Molecularly Imprinted Filtering Adsorbents for Odor Sensing
Abstract
:1. Introduction
2. Materials and Methods
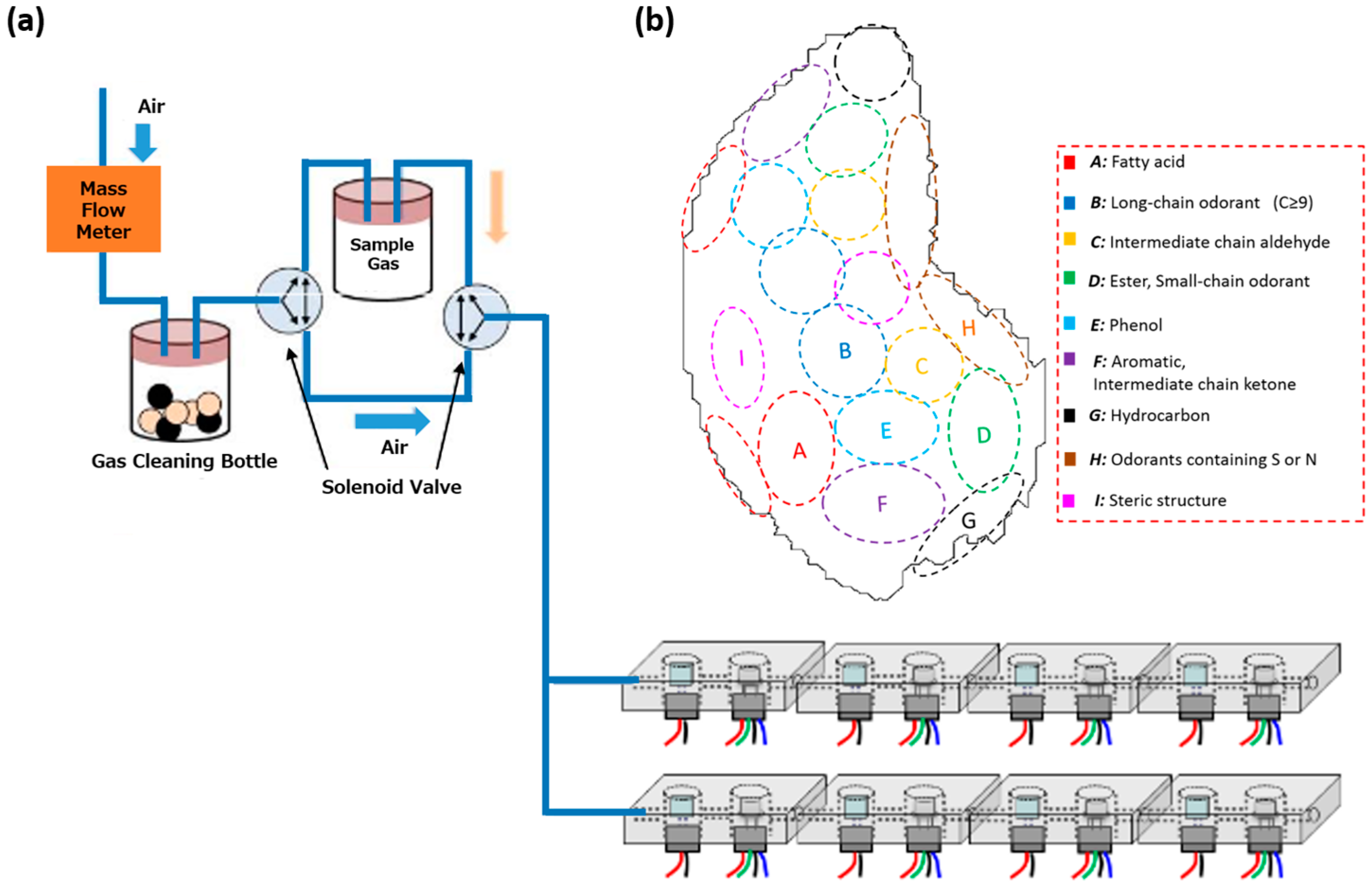
2.1. Preparation of Composite Adsorbents
2.2. Deposition of MIFs on the Adsorbent Substrates
- The polymer solution was prepared by mixing PAA (10 mM), HCl (50 mM), a template molecule (5 mM), and ethanol to obtain a certain concentration, and then stirring the mixture for 4 h.
- The titanium alkoxide solution was prepared by mixing ethanol (1.5 mL), toluene (1.5 mL), and titanium(VI) butoxide (100 mM).
