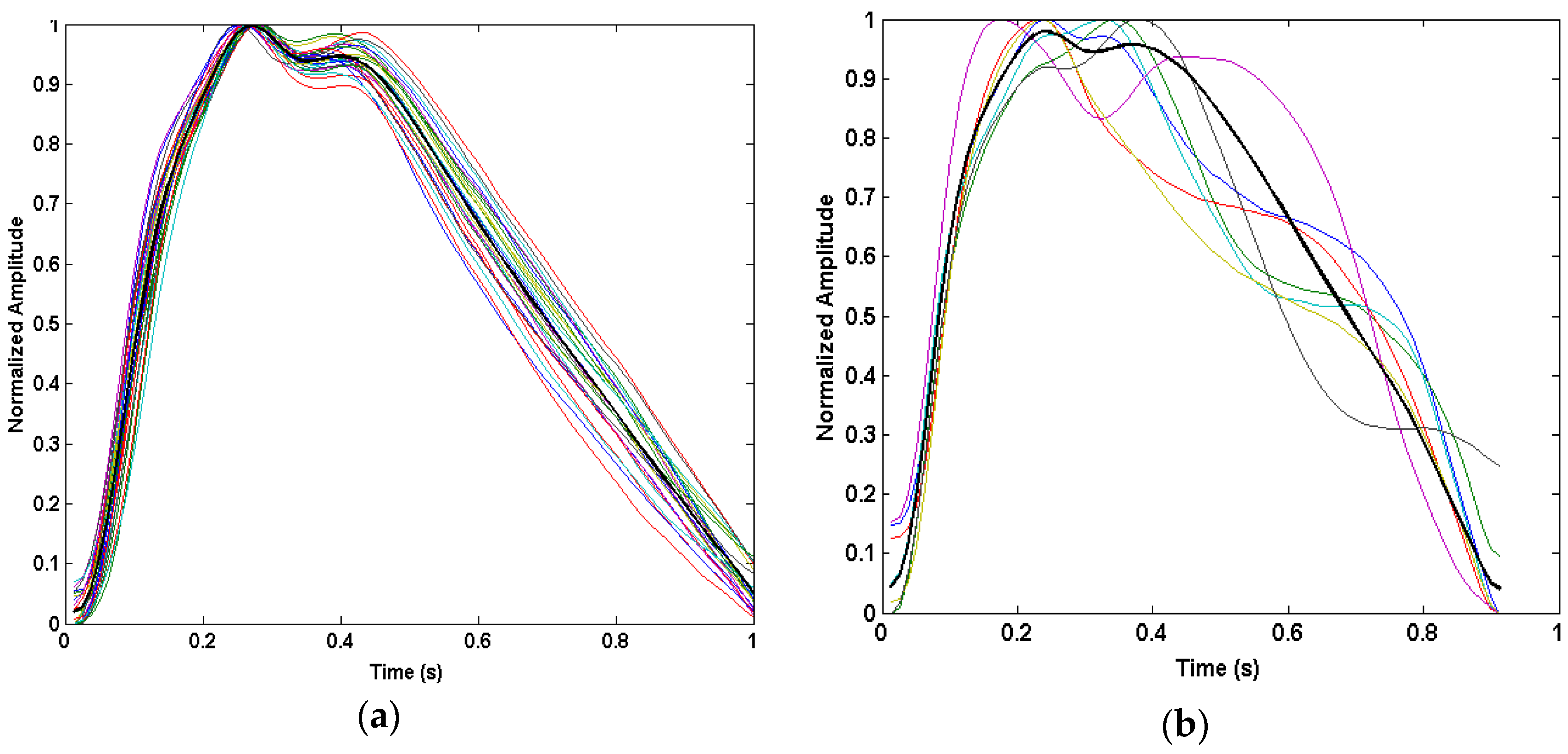
3.1. Time-Domain PPG Waveform Differences During Motion
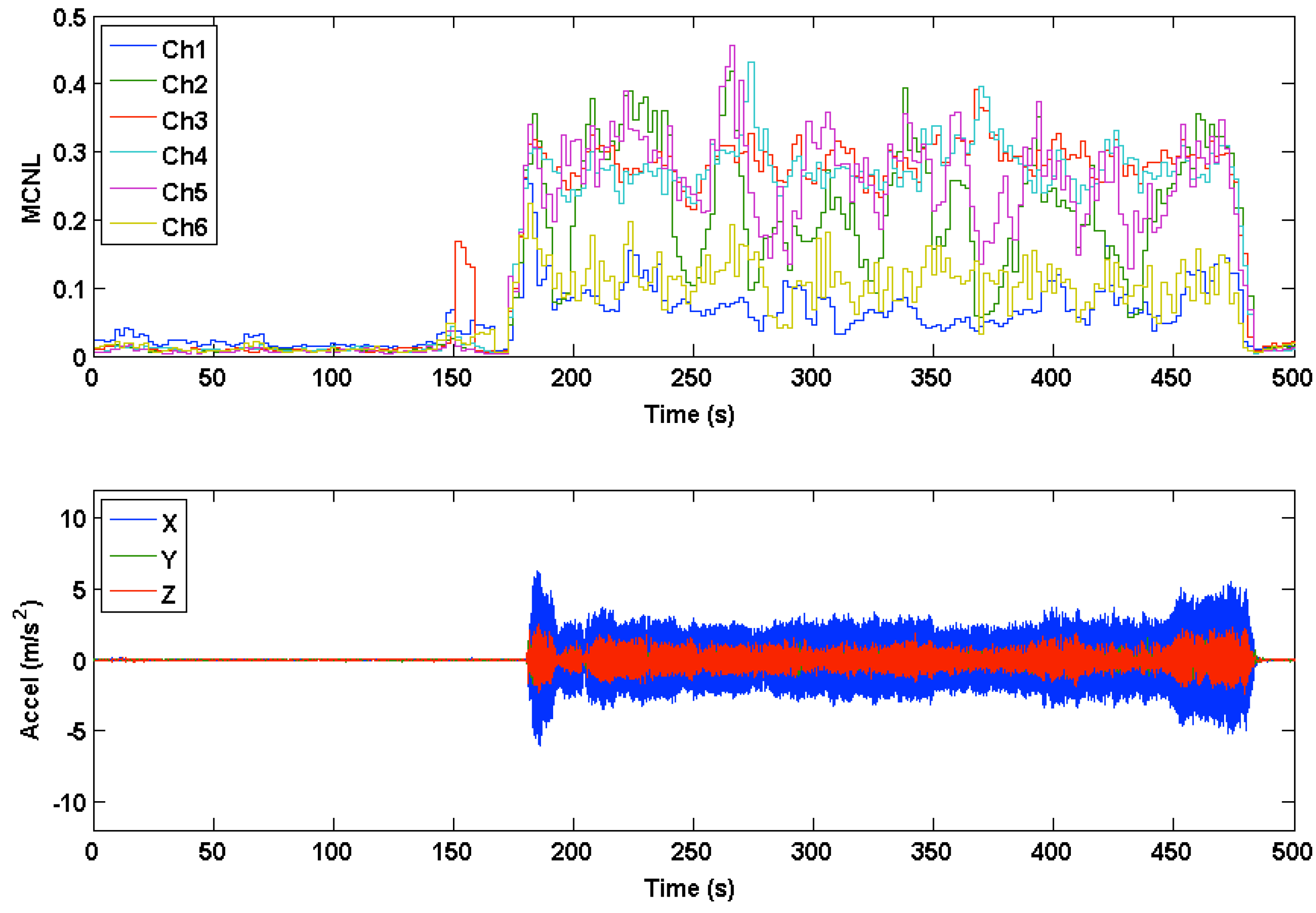
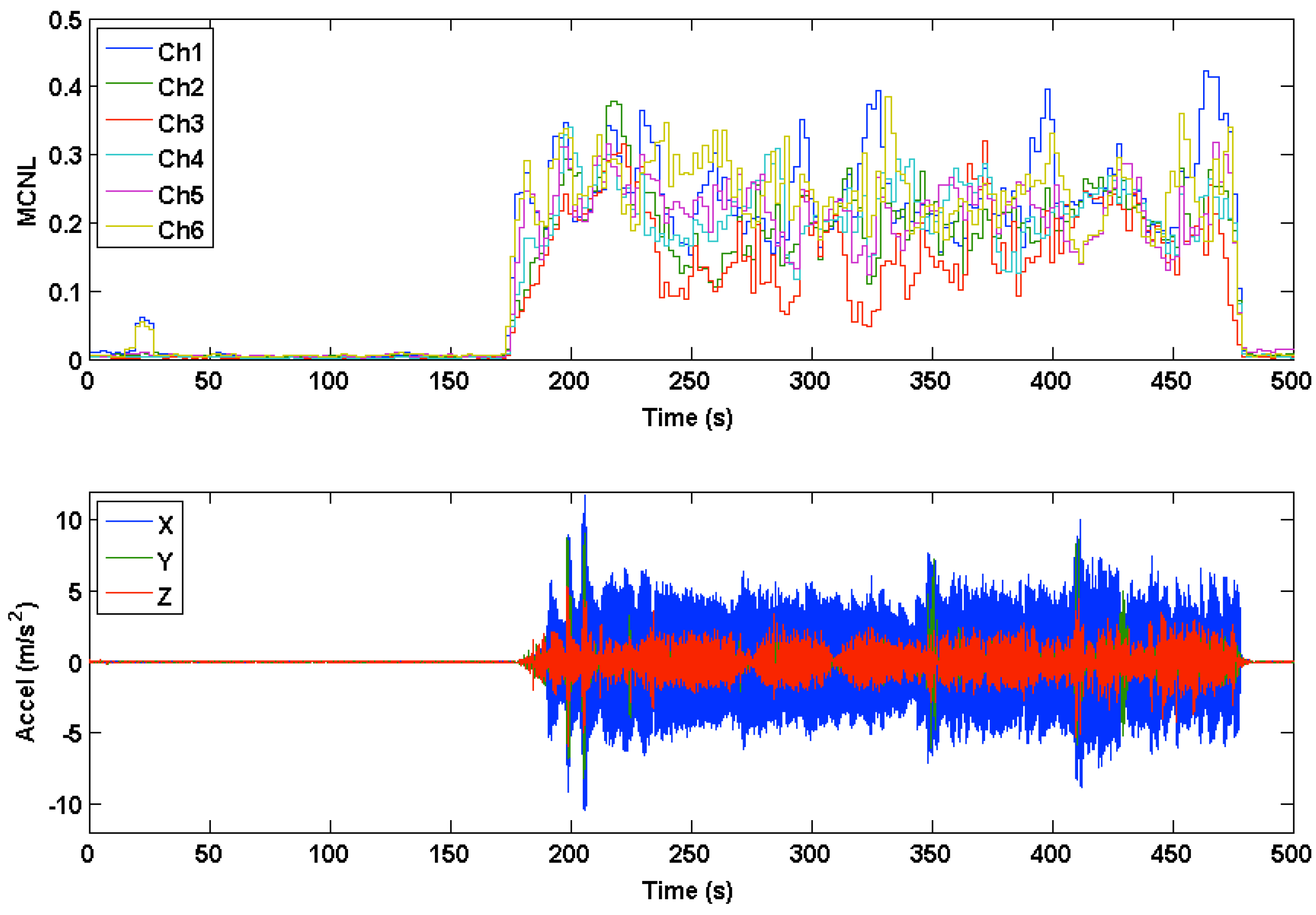
3.2. Accelerometer Range of Motion
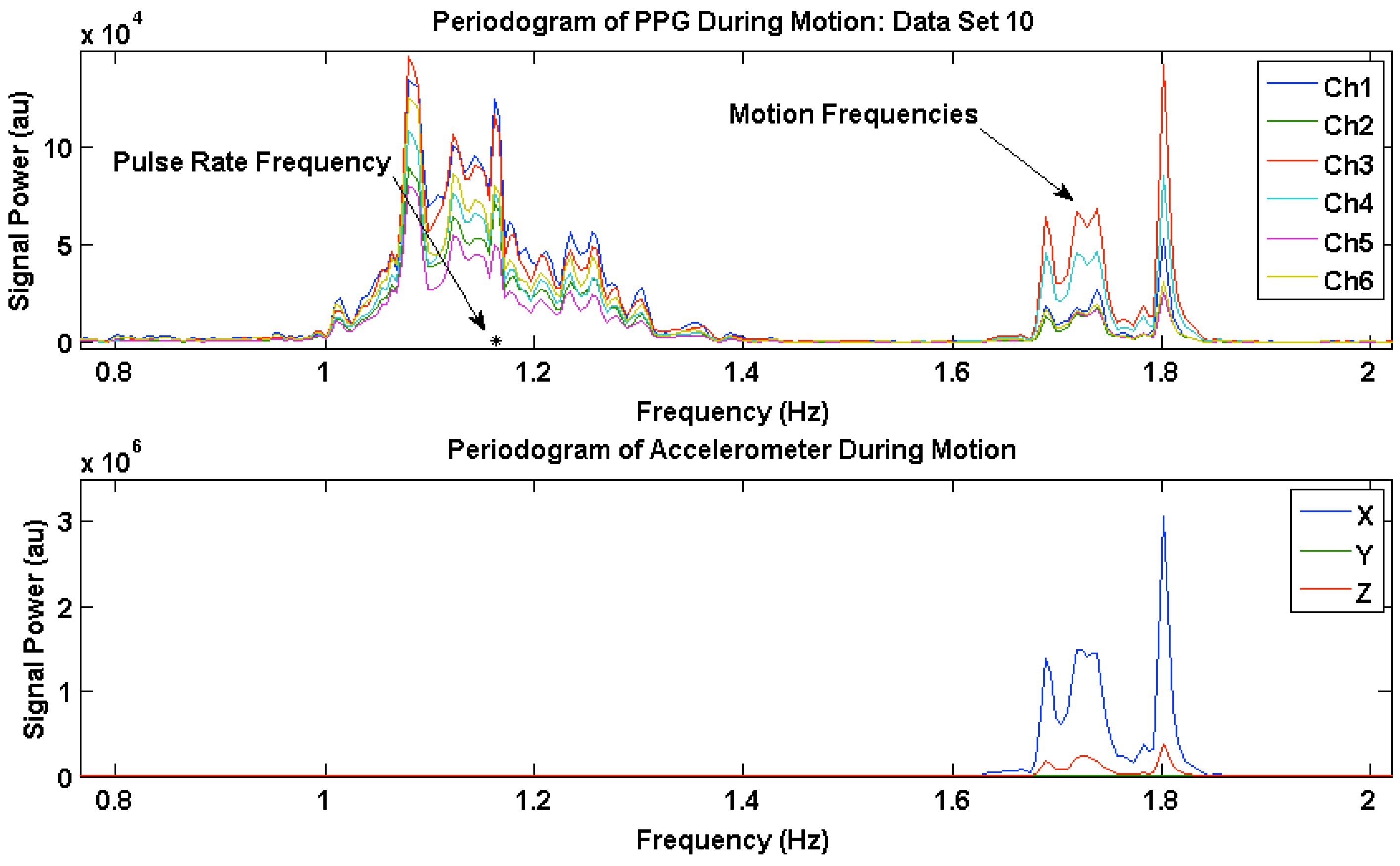
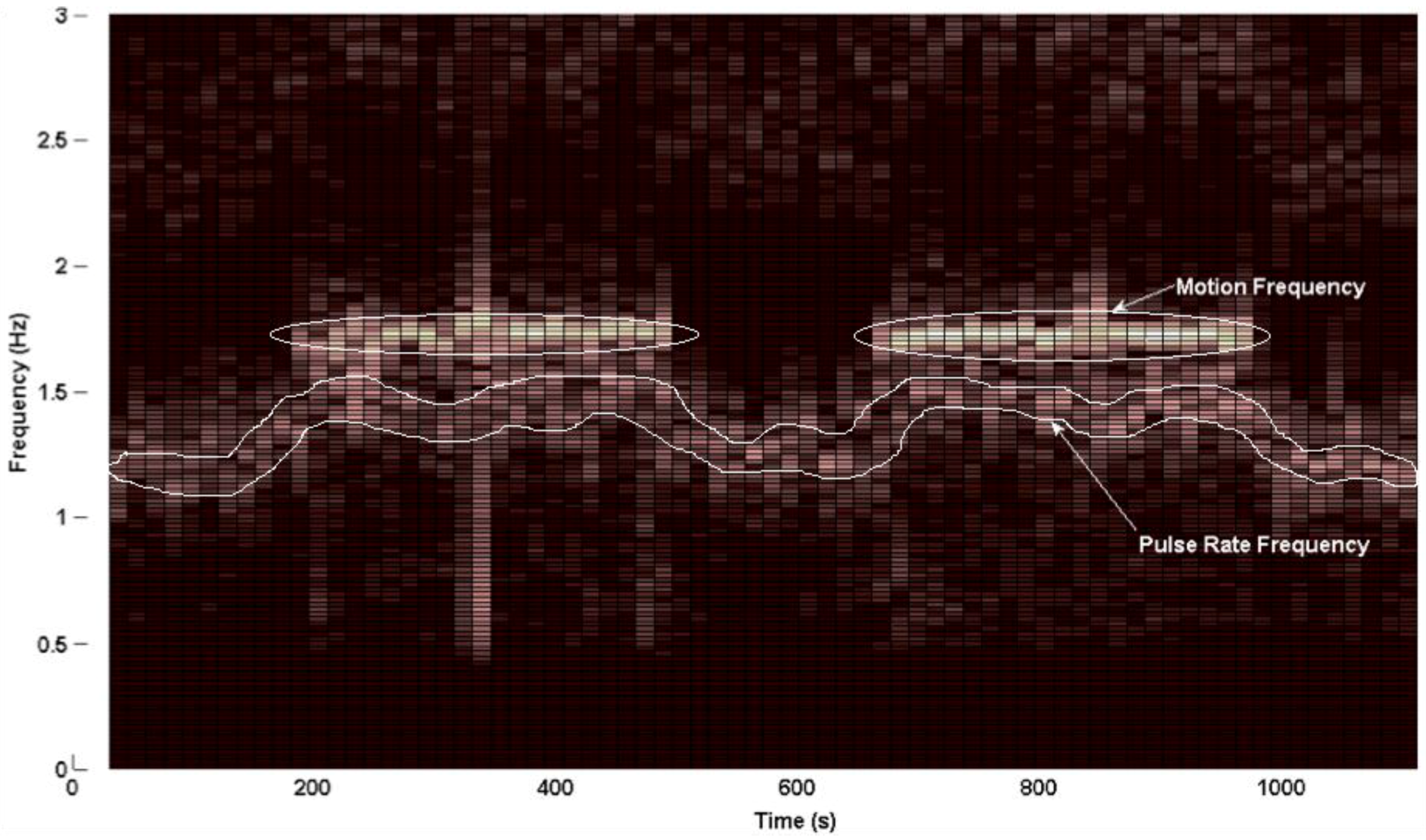
3.3. PPG Motion Frequency Differences
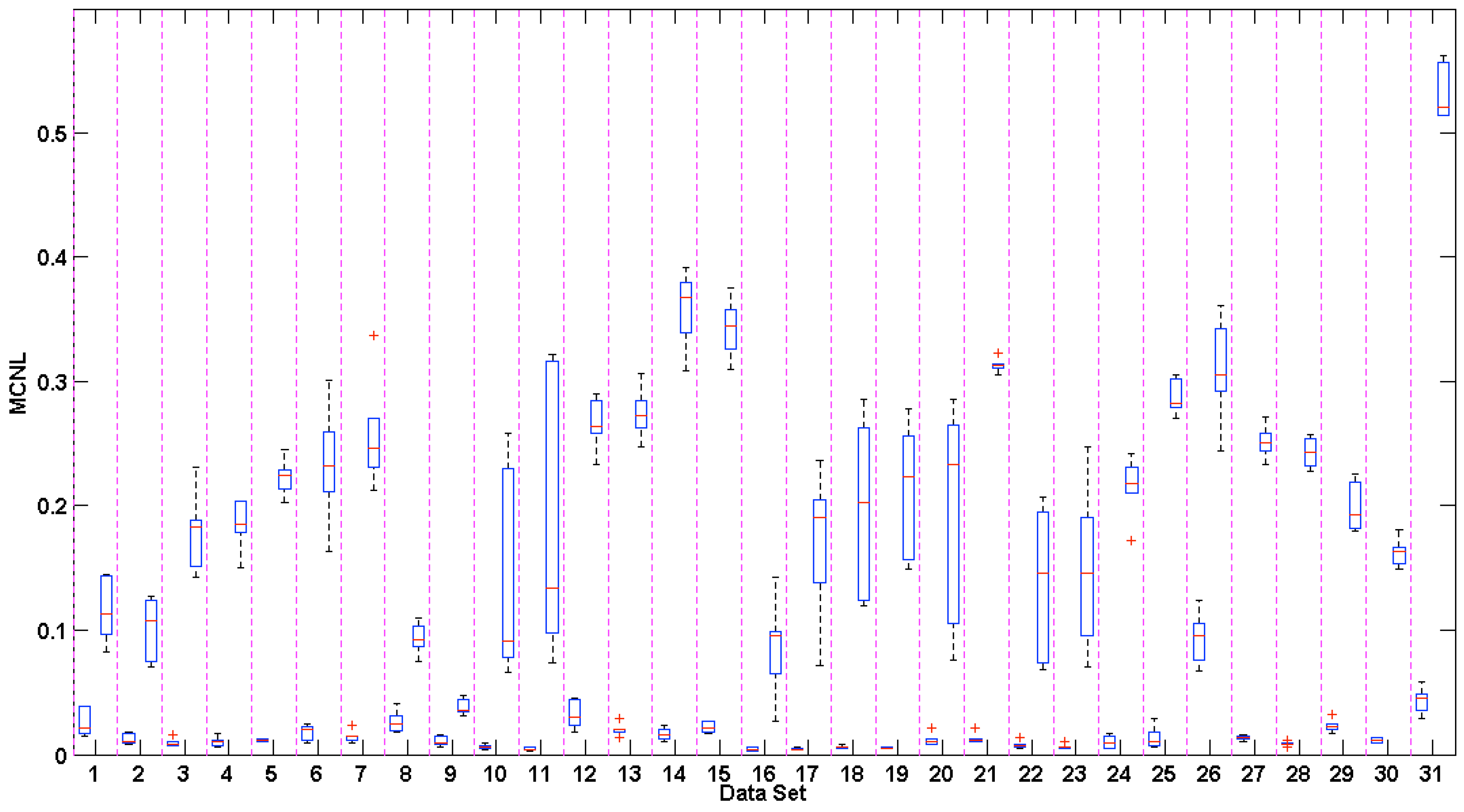
3.4. Multichannel Noise Level (MCNL)
3.5. PR Error During Motion
3.5.1. PR Performance Index (PI)
3.5.2. PR Accuracy
3.5.3. PR Precision
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SpO2 | Arterial Blood Oxygen Saturation |
| PR | Pulse rate |
| PPG | Photoplethysmogram |
| PD | Photodetector |
| LED | Light emitting diode |
| Acc | Accelerometer |
| MA | Motion Artifact |
| ANC | Adaptive Noise Cancellation |
| PCA | Principle Component Analysis |
| ICA | Independent Component Analysis |
| SSA | Singular Spectral Analysis |
| RMS | Root Mean Square |
| SNR | Signal-to-Noise Ratio |
| MPR | Mean Pulse rate |
| PSD | Power Spectral Density |
| MCNL | Multichannel Noise Level |
| IR | Infrared |
| RD | Red |
| PI | Performance Index |
| MC | Multichannel Estimate |
| MCP | Multichannel Photoplethysmogram |
References
