The Impact of Sepiolite on Sensor Parameters during the Detection of Low Concentrations of Alcohols
Abstract
:1. Introduction
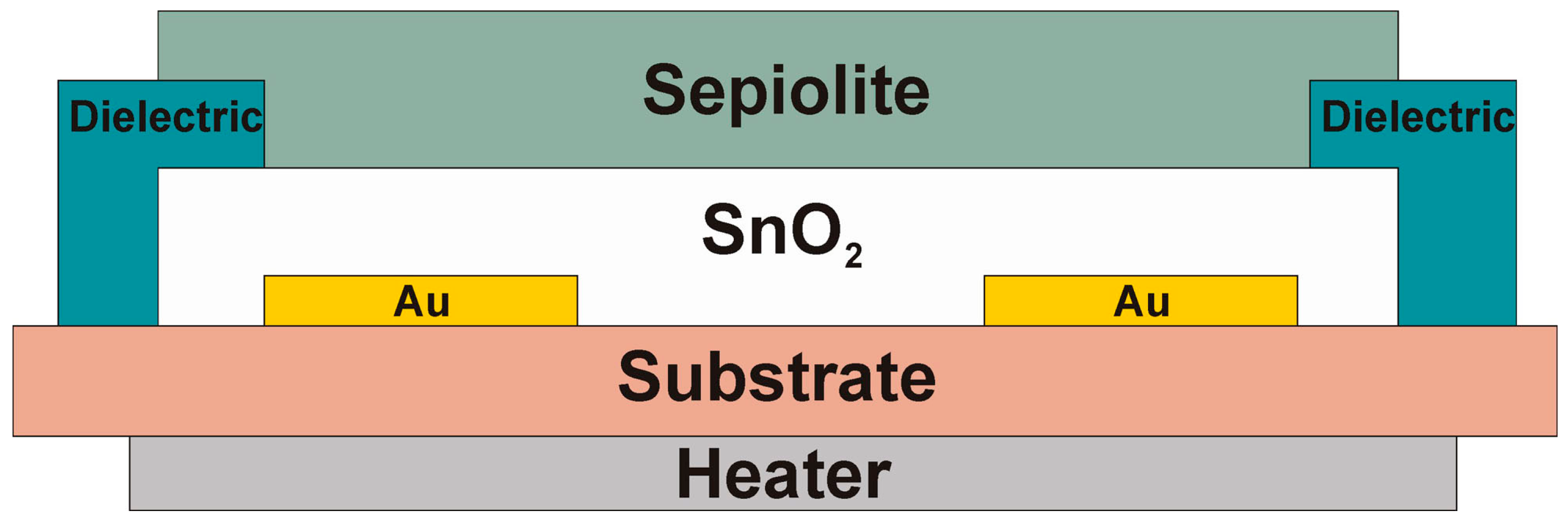
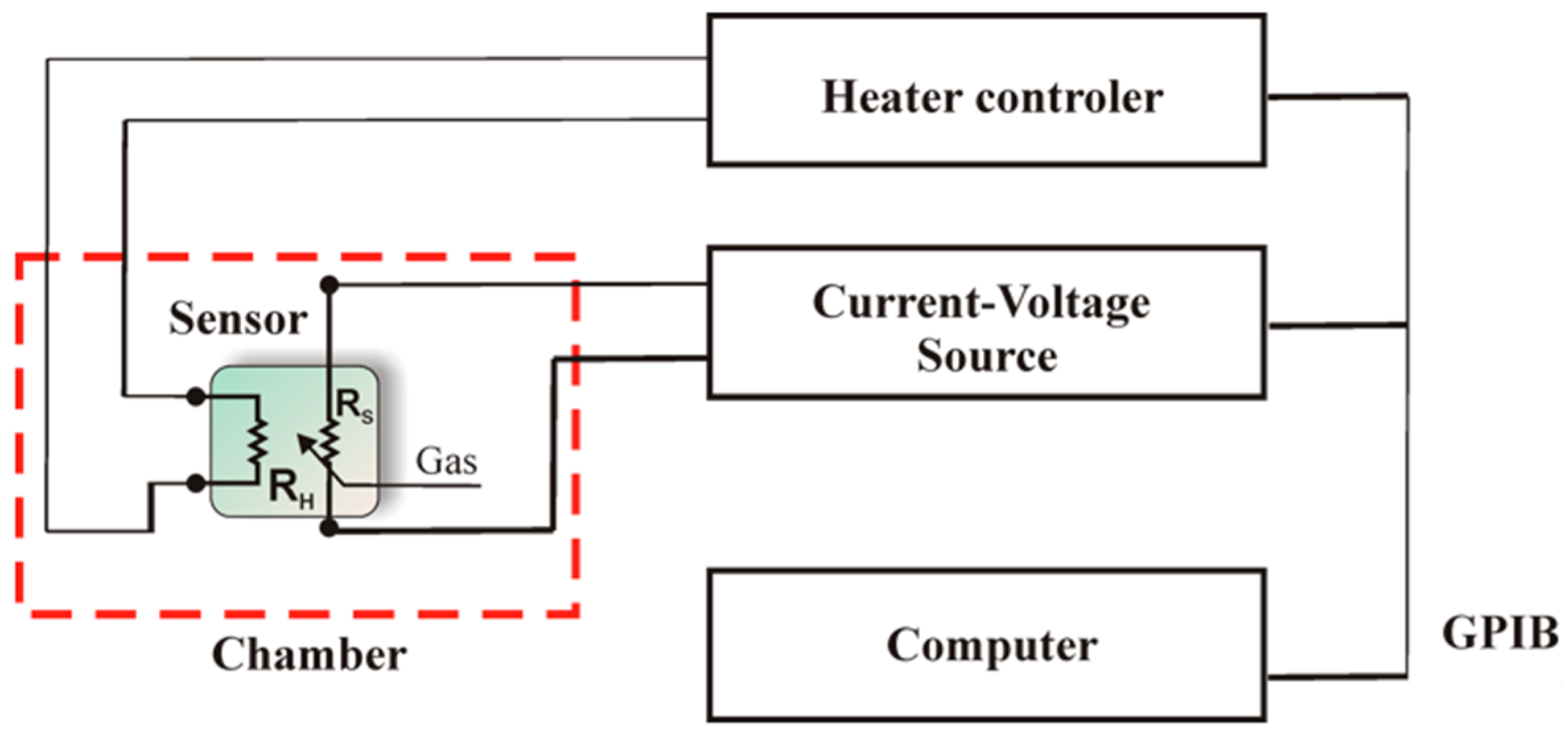