- A gel layer of titanium oxide cannot be attached directly onto the PDMS surface because of its highly water-repellent nature. The surface of PDMS was therefore activated by hydrophilization by treatment with an O2 plasma (Harrick plasma, PDC-002).
- A single titanium alkoxide layer was formed on the PDMS surface by dipping hydrophilization-treated PDMS into a titanium alkoxide solution for 20 min. Thereafter, the surface was rinsed with ethanol and dipped into the polymer solution to give a MIFPAA film including the template molecule.
- The template molecule was removed from the MIF by rinsing with ethanol and heating at 80 °C for 1 h.
- The prepolymer solution was prepared by mixing MAA (2 mmol), EGDMA (1 mmol), a template molecule (2 mmol), acetonitrile (40 mmol) as the diluent, and azobis-isobutyronitrile (2 mg) as the initiator and then stirring this mixture for 4 h.
- A gel layer of titanium oxide was formed on the hydrophilization-treated PDMS using the same method as for the preparation of MIFPAA [15]. The PDMS was then rinsed with ethanol and dipped into the prepolymer solution for 1 h at 80 °C.
- The template molecule was removed as described above for MIFPAA.
2.3. Characterization of the Adsorption Properties of the Adsorbent
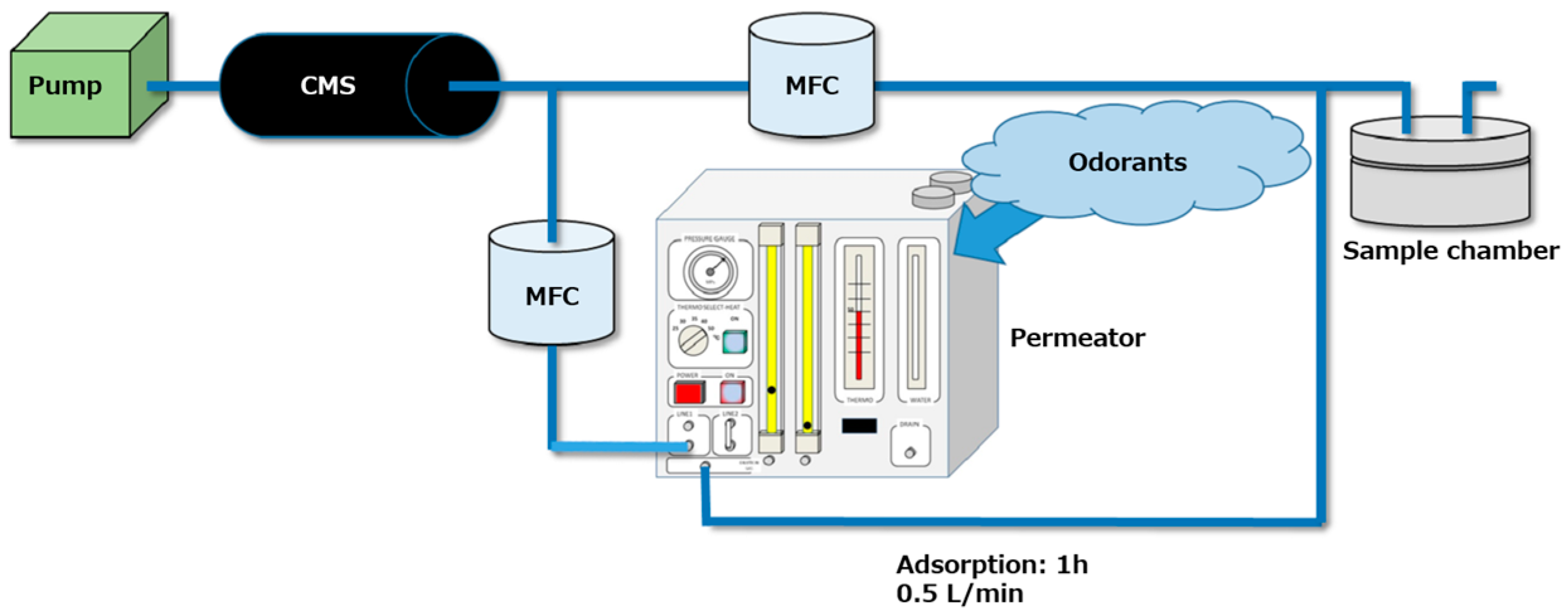
- The adsorbents were placed in a sealed chamber and exposed to odorant gases (Figure 4). The odorants were volatilized at 50 °C in a permeator (PD-1B, GASTEC, Ayase, Japan) or a glass desiccator, and then left in the chamber for 1 h under gas flow at 0.5 L/min.
