Modeling the Insertion Mechanics of Flexible Neural Probes Coated with Sacrificial Polymers for Optimizing Probe Design
Abstract
:1. Introduction
2. Methods
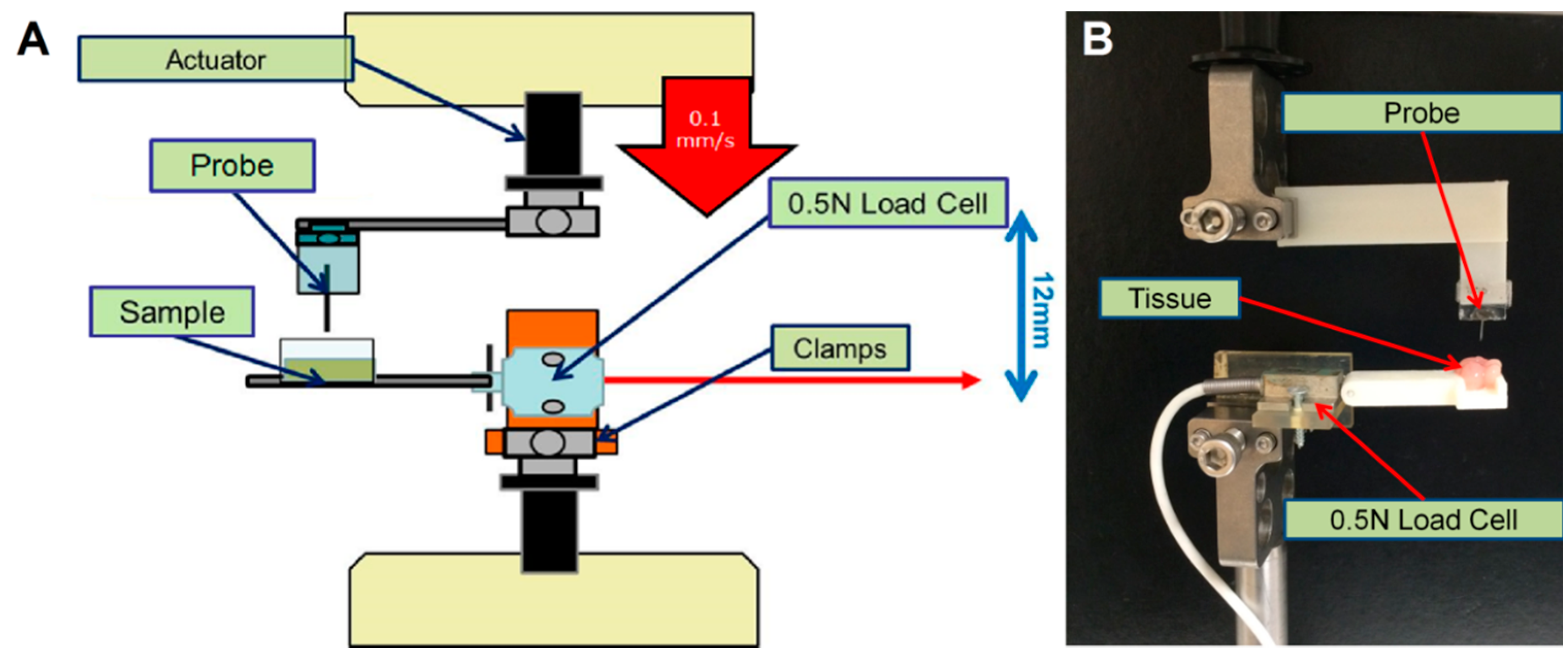
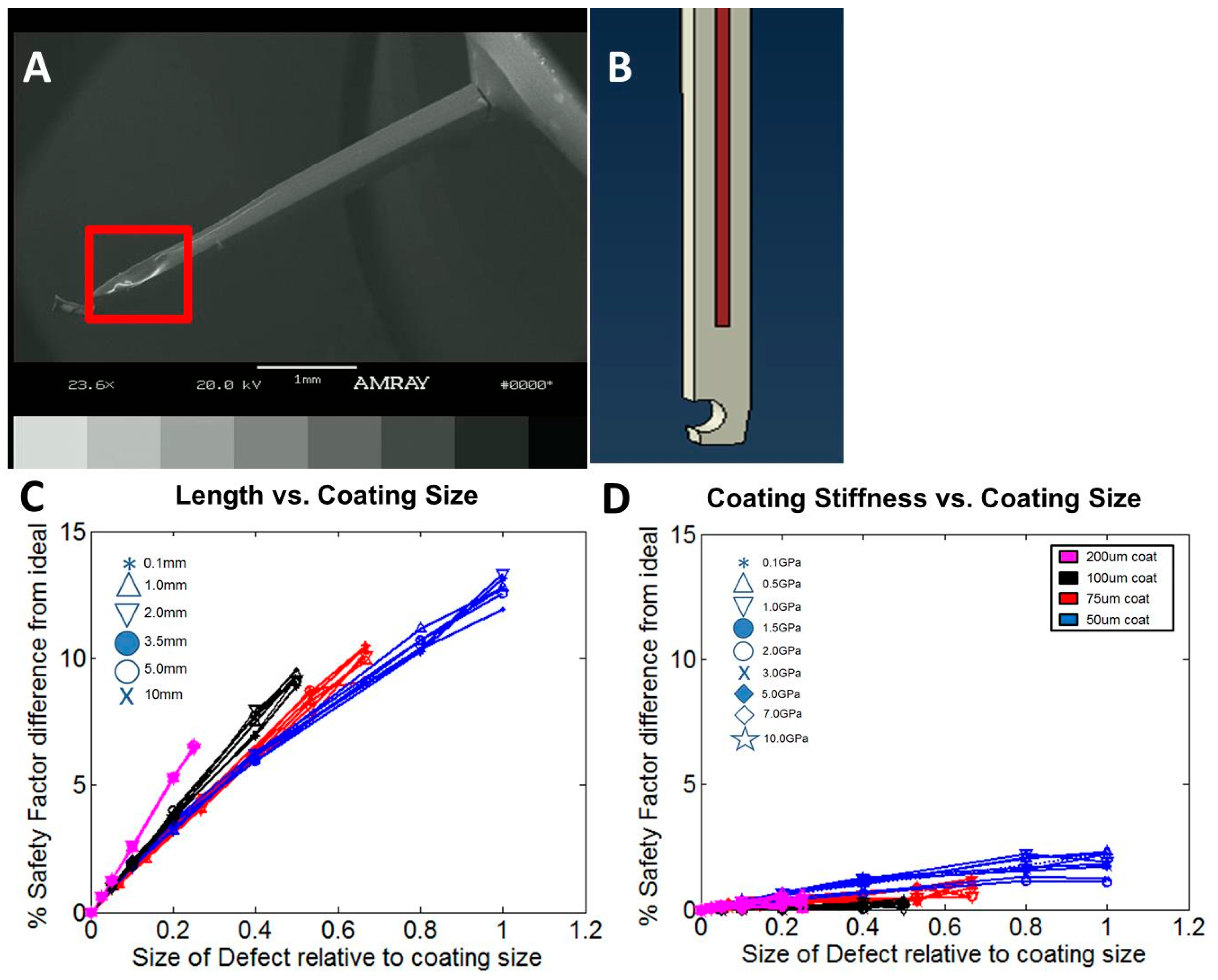
2.1. Buckling and Insertion Tests
2.2. Finite Element Analysis
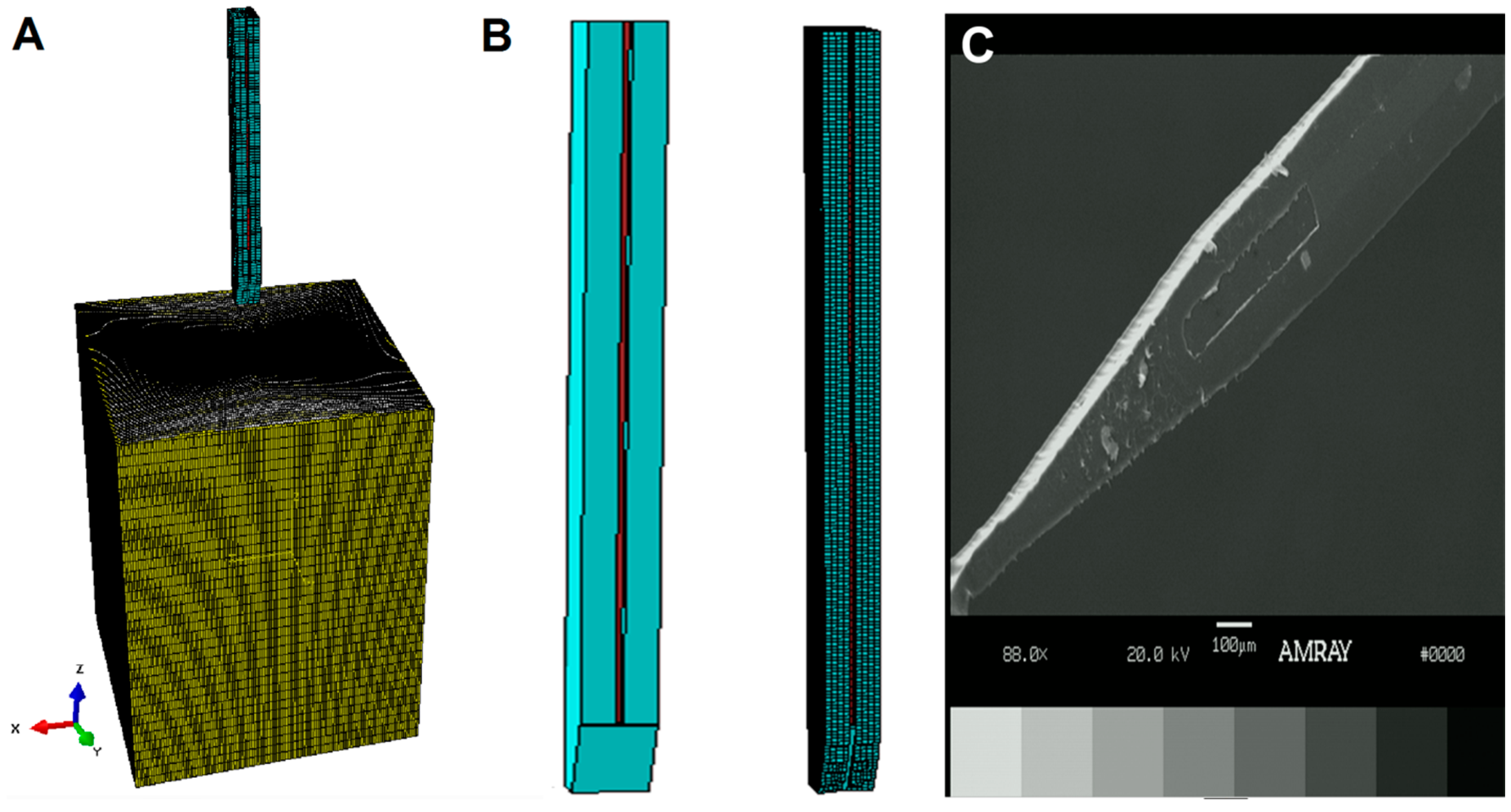
2.2.1. Probe and Coating
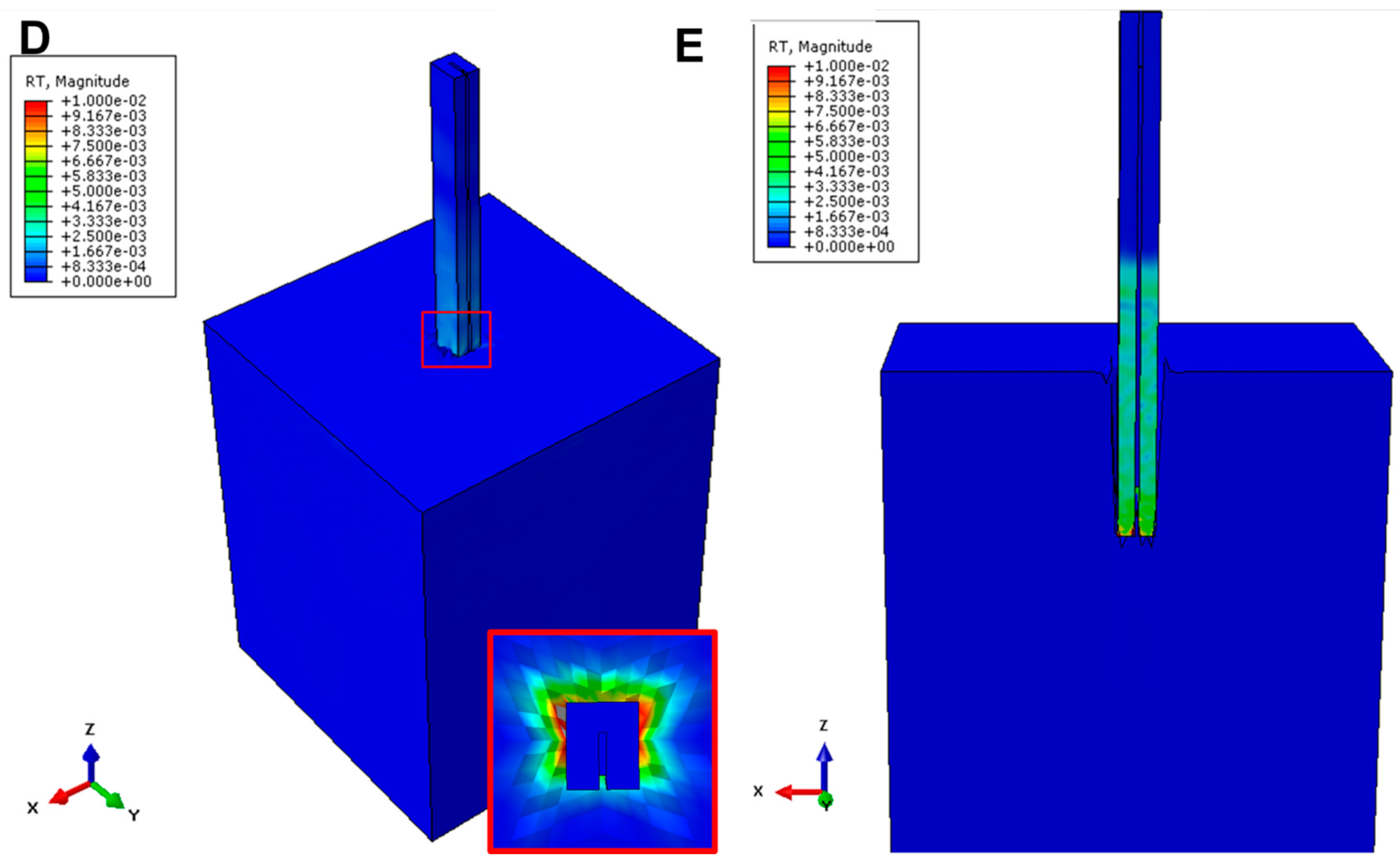
2.2.2. Buckling Model
2.2.3. Insertion Model
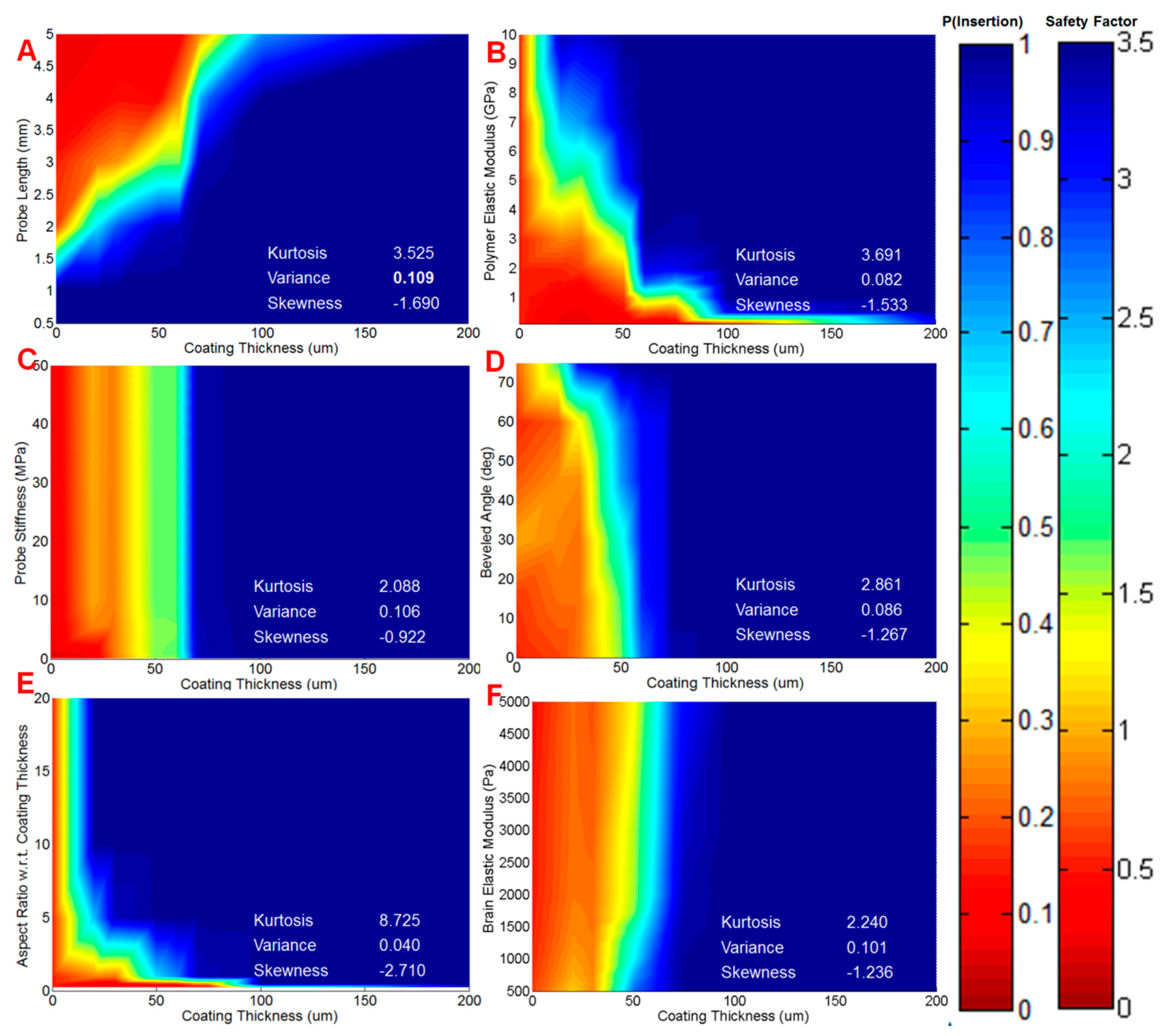
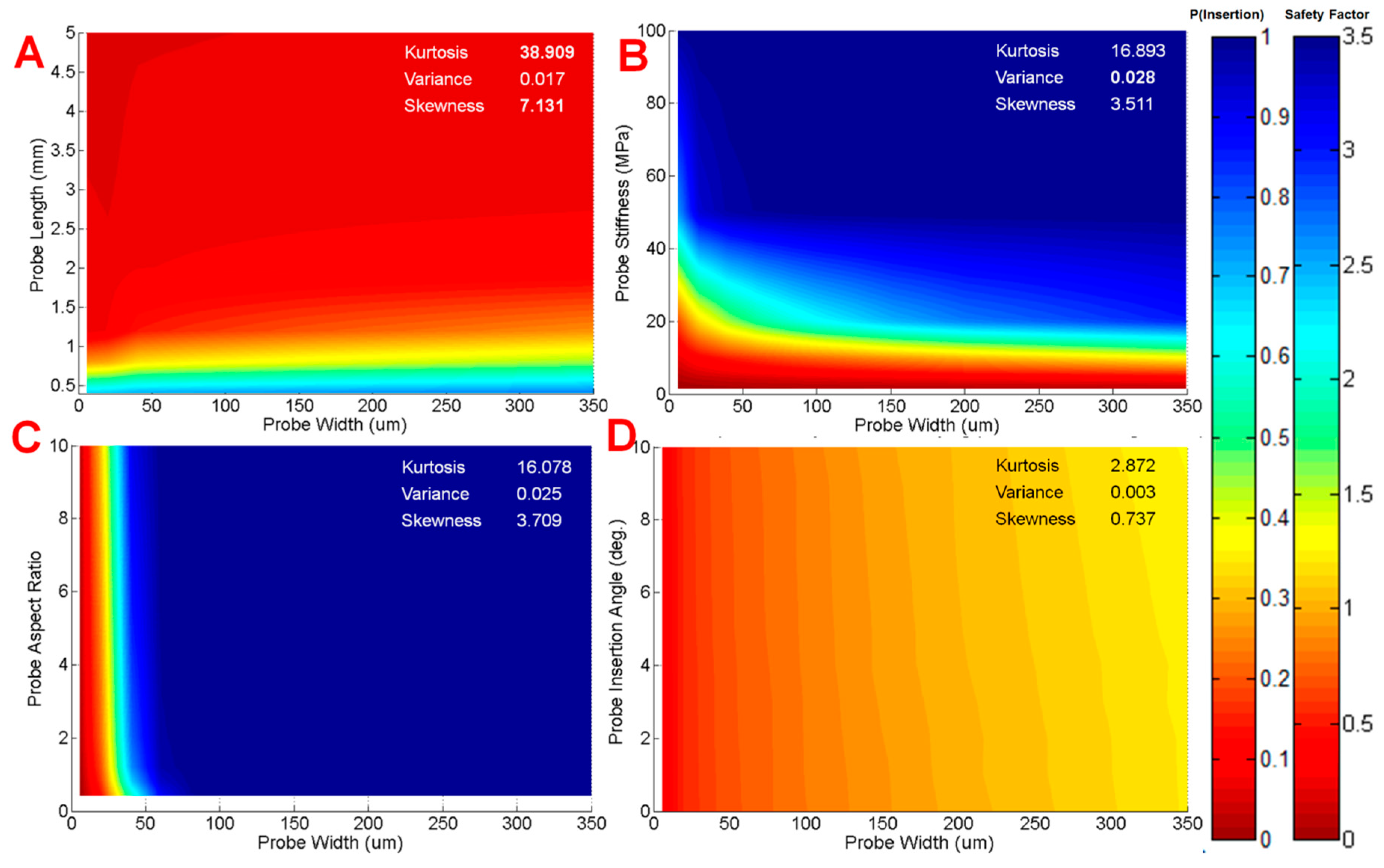
2.2.4. Sensitivity Analysis
2.3. Data Interpretation
3. Results
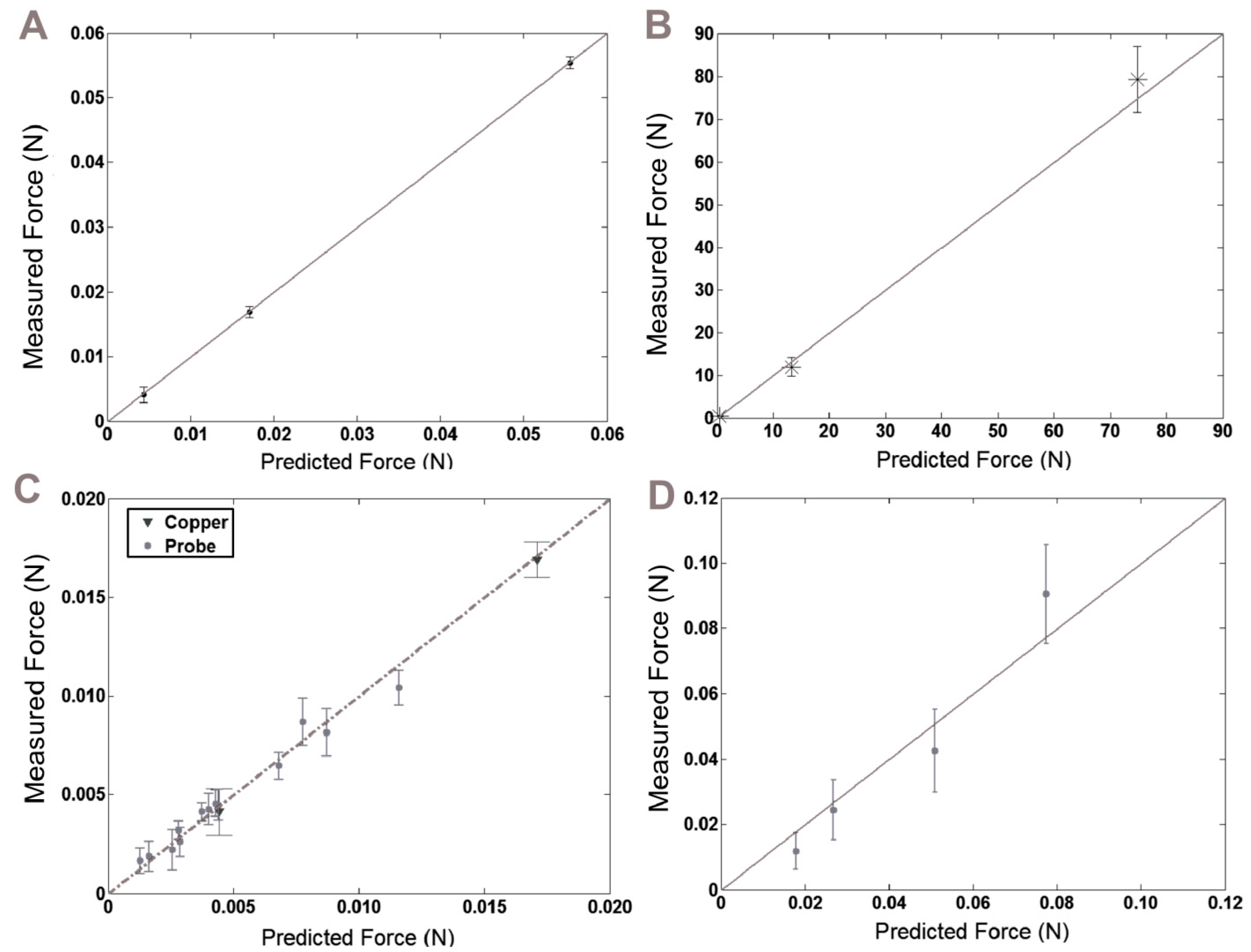
3.1. Model and Fabrication Comparisons
3.2. Insertion and Buckling Tests
3.3. Model Results
3.4. Model Predictions for Probe Performance