- Mannheimer, P.D. The light-tissue interaction of pulse oximetry. Anesth. Analg. 2007, 105, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, Y. Pulse oximetry. In Wiley Encyclopedia of Biomedical Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Asada, H.H.; Hong-Hui, J.; Gibbs, P. Active noise cancellation using mems acceerometers for motion-tolerant wearable bio-sensors. In Engineering in Medicine and Biology Society; IEEE: San Francisco, CA, USA, 2004; Volume 1, pp. 2157–2160. [Google Scholar]
- Comtois, G.; Mendelson, Y.; Ramuka, P. A comparative evaluation of adaptive noise cancellation algorithms for minimizing motion artifacts in a forehead-mounted wearable pulse oximeter. In Engineering in Medicine and Biology Society; IEEE: Lyon, France, 2007; pp. 1528–1531. [Google Scholar]
- Foo, J.Y.; Wilson, S.J. A computational system to optimise noise rejection in photoplethysmography signals during motion or poor perfusion states. Med. Biol. Eng. Comput. 2006, 44, 140–145. [Google Scholar] [PubMed]
- Lee, B.; Han, J.; Baek, H.J.; Shin, J.H.; Park, K.S.; Yi, W.-J. Improved elimination of motion artifacts from a photoplethysmographic signal using a kalman smoother with simultaneous accelerometry. Physiol. Meas. 2010, 31, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.; Nourani, M.; OStadabbas, S.; Panahi, I. A motion-tolerant adaptive algorithm for wearable photoplethysmographic biosensors. IEEE J. Biomed. Health Inf. 2014, 18, 2168–2194. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.M.; Petterson, M.T.; Kopotic, R.J.; Barker, S.J. Masimo signal extraction pulse oximetry. J. Clin. Monit. Comput. 2000, 16, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.R.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. A novel approach for motion artifact reduction in ppg signals based on as-lms adaptive filter. IEEE Trans. Instrum. Meas. 2012, 61, 1445–1457. [Google Scholar] [CrossRef]
- Patterson, J.A.C.; Yang, G.-Z. Ratiometric artifact reduction in low power reflective photoplethysmography. IEEE Trans. Biomed. Circuits Syst. 2011, 5, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T.; Hara, S.; Okuhata, H.; Nakamura, H. Cancellation of motion artifact induced by exercise for ppg-based heart rate sensing. In Engineering in Medicine and Biology Society; IEEE: Chicago, IL, USA, 2014; pp. 3216–3219. [Google Scholar]
- Wijshoff, R.W.; Mischi, M.; Veen, J.; van der Lee, A.M.; Aarts, R.M. Reducing motion artifacts in photoplethysmograms by using relative sensor motion: Phantom study. J. Biomed. Opt. 2012, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, R.H.; Castellanos, M.S.; Rodriguez, J.F.; Caceres, J.L.H. Analysis of the photoplethysmographic signal by means of the decomposition in principle components. Physiol. Meas. 2002, 23, N17–N29. [Google Scholar]