- The adsorbents were transferred to screw tube vials and were introduced into the SPME auto-sampler (AOC5000 plus, Shimadzu, Japan) with an SPME fiber (23-gauge, 50/30 µm, DVB/CAR/PDMS, SPELCO, Bellefonte, PA, USA). The samples were then heated from 40 to 240 °C to desorb the odor molecules from the adsorbents. Finally, the amounts of the detached gases were determined using GC-MS (GCMS-QP2010, Shimadzu, Kyoto, Japan). This method is referred to below as the “GC-MS/SPME method”.
3. Results and Discussion
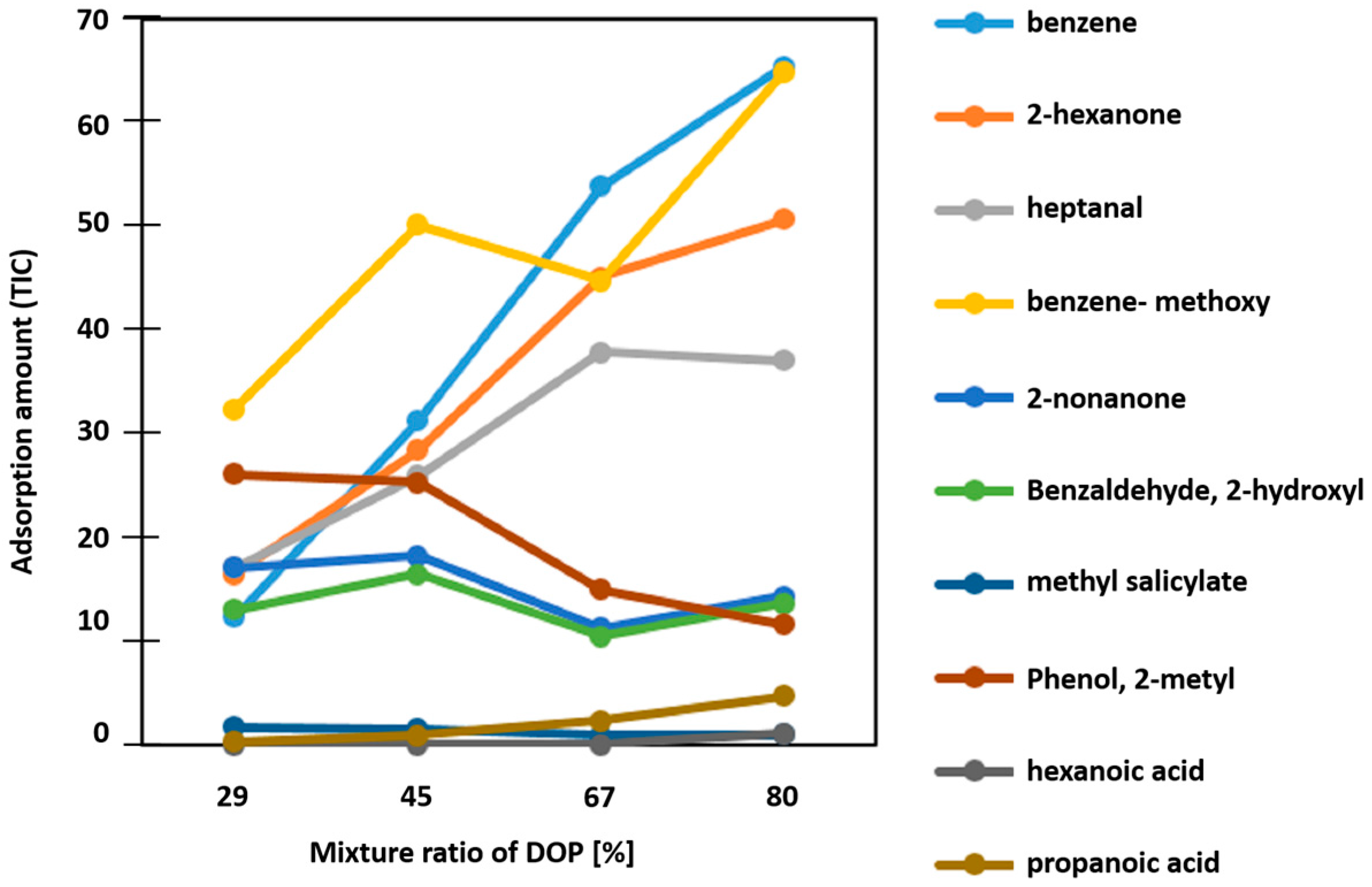
3.1. Influence of DOP Concentration on the Adsorption Properties of PVC-DOP Composite Adsorbents
3.2. Modification of the Adsorption Properties of PDMS by Addition of Other Adsorbents
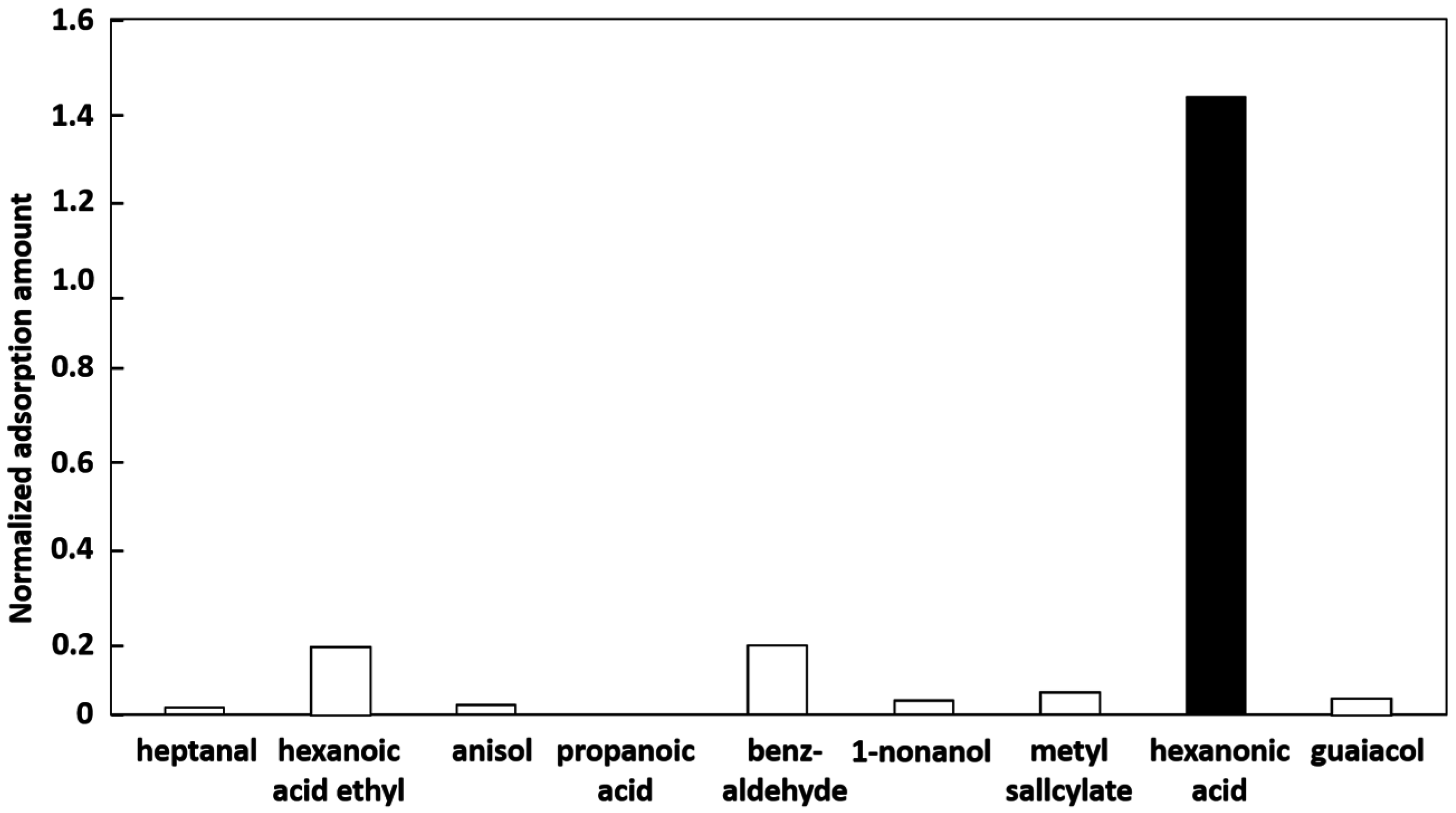
3.3. Controlling the Adsorption Properties of the PVC-DOP Adsorbent with a MIF
3.4. Comparison of the Filtration Efficiency of MIFMAA and MIFPAA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Updike, S.J.; Hicks, G.P. The enzyme electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, N.; Ayazi, F.; Najafi, K. Micromachined inertial sensors. Proc. IEEE 1998, 86, 1640–1659. [Google Scholar] [CrossRef]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans Can Discriminate More than 1 Trillion Olfactory Stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Takahashi, Y.K.; Igarashi, K.M.; Yamaguchi, M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol. Rev. 2006, 86, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Soucy, E.R.; Albeanu, D.F.; Fantana, A.L.; Murthy, V.N.; Meister, M. Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 2009, 12, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.B. Olfactory receptors and odor coding in mammals. Nutr. Rev. 2004, 62, S184–S188. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Leon, M. Chemotopic odorant coding in a mammalian olfactory system. J. Comp. Neurol. 2007, 503, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Kobayakawa, K.; Kobayakawa, R.; Tashiro, T.; Mori, K.; Sakano, H. Spatial Arrangement of Glomerular Molecular-Feature Clusters in the Odorant-Receptor Class Domains of the Mouse Olfactory Bulb. J. Neurophysiol. 2010, 103, 3490–3500. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Yoshihara, Y. Molecular recognition and olfactory processing in the mammalian olfactory system. Prog. Neurobiol. 1995, 46, 585–619. [Google Scholar] [CrossRef]
- Gottfried, J.A. Function follows form: Ecological constraints on odor codes and olfactory percepts. Curr. Opin. Neurobiol. 2009, 19, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Imahashi, M.; Hayashi, K. Odor clustering and discrimination using an odor separating system. Sens. Actuators B Chem. 2012, 166, 685–694. [Google Scholar] [CrossRef]
- Bozza, T.; Vassalli, A.; Fuss, S.; Zhang, J.J.; Weiland, B.; Pacifico, R.; Feinstein, P.; Mombaerts, P. Mapping of Class I and Class II Odorant Receptors to Glomerular Domains by Two Distinct Types of Olfactory Sensory Neurons in the Mouse. Neuron 2009, 61, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Imahashi, M.; Watanabe, M.; Jha, S.K.; Hayashi, K. Olfaction-Inspired Sensing Using a Sensor System with Molecular Recognition and Optimal Classification Ability for Comprehensive Detection of Gases. Sensors 2014, 14, 5221–5238. [Google Scholar] [CrossRef] [PubMed]
- Plastic Material Dictionary. Available online: http://www.plastics-material.com/ (accessed on 9 September 2016).