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mak, J.; Wolpaw, J. Clinical applications of brain-computer interfaces: Current state and future prospects. IEEE Rev. Biomed. Eng. 2009, 2, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces, a review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Fazel-Rezai, R.; Asadpour, V. A review of hybrid brain-computer interface systems. Adv. Hum. Comput. Interact. 2013, 2013. [Google Scholar] [CrossRef]
- Maynard, E.M.; Nordhausen, C.T.; Normann, R.A. The utah intracortical electrode array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 228–239. [Google Scholar] [CrossRef]
- Cheung, K.C. Implantable microscale neural interfaces. Biomed. Microdevices 2007, 9, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Hoogerwerf, A.C.; Wise, K.D. A three-dimensional microelectrode array for chronic neural recording. IEEE Trans. Biomed. Eng. 1994, 41, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Bareket-Keren, L.; Hanein, Y. Carbon nanotube-based multi electrode arrays for neuronal interfacing: Progress and prospects. Front. Neural Circuits 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.N.; Shain, W.; Szarowski, D.H.; Andersen, M.; Martins, S.; Isaacson, M.; Craighead, H. Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 1999, 156, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Aregueta-Robles, U.A.; Woolley, A.J.; Poole-Warren, L.A.; Lovell, N.H.; Green, R.A. Organic electrode coatings for next-generation neural interfaces. Front. Neuroeng. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Rousche, P.J.; Normann, R.A. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J. Neurosci. Methods 1998, 82, 1–15. [Google Scholar] [CrossRef]