- Kim, B.S.; Yoo, S.K. Motion artifact reduction in photoplethysmography using independent component analysis. IEEE Trans. Biomed. Eng. 2006, 53, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Natarajan, B.; Warren, S. Two-stage approach for detection and reduction of motion artifacts in photoplethysmographic data. IEEE Trans. Biomed. Eng. 2010, 57, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhang, Z.; Gou, X.; Liu, H.; Wang, W. Motion artifact removal from photoplethysmographic signals by combining temporally constrained independent component analysis and adaptive filter. Biomed. Eng. Online 2014, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, S.M.A.; Dao, D.K.; Chong, J.W.; McManus, D.; Darling, C.; Mendelson, Y.; Chon, K.H. Photoplethysmograph signal reconstruction based on a novel motion aritfact detection-reduction approach part II: Motion and noise artifact removal. Ann. Biomed. Eng. 2014, 42, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.W.; Dao, D.K.; Salehizadeh, S.M.; McManus, D.D.; Darling, C.E.; Chon, K.H.; Mendelson, Y. Photoplethysmograph signal reconstruction based on a novel hybrid motion artifact detection-reduction approach. Part I: Motion and noise artifact detection. Ann. Biomed. Eng. 2014, 42, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Warren, S. A wireless reflectance pulse oximeter with digital baseline control for unfiltered photoplethysmograms. IEEE Trans. Biomed. Circuits Syst. 2011, 6, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, W.; Kang, I.; Kim, Y.; Lee, G. Design of filter to reject motion artifact of pulse oximetry. Comput. Stand. Interfaces 2004, 26, 241–249. [Google Scholar] [CrossRef]
- Li, K.; Warren, S.; Natarajan, B. Onboard tagging for real-time quality assesment of photoplethysmograms acquired by a wireless reflectance pulse oximeter. IEEE Trans. Biomed. Circuits Syst. 2011, 6, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Lee, J.-W.; Jung, W.-G.; Lee, G.-K. The periodic moving average filter for removing motion artifacts from ppg signals. Int. J. Control Autom. Syst. 2007, 5, 701–706. [Google Scholar]
- Baca, A.; Biagetti, G.; Camilletti, M.; Crippa, P.; Falaschetti, L.; Orcioni, S.; Rossini, L.; Tonelli, D.; Turchetti, C. Carma: A robust motion artifact reduction algorithm for heart rate monitoring from ppg. In European Signal Processing Conference; IEEE: Nice, France, 2015. [Google Scholar]
