Polypyrrole Nanotubes and Their Carbonized Analogs: Synthesis, Characterization, Gas Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Carbonization of Polypyrrole
2.3. Characterization
2.4. Vapor Response
3. Results and Discussion
3.1. Analytical Carbonization
3.2. FTIR Spectroscopy
3.2.1. Polypyrrole Bases
3.2.2. Carbonized Materials
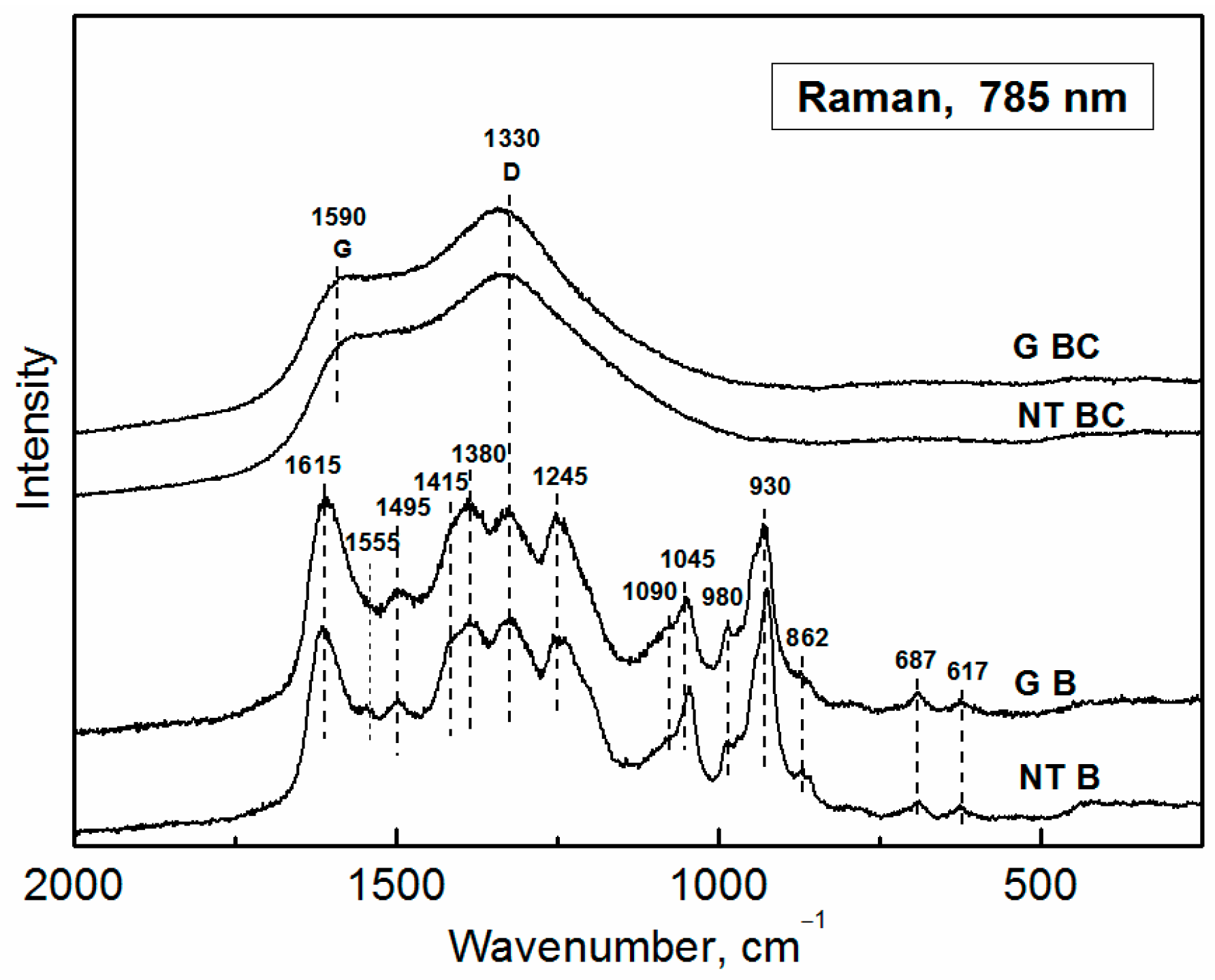
3.3. Raman Spectroscopy
3.3.1. Polypyrrole Bases
3.3.2. Carbonized Materials
3.4. Vapor Response
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abraham, J.K.; Philip, B.; Witchurch, A.; Varadan, V.K.; Reddy, C.C. A compact wireless gas sensor using a carbon nanotube/PMMA thin film chemiresistor. Smart Mater. Struct. 2004, 13, 1045–1049. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Olejník, R.; Slobodian, P.; Říha, P.; Machovský, M. Increased sensitivity of multiwalled carbon nanotube network by PMMA functionalization to vapors with affine polarity. J. Appl. Polym. Sci. 2012, 126, 21–29. [Google Scholar] [CrossRef]
- Philip, B.; Abraham, J.K.; Chandrasekhar, A.; Varadan, V.K. Carbon nanotube/PMMA composite thin films for gas-sensing applications. Smart Mater. Struct. 2003, 12, 935–939. [Google Scholar] [CrossRef]
- Slobodian, P.; Říha, P.; Lengalová, A.; Svoboda, P.; Sáha, P. Multi-wall carbon nanotube networks as potential resistive gas sensors for organic vapor detection. Carbon 2011, 49, 2499–2507. [Google Scholar] [CrossRef]
- Su, P.G.; Ho, C.J.; Sun, Y.L.; Chen, I.C. A micromachined resistive-type humidity sensor with a composite material as sensitive film. Sens. Actuators B Chem. 2006, 113, 837–842. [Google Scholar] [CrossRef]