- Hamzavi, N.; Tsang, W.M.; Shim, V.P.W. Nonlinear elastic brain tissue model for neural probe-tissue mechanical interaction. In Proceedings of the 6th International IEEE/EMBS Conference on Neural Engineering (NER), San Diego, CA, USA, 6–8 November 2013; pp. 1119–1122.
- Stice, P.; Muthuswamy, J. Assessment of gliosis around moveable implants in the brain. J. Neural Eng. 2009, 6, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Subbaroyan, J.; Martin, D.C.; Kipke, D.R. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J. Neural Eng. 2005, 2, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.; Andersen, M.; Retterer, S. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef]
- Zhu, R.; Huang, G.L.; Yoon, H.; Smith, C.S.; Varadan, V.K. Biomechanical Strain Analysis at the Interface of Brain and Nanowire Electrodes on a Neural Probe. J. Nanotechnol. Eng. Med. 2011, 2, 31–41. [Google Scholar] [CrossRef]
- Lind, G.; Linsmeier, C.E.; Schouenborg, J. The density difference between tissue and neural probes is a key factor for glial scarring. Sci. Rep. 2013, 3, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Lind, G.; Linsmeier, C.E.; Thelin, J.; Schouenborg, J. Gelatine-embedded electrodes—A novel biocompatible vehicle allowing implantation of highly flexible microelectrodes. J. Neural Eng. 2010, 7, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Felix, S.H.; Shah, K.G.; Tolosa, V.M.; Sheth, H.J.; Tooker, A.C.; Delima, T.L.; Jadhav, S.P.; Frank, L.M.; Pannu, S.S. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable adhesive. J. Vis. Exp. 2013, 79, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007, 404, 15–27. [Google Scholar] [CrossRef] [PubMed]
- CFoley, P.; Nishimura, N.; Neeves, K.B.; Schaffer, C.B.; Olbricht, W.L. Flexible microfluidic devices supported by biodegradable insertion scaffolds for convection-enhanced neural drug delivery. Biomed. Microdevices 2009, 11, 915–924. [Google Scholar]
- Lewitus, D.Y.; Smith, K.L.; Shain, W.; Bolikal, D.; Kohn, J. The fate of ultrafast degrading polymeric implants in the brain. Biomaterials 2011, 32, 5543–5550. [Google Scholar] [CrossRef] [PubMed]
- Lewitus, D.; Smith, K.L.; Shain, W.; Kohn, J. Ultrafast resorbing polymers for use as carriers for cortical neural probes. Acta Biomater. 2011, 7, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, N.; Patel, R.V. Deflection of a flexible needle during insertion into soft tissue. In Proceedings of the 27th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Shanghai, China, 17–18 January 2006; pp. 3858–3861.