- Salehizadeh, S.M.A.; Dao, D.; Bolkhovsky, J.; Cho, C.; Mendelson, Y.; Chon, K.H. A novel time-varying spectral filtering algorithm for reconstruction of motion artifact corrupted heart rate signals during intense physical activities using a wearable photoplethysmogram sensor. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Vetter, R.; Rossini, L.; Ridolfi, A.; Sola, J.; Chetelat, O.; Correvon, M.; Krauss, J. Frequency domain SpO2 estimation based on multichannel photoplethysmographic measurements at the sternum. In World Congress on Medical Physics and Biomedical Engineering; Olaf Dossel, W.C.S., Ed.; Spinger: Munich, Germany, 2009; Volume 25, pp. 326–329. [Google Scholar]
- Wang, L.; Lo, B.P.L.; Yang, G.Z. Multichannel reflective ppg earpiece sensor with passive motion cancellation. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Petterson, M.T.; Begnoche, V.L.; Graybeal, J.M. The effect of motion on pulse oximetry and its clinical significance. Anesth. Analg. 2007, 105, S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.K.; Mendelson, Y.; Chon, K.H. Multi-channel pulse oximetry for wearable physiological monitoring. In IEEE International Conference on Body Sensor Networks (BSN); IEEE: Cambridge, MA, USA, 2013; pp. 1–6. [Google Scholar]