- Su, P.G.; Huang, S.C. Electrical and humidity sensing properties of carbon nanotubes-SiO2-poly(2-acrylamido-2-methylpropane sulfonate) composite material. Sens. Actuators B Chem. 2006, 113, 142–149. [Google Scholar] [CrossRef]
- Goldoni, A.; Larciprete, R.; Petaccia, L.; Lizzit, S. Single-wall carbon nanotube interaction with gases: Sample contaminants and environmental monitoring. J. Am. Chem. Soc. 2003, 125, 11329–11333. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Cho, K.J. Ab initio study of doped carbon nanotube sensors. Nano Lett. 2003, 3, 513–517. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Liu, P. Modifications of carbon nanotubes with polymers. Eur. Polym. J. 2005, 41, 2693–2703. [Google Scholar] [CrossRef]
- Ramanathan, T.; Liu, H.; Brinson, L.C. Functionalized SWNT/polymer nanocomposites for dramatic property improvement. J. Polym. Sci. B Polym. Phys. 2005, 43, 2269–2279. [Google Scholar] [CrossRef]
- Wang, C.C.; Guo, Z.X.; Fu, S.K.; Wu, W.; Zhu, D.B. Polymers containing fullerene or carbon nanotube structures. Prog. Polym. Sci. 2004, 29, 1079–1141. [Google Scholar] [CrossRef]
- Yao, Z.L.; Braidy, N.; Botton, G.A.; Adronov, A. Polymerization from the surface of single-walled carbon nanotubes—Preparation and characterization of nanocomposites. J. Am. Chem. Soc. 2003, 125, 16015–16024. [Google Scholar] [CrossRef] [PubMed]
- Posudievsky, O.Y.; Konoschuk, N.V.; Kukla, A.L.; Pavluchenko, A.S.; Shirshov, Y.M.; Pokhodenko, V.D. Comparative analysis of sensor responses of thin conducting polymer films to organic solvent vapors. Sens. Actuators B Chem. 2011, 151, 351–359. [Google Scholar] [CrossRef]