- Okamura, A.M.; Simone, C.; O'Leary, M.D. Force modeling for needle insertion into soft tissue. IEEE Trans. Biomed. Eng. 2004, 51, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, N.; Patel, R.; Moallem, M. Needle insertion into soft tissue: A survey. Med. Eng. Phys. 2007, 29, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Nguyen, J.K.; Capadona, J.R.; Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J. Neural Eng. 2015, 12, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Polanco, M.; Yoon, H.; Bawab, S. Micromotion-induced dynamic effects from a neural probe and brain tissue interface. J. Micro/Nanolithogr. Microfabr. Microsyst. 2014, 13, 23–29. [Google Scholar] [CrossRef]
- Lo, M.; Wang, S.; Singh, S.; Damodaran, V.B.; Kaplan, H.M.; Kohn, J.; Shreiber, D.I.; Zahn, J.D. Coating flexible probes with an ultra fast degrading polymer to aid in tissue insertion. Biomed. Microdevices 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Salcman, M. Mechanical factors in the design of chronic recording intracortical microelectrodes. IEEE Trans. Biomed. Eng. 1973, 20, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gillies, G.; Broaddus, W. A realistic brain tissue phantom for intraparenchymal infusion studies. J. Neurosurg. 2004, 101, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Prange, M.T.; Margulies, S.S. Regional, directional, and age-dependent properties of the brain undergoing large deformation. J. Biomech. Eng. 2002, 124, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Spratley, J.P.F.; Ward, M.C.L.; Hall, P.S. Bending characteristics of SU-8. Micro Nano Lett. 2007, 2, 20–23. [Google Scholar] [CrossRef]
- Hopcroft, M.; Kramer, T.; Kim, G.; Takashima, K.; Higo, Y.; Moore, D.; Brugger, J. Micromechanical testing of SU-8 cantilevers. Fatigue Fract. Eng. Mater. Struct. 2003, 28, 10–12. [Google Scholar] [CrossRef]
- Rizzi, F.; Qualtieri, A.; Chambers, L.D.; Megill, W.M.; de Vittorio, M. Parylene conformal coating encapsulation as a method for advanced tuning of mechanical properties of an artificial hair cell. Soft Matter 2013, 9, 2584–2588. [Google Scholar] [CrossRef]
- Chung, C.; Allen, M. Uncrosslinked SU-8 as a sacrificial material. J. Micromech. Microeng. 2005, 15, N1–N5. [Google Scholar] [CrossRef]
- Sim, W.; Kim, B.; Choi, B.; Park, J.O. Theoretical and experimental studies on the parylene diaphragms for microdevices. Microsyst. Technol. 2005, 11, 11–15. [Google Scholar] [CrossRef]
- Kalpakjian, S.; Schmid, S.R. Manufacturing, Engineering and Technology, 5th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Normand, V.; Lootens, D.; Amici, E. New insight into agarose gel mechanical properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, T.; Mota, A.; Fraternali, F.; Ortiz, M. Biomechanics of traumatic brain injury. Comput. Methods Appl. Mech. Eng. 2008, 197, 4692–4701. [Google Scholar] [CrossRef]
- Persson, C.; Evans, S.; Marsh, R.; Summers, J.L.; Hall, R.M. Poisson’s ratio and strain rate dependency of the constitutive behavior of spinal dura mater. Ann. Biomed. Eng. 2010, 38, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, K.H.; King, A.I. A proposed injury threshold for mild traumatic brain injury. J. Biomech. Eng. 2004, 126, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Cater, H.L.; Sundstrom, L.E.; Morrison, B. Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. J. Biomech. 2006, 39, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, C.S.; Oh, S.J.; Al-Kofahi, Y.A.; Lim, Y.J.; Smith, K.L.; Turner, J.N.; De, S.; Roysam, B.; Shain, W.; Kim, S.J. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J. Neural Eng. 2006, 3, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Dong, R.G.; Schopper, A.W. Analysis of effects of friction on the deformation behavior of soft tissues in unconfined compression tests. J. Biomech. 2004, 37, 147–155. [Google Scholar] [CrossRef]