- Mendelson, Y.; Ochs, B.D. Noninvasive pulse oximetry utilizing skin reflectance photoplethysmography. IEEE Trans. Biomed. Eng. 1988, 35, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Orphanidou, C.; Bonnici, T.; Charlton, P.; Clifton, D.; Vallance, D.; Tarassenko, L. Signal-quality indices for the electrocardiogram and photoplethysmogram: Derivation and applications to wireless monitoring. IEEE J. Biomed. Health Inf. 2015, 19, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Masimo. Masimo Radical-57: Operator’s Manual; Masimo Corporation: Irvine, CA, USA, 2012. [Google Scholar]






| Ch 1 | Ch 2 | Ch 3 | Ch 4 | Ch 5 | Ch 6 | Mean | Median | MC | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (all) | 57.8% | 56.2% | 53.3% | 54.9% | 56.4% | 57.9% | 48.8% | 55.6% | 66.4% |
| Mean (excluding 21 and 31) | * 61.7% | * 59.9% | * 56.7% | * 58.5% | * 60.1% | * 61.7% | * 52.0% | * 59.2% | 70.9% |
| Mean diff. | 9.24% | 11.02% | 14.15% | 12.35% | 10.78% | 9.15% | 18.85% | 11.66% | |
| Std Err diff. | 3.05% | 3.39% | 4.94% | 4.99% | 3.98% | 2.53% | 4.56% | 3.55% |
| Ch 1 | Ch 2 | Ch 3 | Ch 4 | Ch 5 | Ch 6 | Mean | Median | MC | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (all) | 9.7 | 9.5 | 10.2 | 9.6 | 9.4 | 9.6 | 9.3 | 9.0 | 7.7 |
| Mean (excluding 21 and 31) | * 8.1 | * 8.0 | * 8.8 | * 8.1 | * 7.9 | * 8.1 | * 7.8 | * 7.5 | 6.1 |