- Vrňata, M.; Kopecký, D.; Vysloužil, F.; Myslík, V.; Fitl, P.; Ekrt, O.; Hofmann, J.; Kučera, L. Impedance properties of polypyrrolic sensors prepared by MAPLE technology. Sens. Actuators B Chem. 2009, 137, 88–93. [Google Scholar] [CrossRef]
- Ma, Y.W.; Zhang, L.R.; Li, J.J.; Ni, H.T.; Li, M.; Zhang, J.L.; Feng, X.M.; Fan, Q.L.; Hu, Z.; Huang, W. Carbon-nitrogen/graphene composite as metal-free electrocatalyst for the oxygen reduction reaction. Chin. Sci. Bull. 2011, 56, 3583–3589. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Y.; Ding, B.; Nie, P.; Shen, L.F.; Wang, J.; Zhang, X.G. Porous nitrogen-doped carbon nanotubes derived from tubular polypyrrole for energy-storage applications. Chem. Eur. J. 2013, 19, 12306–12312. [Google Scholar] [CrossRef] [PubMed]
- Ćirić-Marjanović, G.; Pašti, I.; Gavrilov, N.; Janošević, A.; Mentus, S. Carbonised polyaniline and polypyrrole: Towards advanced nitrogen-containing carbon materials. Chem. Pap. 2013, 67, 781–813. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Kovářová, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Stejskal, J.; Omastová, M.; Fedorová, S.; Prokeš, J.; Trchová, M. Polyaniline and polypyrrole prepared in the presence of surfactants: A comparative conductivity study. Polymer 2003, 44, 1353–1358. [Google Scholar] [CrossRef]