- Bain, A.; Meaney, D. Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J. Biomech. Eng. 2000, 122, 615–622. [Google Scholar] [CrossRef] [PubMed]
- LaPlaca, M.C.; Simon, C.M.; Prado, G.R.; Cullen, D.K. CNS injury biomechanics and experimental models. Prog. Brain Res. 2007, 161, 13–26. [Google Scholar] [PubMed]


| Cohort | Probe Material | Probe Dimensions (μm) | Coating Dimensions (μm) | Total | Pass | Fail | % Success Rate |
|---|---|---|---|---|---|---|---|
| 1 | SU-8 | 320 × 5 × 3500 | - | 8 | 8 | 0 | 100.0 |
| 2 | SU-8 | 320 × 20 × 3500 | - | 8 | 8 | 0 | 100.0 |
| 3 | SU-8 | 320 × 20 × 3500 | 50 × 50 × 4000 | 8 | 8 | 0 | 100.0 |
| 4 | SU-8 | 320 × 20 × 3500 | 75 × 100 × 4000 | 8 | 8 | 0 | 100.0 |
| 5 | SU-8 | 320 × 20 × 3500 | 100 × 100 × 4000 | 8 | 8 | 0 | 100.0 |
| 6 | Parylene C | 20 × 5 × 3500 | - | 8 | 0 | 8 | 0.0 |
| 7 | Parylene C | 100 × 5 × 3500 | - | 8 | 0 | 8 | 0.0 |
| 8 | Parylene C | 320 × 5 × 3500 | - | 8 | 0 | 8 | 0.0 |
| 9 | Parylene C | 320 × 20 × 3500 | - | 8 | 0 | 8 | 0.0 |
| 10 | Parylene C | 20 × 5 × 3500 | 50 × 50 × 4000 | 8 | 4 | 4 | 50.0 |
| 11 | Parylene C | 20 × 5 × 3500 | 50 × 100 × 4000 | 8 | 7 | 1 | 87.5 |
| 12 | Parylene C | 20 × 5 × 3500 | 75 × 100 × 4000 | 8 | 8 | 0 | 100.0 |
| 13 | Parylene C | 20 × 5 × 3500 | 100 × 100 × 4000 | 8 | 8 | 0 | 100.0 |
| 14 | Parylene C | 20 × 5 × 3500 | 250 × 100 × 4000 | 8 | 8 | 0 | 100.0 |
| 15 | Parylene C | 20 × 5 × 3500 | 350 × 100 × 4000 | 8 | 8 | 0 | 100.0 |
| Cohort | Probe Material | Probe Dimensions (μm) | Coating Dimensions (μm) | Total | Pass | Fail | % Success Rate |
|---|---|---|---|---|---|---|---|
| 1 | SU-8 | 40 × 10 × 3500 | - | 11 | 6 | 5 | 54.5 |
| 2 | SU-8 | 40 × 10 × 3500 | 100 × 100 × 4000 | 12 | 12 | 0 | 100.0 |
| 3 | Parylene C | 40 × 2.5 × 3500 | - | 8 | 0 | 8 | 0.0 |
| 4 | Parylene C | 40 × 2.5 × 3500 | 50 × 100 × 4000 | 8 | 5 | 3 | 62.5 |
| 5 | Parylene C | 40 × 2.5 × 3500 | 75 × 100 × 4000 | 8 | 7 | 1 | 87.5 |
| 6 | Parylene C | 40 × 2.5 × 3500 | 100 × 100 × 4000 | 12 | 12 | 0 | 100.0 |
| 7 | Polymer Shank | - | 100 × 100 × 4000 | 8 | 8 | 0 | 100.0 |
| Parameter | Range Modeled | Coefficient Value | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| Coating Thickness | 0–200 µm | 15,513 µm−1 | [11928, 19113] | <0.0001 |
| Probe Length | 0.01–10 mm | −33,037 mm−1 | [−41029, −26755] | <0.0001 |
| Coating Stiffness | 0.001–100 GPa | 1857 (GPa)−1 | [1322, 2274] | <0.0001 |
| Coating Aspect Ratio | 0.1–10 | 5480 | [3922, 7034] | <0.0001 |
| Probe Width | 5–350 µm | 62 µm−1 | [54, 69] | <0.0001 |
| Beveled Angle | 0–75° | 1398 | [515, 2236] | 0.0043 |
| Probe Stiffness | 1–5000 MPa | 434 (MPa)−1 | [131, 768] | 0.0056 |
| Brain Stiffness | 0.5–5 kPa | −25 (kPa)−1 | [−39, −11] | 0.0114 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Lo, M.-C.; Damodaran, V.B.; Kaplan, H.M.; Kohn, J.; Zahn, J.D.; Shreiber, D.I. Modeling the Insertion Mechanics of Flexible Neural Probes Coated with Sacrificial Polymers for Optimizing Probe Design. Sensors 2016, 16, 330. https://doi.org/10.3390/s16030330
Singh S, Lo M-C, Damodaran VB, Kaplan HM, Kohn J, Zahn JD, Shreiber DI. Modeling the Insertion Mechanics of Flexible Neural Probes Coated with Sacrificial Polymers for Optimizing Probe Design. Sensors. 2016; 16(3):330. https://doi.org/10.3390/s16030330
Chicago/Turabian StyleSingh, Sagar, Meng-Chen Lo, Vinod B. Damodaran, Hilton M. Kaplan, Joachim Kohn, Jeffrey D. Zahn, and David I. Shreiber. 2016. "Modeling the Insertion Mechanics of Flexible Neural Probes Coated with Sacrificial Polymers for Optimizing Probe Design" Sensors 16, no. 3: 330. https://doi.org/10.3390/s16030330
APA StyleSingh, S., Lo, M.-C., Damodaran, V. B., Kaplan, H. M., Kohn, J., Zahn, J. D., & Shreiber, D. I. (2016). Modeling the Insertion Mechanics of Flexible Neural Probes Coated with Sacrificial Polymers for Optimizing Probe Design. Sensors, 16(3), 330. https://doi.org/10.3390/s16030330