- Kunitake, T.; Lee, S.W. Molecular imprinting in ultrathin titania gel films via surface sol-gel process. Anal. Chim. Acta 2004, 504, 1–6. [Google Scholar] [CrossRef]
- Snow, N.H.; Slack, G.C. Head-space analysis in modern gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Imahashi, M.; Hayashi, K. Concentrating materials covered by molecular imprinted nanofiltration layer with reconfigurability prepared by a surface sol–gel process for gas-selective detection. J. Colloid Interface Sci. 2013, 406, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Imahashi, M.; Kenshi, H. Odor Clustering Based on Molecular Parameter for Odor Sensing. Sens. Mater. 2014, 26, 171–180. [Google Scholar]





| 2-Heptanone | 1-Heptanol | Heptanoic Acid | |
|---|---|---|---|
| PDMS-DVB/pure PDMS | 0.82 | 0.92 | 8.71 |
| PEG-PVA/pure PDMS | 0.3 | 1.1 | 6.33 |
| PDMS-PVA/pure PDMS | 1.0 | 1.08 | 3.02 |
| 1-Heptanol/2-Heptanone | Heptanoic Acid/2-Heptanone | |
|---|---|---|
| PDMS | 0.479 | 0.00215 |
| PDMS-DVB | 0.541 | 0.0229 |
| PEG-PVA | 1.779 | 0.046 |
| PDMS-PVA | 0.515 | 0.00647 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinohara, S.; Chiyomaru, Y.; Sassa, F.; Liu, C.; Hayashi, K. Molecularly Imprinted Filtering Adsorbents for Odor Sensing. Sensors 2016, 16, 1974. https://doi.org/10.3390/s16111974
Shinohara S, Chiyomaru Y, Sassa F, Liu C, Hayashi K. Molecularly Imprinted Filtering Adsorbents for Odor Sensing. Sensors. 2016; 16(11):1974. https://doi.org/10.3390/s16111974
Chicago/Turabian StyleShinohara, Sho, You Chiyomaru, Fumihiro Sassa, Chuanjun Liu, and Kenshi Hayashi. 2016. "Molecularly Imprinted Filtering Adsorbents for Odor Sensing" Sensors 16, no. 11: 1974. https://doi.org/10.3390/s16111974