- Blínová, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J.; Omastová, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Peng, Y.J.; Qiu, L.H.; Pan, C.T.; Wang, C.C.; Shang, S.M.; Yan, F. Facile preparation of water dispersible polypyrrole nanotube-supported silver nanoparticles for hydrogen peroxide reduction and surface-enhanced Raman scattering. Electrochim. Acta 2012, 75, 399–405. [Google Scholar] [CrossRef]
- Shang, S.M.; Yang, X.M.; Tao, X.M. Easy synthesis of carbon nanotubes with polypyrrole nanotubes as the carbon precursor. Polymer 2009, 50, 2815–2818. [Google Scholar] [CrossRef]
- Škodová, J.; Kopecký, D.; Vrňata, M.; Varga, M.; Prokeš, J.; Cieslar, M.; Bober, P.; Stejskal, J. Polypyrrole-silver composites prepared by the reduction of silver ions with polypyrrole nanotubes. Polym. Chem. 2013, 4, 3610–3616. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, L.; Yan, F. Fabrication of polypyrrole/Ag composite nanotubes via in situ reduction of AgNO3 on polypyrrole nanotubes. Chem. Lett. 2010, 39, 118–119. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, L.; Yan, F. Polypyrrole/silver composite nanotubes for gas sensors. Sens. Actuators B Chem. 2010, 145, 495–500. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, L.A.; Zhao, Y. Ag/AgCl-decorated polypyrrole nanotubes and their sensory properties. Synth. Met. 2010, 160, 1822–1825. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Kopecký, D.; Vrňata, M.; Prokeš, J.; Varga, M.; Watzlová, E. Polypyrrole salts and bases: Superior conductivity of nanotubes and their stability towards the loss of conductivity by deprotonation. RSC Adv. 2016, 6, 88382–88391. [Google Scholar] [CrossRef]