| Mean diff. | 2.0 | 1.9 | 2.7 | 2.0 | 1.8 | 2.0 | 1.7 | 1.3 | |
| Std Err diff. | 1.0 | 0.7 | 1.1 | 1.1 | 0.6 | 0.9 | 0.5 | 0.5 |
| Ch 1 | Ch 2 | Ch 3 | Ch 4 | Ch 5 | Ch 6 | Mean | Median | MC | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (all) | 5.7 | 6.4 | 7.3 | 6.6 | 6.5 | 5.7 | 5.4 | 5.8 | 5.7 |
| Mean (excluding 21 and 31) | 5.6 | 6.3 | * 7.3 | 6.6 | 6.5 | 5.7 | 5.3 | 5.8 | 5.6 |
| Mean diff. | 0.0 | 0.7 | 1.7 | 0.9 | 0.9 | 0.1 | −0.3 | 0.2 | |
| Std Err diff. | 0.4 | 0.5 | 0.8 | 0.8 | 0.5 | 0.4 | 0.5 | 0.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warren, K.M.; Harvey, J.R.; Chon, K.H.; Mendelson, Y. Improving Pulse Rate Measurements during Random Motion Using a Wearable Multichannel Reflectance Photoplethysmograph. Sensors 2016, 16, 342. https://doi.org/10.3390/s16030342
Warren KM, Harvey JR, Chon KH, Mendelson Y. Improving Pulse Rate Measurements during Random Motion Using a Wearable Multichannel Reflectance Photoplethysmograph. Sensors. 2016; 16(3):342. https://doi.org/10.3390/s16030342
Chicago/Turabian StyleWarren, Kristen M., Joshua R. Harvey, Ki H. Chon, and Yitzhak Mendelson. 2016. "Improving Pulse Rate Measurements during Random Motion Using a Wearable Multichannel Reflectance Photoplethysmograph" Sensors 16, no. 3: 342. https://doi.org/10.3390/s16030342