- Kudoh, Y. Properties of polypyrrole prepared by chemical polymerization using aqueous solutions containing Fe2(SO4)3 and anionic surfactants. Synth. Met. 1996, 79, 17–22. [Google Scholar] [CrossRef]
- Omastová, M.; Pionteck, J.; Trchová, M. Properties and morphology of polypyrrole containing a surfactant. Synth. Met. 2003, 135, 437–438. [Google Scholar] [CrossRef]
- Mrlík, M.; Pavlínek, V.; Cheng, Q.L.; Sáha, P. Synthesis of titanate/polypyrrole composite rod-like particles and the role of conducting polymer on electrorheological efficiency. Int. J. Mod. Phys. B 2012, 26, 280–286. [Google Scholar] [CrossRef]
- Quadrat, O.; Stejskal, J. Polyaniline in electrorheology. J. Ind. Eng. Chem. 2006, 12, 352–361. [Google Scholar]
- Sedlačík, M.; Mrlík, M.; Pavlínek, V.; Sáha, P.; Quadrat, O. Electrorheological properties of suspensions of hollow globular titanium oxide/polypyrrole particles. Colloid Polym. Sci. 2012, 290, 41–48. [Google Scholar] [CrossRef]
- Kostić, R.; Raković, D.; Stepanyan, S.A.; Davidova, I.E.; Gribov, L.A. Vibrational Spectroscopy of Polypyrrole, Theoretical-Study. J. Chem. Phys. 1995, 102, 3104–3109. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Alizadeh, N. Design and fabrication of gas sensor based on nanostructure conductive polypyrrole for determination of volatile organic solvents. Sens. Actuators B Chem. 2010, 147, 461–466. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Patil, S.V.; Deshmukh, P.R.; Shinde, N.M.; Lokhande, C.D. Fabrication and performance of polypyrrole (Ppy)/TiO2 heterojunction for room temperature operated LPG sensor. Sens. Actuators B Chem. 2013, 181, 417–423. [Google Scholar] [CrossRef]
- De Souza, J.E.G.; dos Santos, F.L.; Barros-Neto, B.; dos Santos, C.G.; de Melo, C.P. Polypyrrole thin films gas sensors. Synth. Met. 2001, 119, 383–384. [Google Scholar] [CrossRef]
- Babaei, M.; Alizadeh, N. Methanol selective gas sensor based on nano-structured conducting polypyrrole prepared by electrochemically on interdigital electrodes for biodiesel analysis. Sens. Actuators B Chem. 2013, 183, 617–626. [Google Scholar] [CrossRef]
- De Melo, C.P.; Neto, B.B.; de Lima, E.G.; de Lira, L.F.B.; de Souza, J.E.G. Use of conducting polypyrrole blends as gas sensors. Sens. Actuators B Chem. 2005, 109, 348–354. [Google Scholar] [CrossRef]
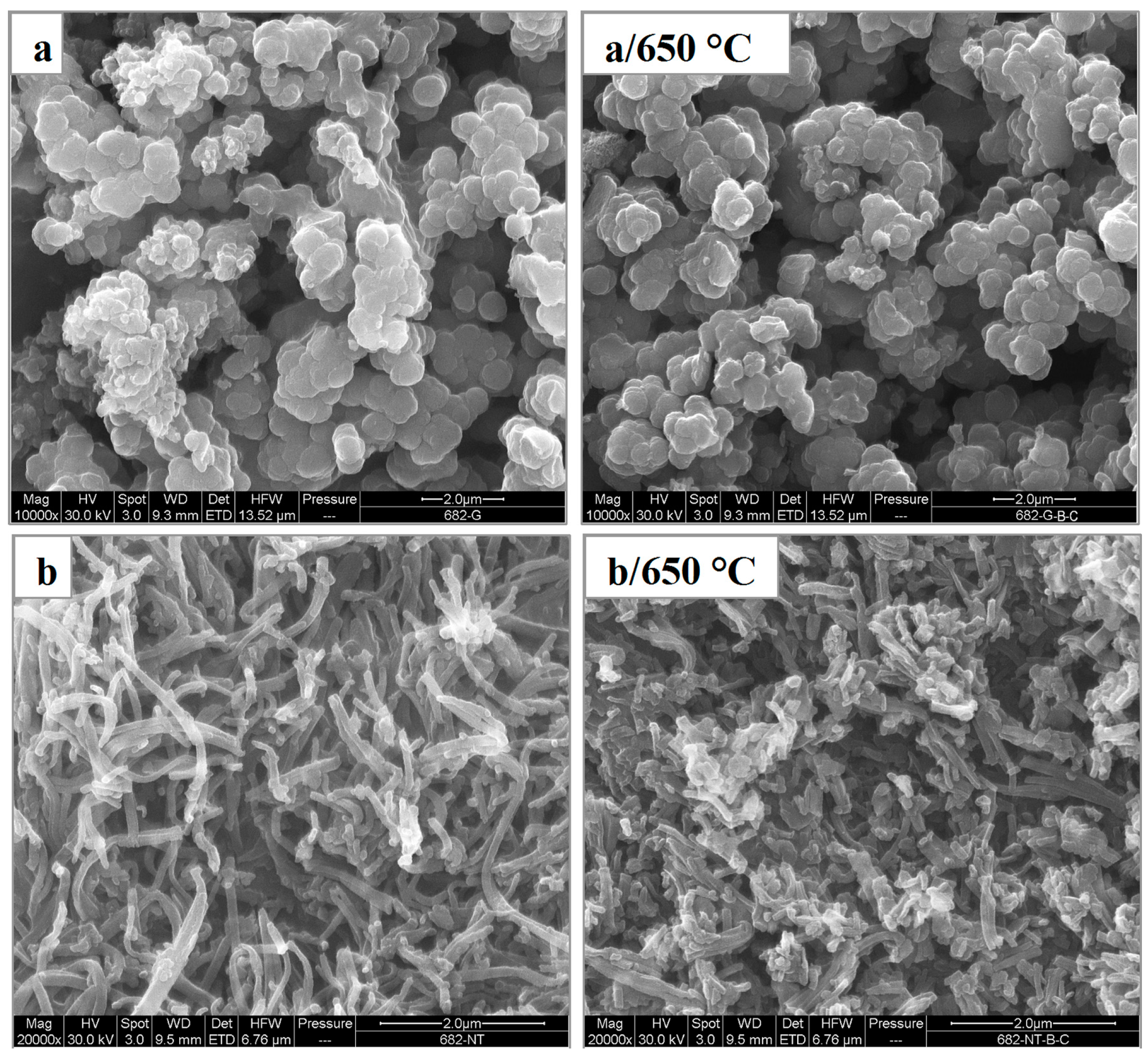
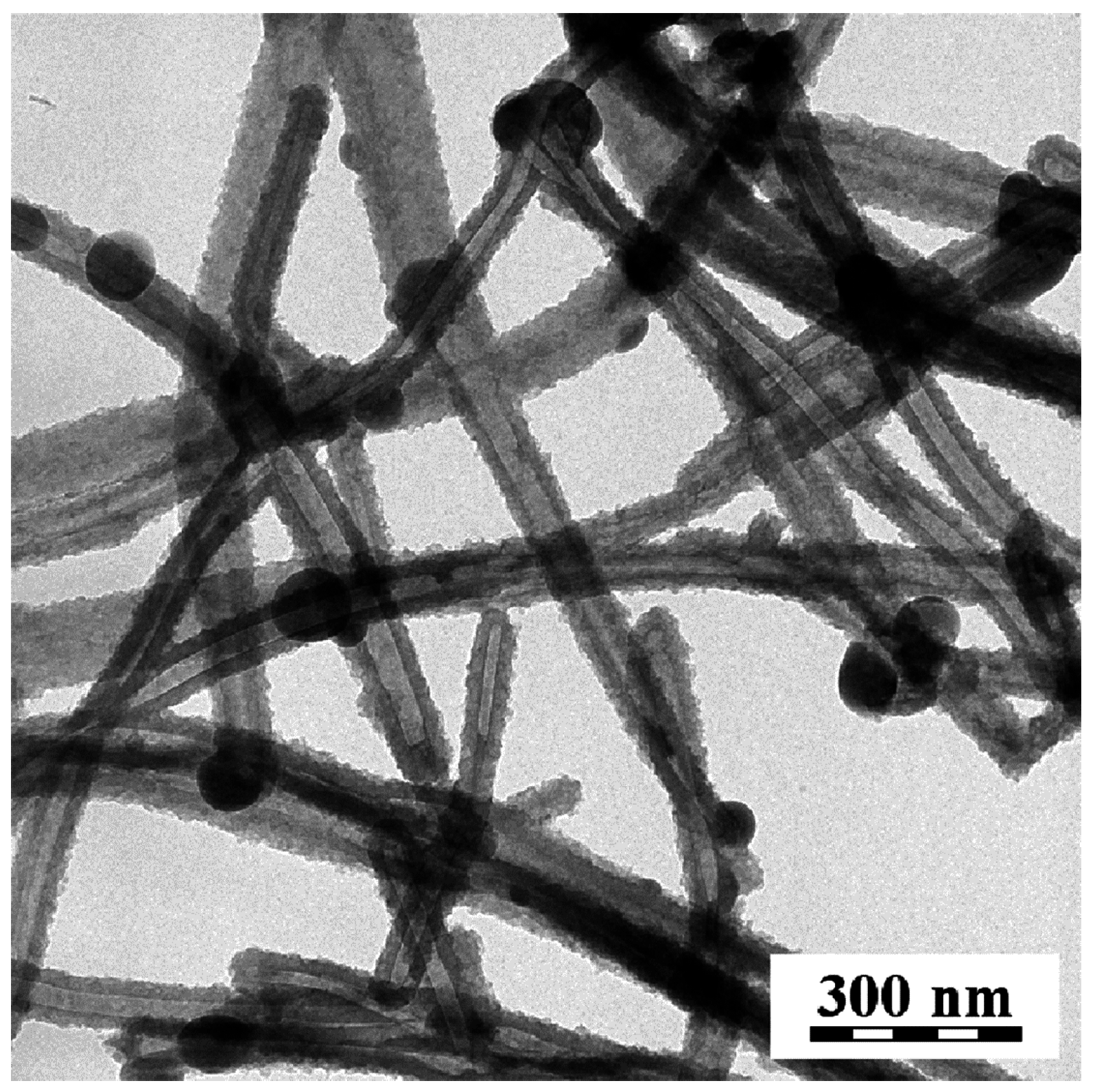
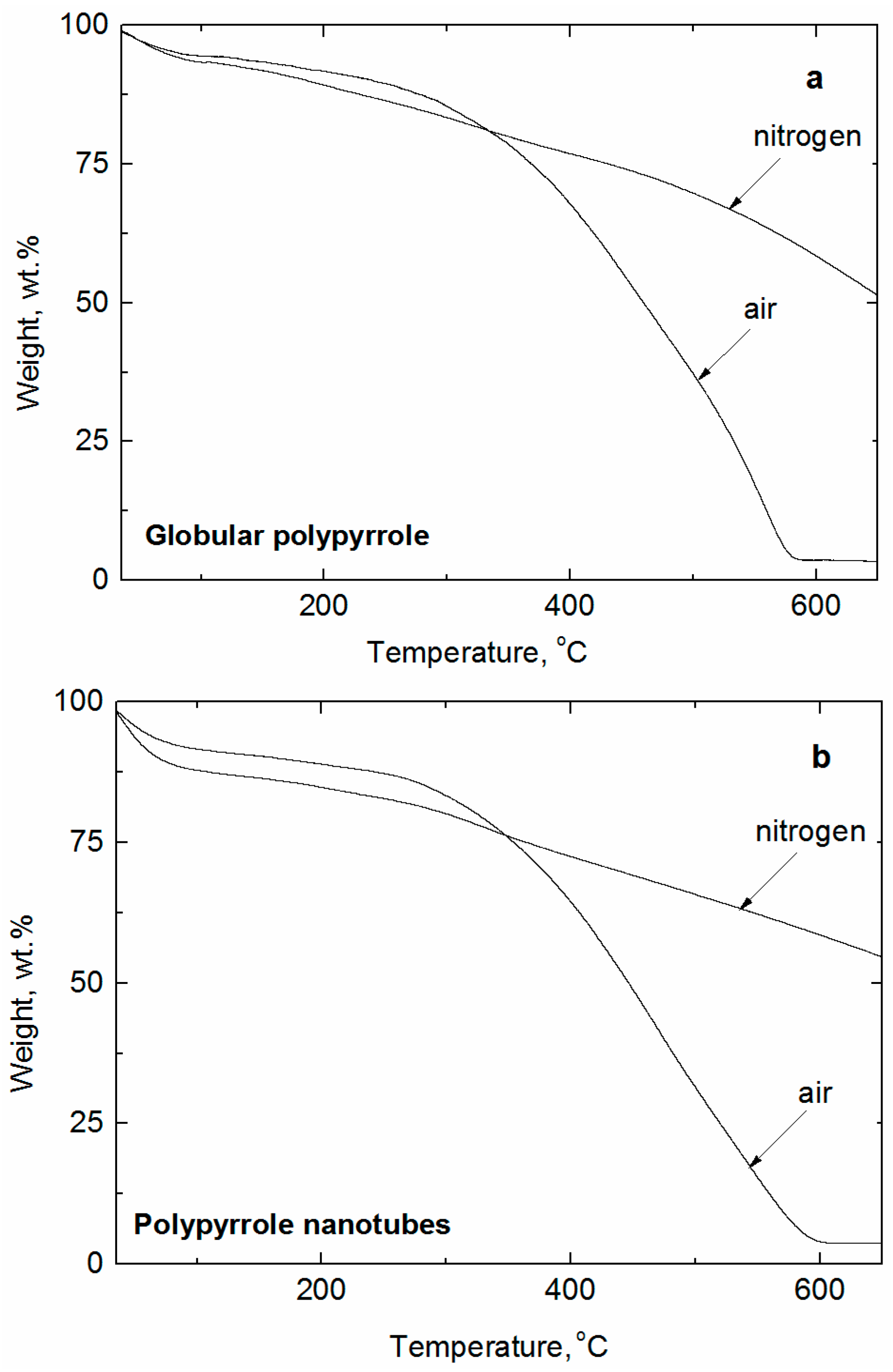
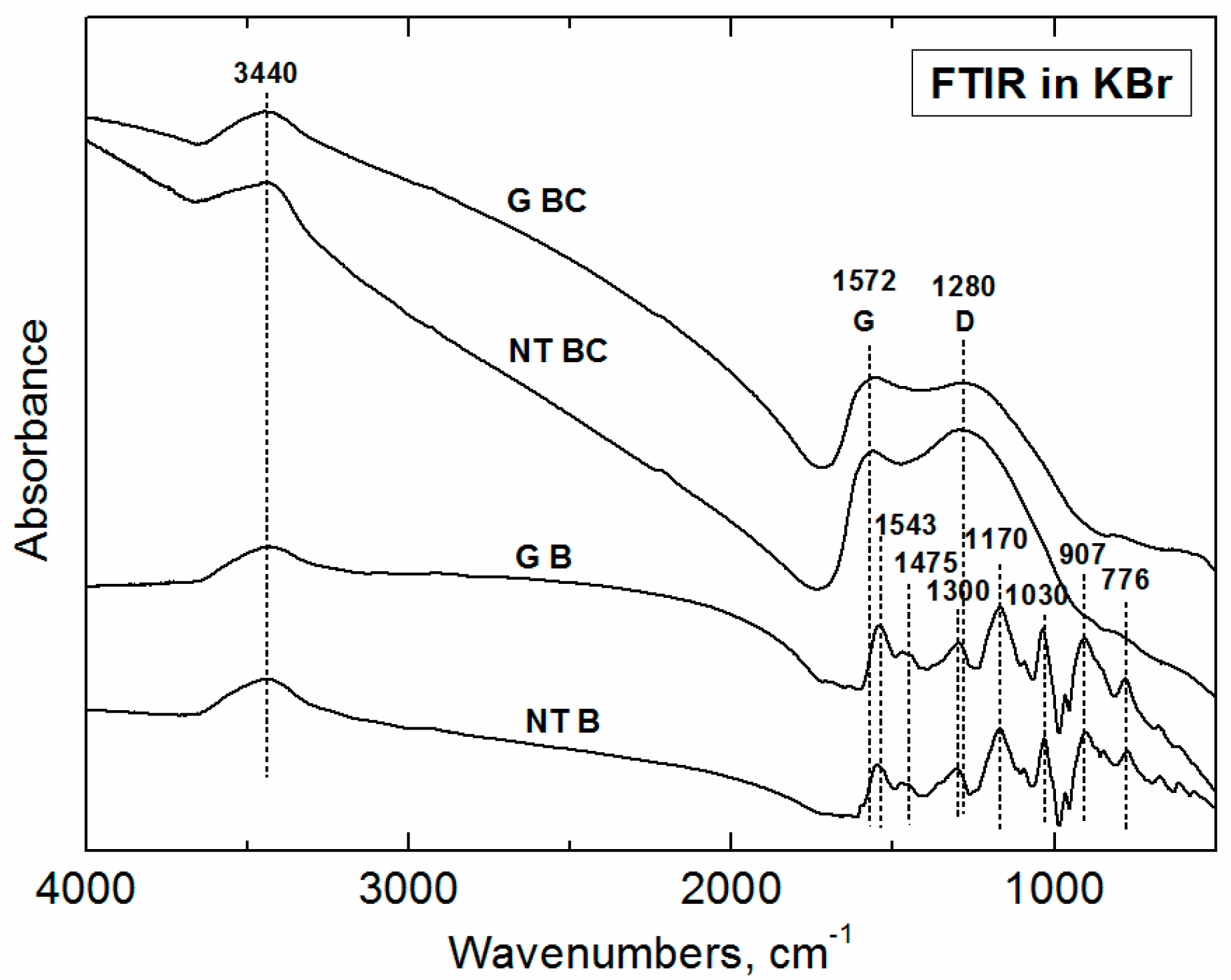


| Sample | Conductivity (S·cm−1) | Specific Surface Area (m2·g−1) | ||||
|---|---|---|---|---|---|---|
| Salt | Base | Carbonized Base | Salt | Base | Carbonized Base | |
| PPy nanotubes | 60 a | 6.7 × 10−2 a | 6.7 × 10−6 | 75 | 63 | 211 |
| Globular PPy | 0.011 | 3.4 × 10−5 | 1.4 × 10−7 | 26 | 25 | 150 |
| Sensor Design | Detection Conditions | ||||
|---|---|---|---|---|---|
| Sensitive Material | Analyte | Concentration | S (%) | Temp./Hum. (°C)/(% RH) | References |
| Sensors of this work | |||||
| PPy nanotubes deprotonated | ethanol | saturated vapors at 25 °C | 18 | 25/0 | This work |
| n-heptane | 110 | ||||
| PPy nanotubes carbonized | ethanol | 24 | |||
| n-heptane | 20 | ||||
| Polypyrrole based sensors a | |||||
| PPy/sulfate | propane/butane | 1040 ppm | 55 | 27/35 | [39] |
| PPy/Cl– | hexane | - | 0.8 | 100/0 | [38] |
| methanol | 5 | ||||
| PPy/ClO4− | iso-butanol | saturated vapors at 25 °C | 15.5 | 25/0 | [15] |
| ethanol | 10.4 | ||||
| iso-propanol | 15.8 | ||||
| n-pentanol | 11.2 | ||||
| PPy/PF6− | iso-butanol | 0.5 | |||
| ethanol | 3.2 | ||||
| iso-propanol | 0.6 | ||||
| n-pentanol | 1.1 | ||||
| PPy/CF3SO3− | iso-butanol | 3.0 | |||
| ethanol | 7.5 | ||||
| iso-propanol | 4.4 | ||||
| n-pentanol | 1.3 | ||||
| PPy/camphorsulfonate | iso-butanol | 6.1 | |||
| ethanol | 5.8 | ||||
| iso-propanol | 5.7 | ||||
| n-pentanol | 5.1 | ||||
| PPy/p-toluenesulfonate | methanol | - | 18 | - | [40] |
| ethanol | 10 | ||||
| PPy/3-nitrobenzenesulfonate | methanol | 11 | |||
| ethanol | 6 | ||||
| Nanostructured PPy/ClO4− | methanol | 11% wt. of VOC in n-hexane | 2.9 | 120/0 | [41] |
| ethanol | 0.58 | ||||
| n-propanol | 0.22 | ||||
| iso-propanol | 0.18 | ||||
| Nanostructured PPy/p-toluenesulfonate | methanol | 1.5 | |||
| ethanol | 0.75 | ||||
| n-propanol | – | ||||
| iso-propanol | – | ||||
| PPy/PCP a | methanol | – | 7 | –/0 | [42] |
| ethanol | 7 | ||||
| PPy/PEO | methanol | 3.5 | |||
| ethanol | 5.5 | ||||
| PPy/PMMA | methanol | 65 | |||
| ethanol | 20 | ||||
| PPy/PVAL | methanol | 14 | |||
| ethanol | 14 | ||||
| PPy/PVAc | methanol | 27.5 | |||
| ethanol | 37.5 | ||||
| CNT based sensors | |||||
| MWCNT | iso-pentane | saturated vapors at 25 °C | 20.3 | 25/60 | [3] |
| methanol | 13.6 | ||||
| MWCNT/PMMA | iso-pentane | 12.6 | |||
| methanol | 14.7 | ||||
| MWCNT/PMMA | methanol | saturated vapors at 25 °C | 429 | –/0 | [4] |
| hexane | – | ||||
| Surface modified MWCNT/PMMA | methanol | 4500 | |||
| hexane | – | ||||
| MWCNT | iso-pentane | saturated vapors at 25 °C | 20.6 | 25/60 | [5] |
| methanol | 12.9 | ||||
| Oxidized MWCNT | iso-pentane | 12.0 | |||
| methanol | 46.6 | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopecká, J.; Mrlík, M.; Olejník, R.; Kopecký, D.; Vrňata, M.; Prokeš, J.; Bober, P.; Morávková, Z.; Trchová, M.; Stejskal, J. Polypyrrole Nanotubes and Their Carbonized Analogs: Synthesis, Characterization, Gas Sensing Properties. Sensors 2016, 16, 1917. https://doi.org/10.3390/s16111917
Kopecká J, Mrlík M, Olejník R, Kopecký D, Vrňata M, Prokeš J, Bober P, Morávková Z, Trchová M, Stejskal J. Polypyrrole Nanotubes and Their Carbonized Analogs: Synthesis, Characterization, Gas Sensing Properties. Sensors. 2016; 16(11):1917. https://doi.org/10.3390/s16111917
Chicago/Turabian StyleKopecká, Jitka, Miroslav Mrlík, Robert Olejník, Dušan Kopecký, Martin Vrňata, Jan Prokeš, Patrycja Bober, Zuzana Morávková, Miroslava Trchová, and Jaroslav Stejskal. 2016. "Polypyrrole Nanotubes and Their Carbonized Analogs: Synthesis, Characterization, Gas Sensing Properties" Sensors 16, no. 11: 1917. https://doi.org/10.3390/s16111917
APA StyleKopecká, J., Mrlík, M., Olejník, R., Kopecký, D., Vrňata, M., Prokeš, J., Bober, P., Morávková, Z., Trchová, M., & Stejskal, J. (2016). Polypyrrole Nanotubes and Their Carbonized Analogs: Synthesis, Characterization, Gas Sensing Properties. Sensors, 16(11), 1917. https://doi.org/10.3390/s16111917