2. Materials and Methods
- with a layer made only from sepiolite (without gas sensitive layer of tin dioxide),
- with a layer made of undoped tin dioxide,
- double layered with a gas-sensitive layer made of undoped tin dioxide (active layer) and a natural filter made of sepiolite.
3. Results and Discussion
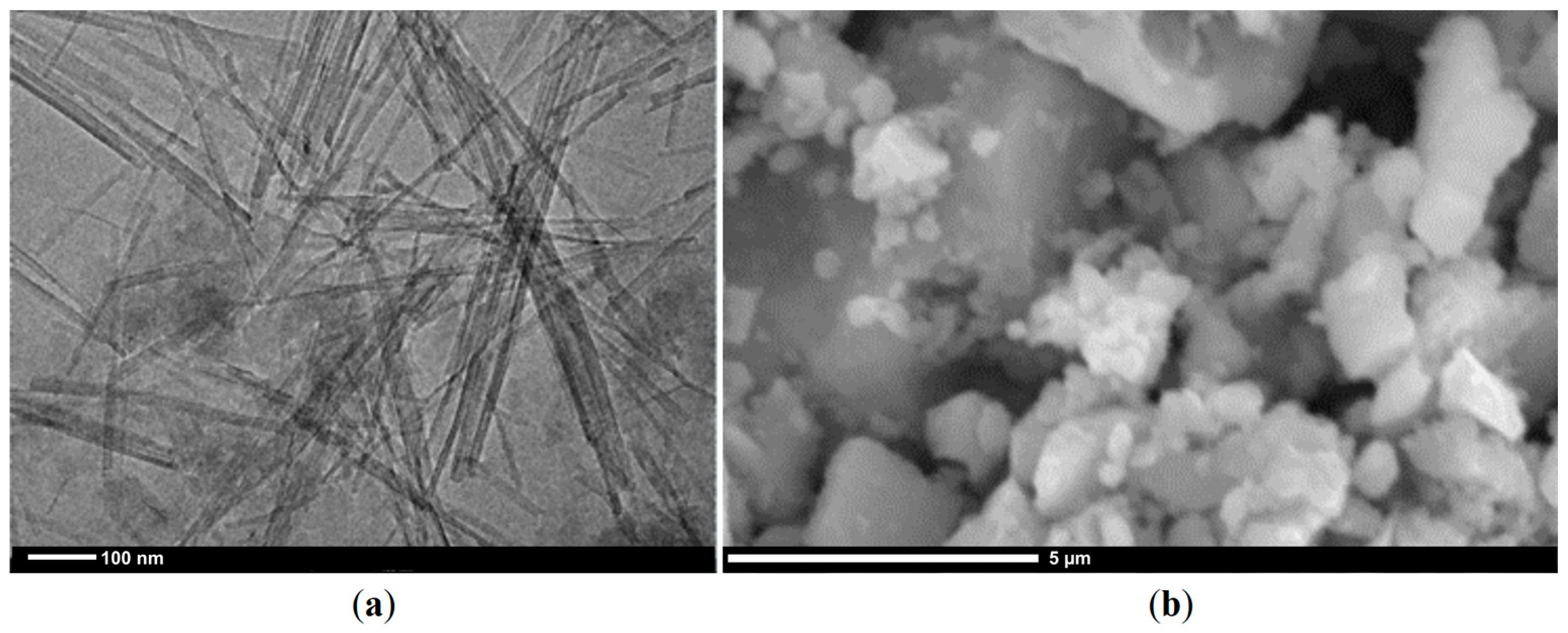
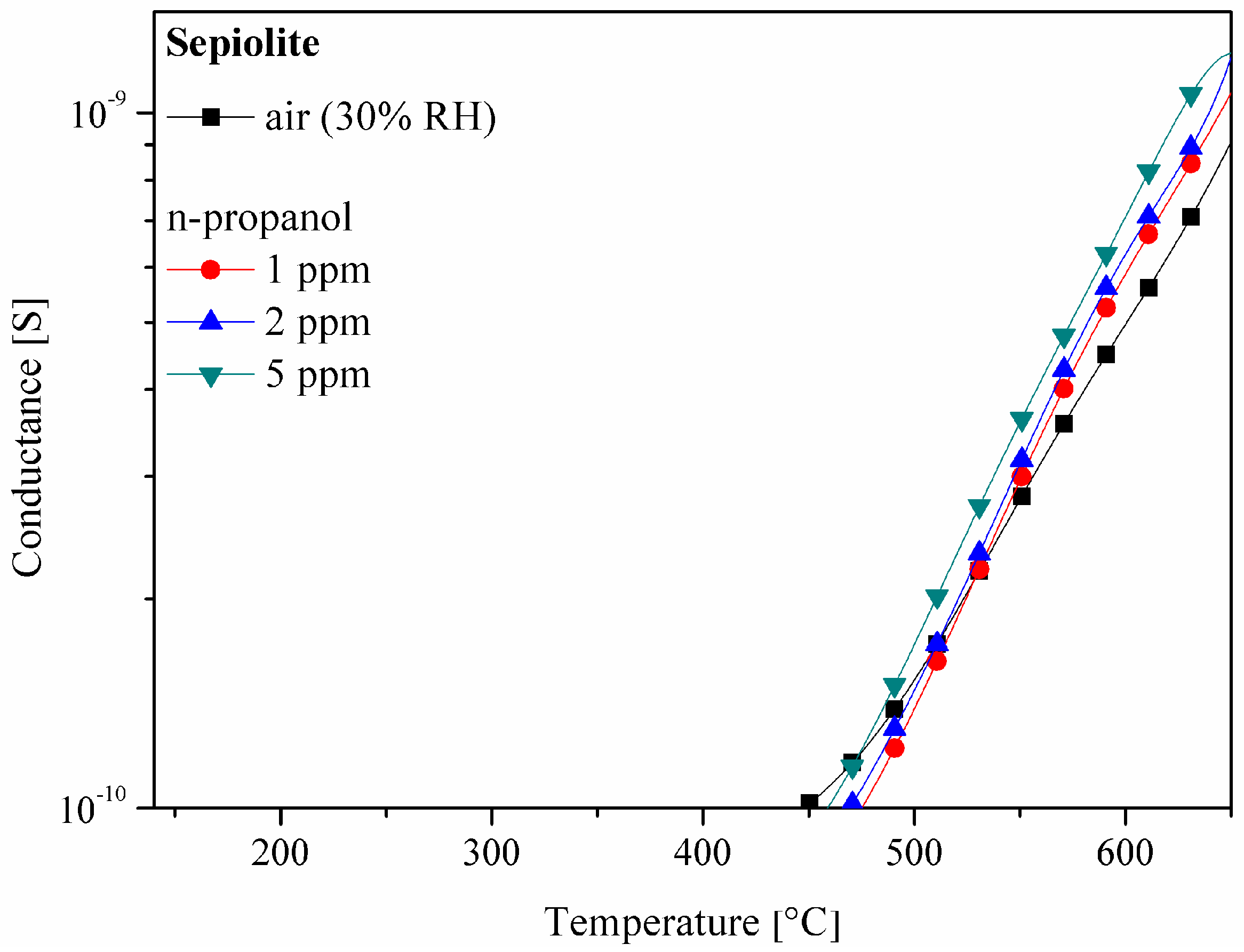
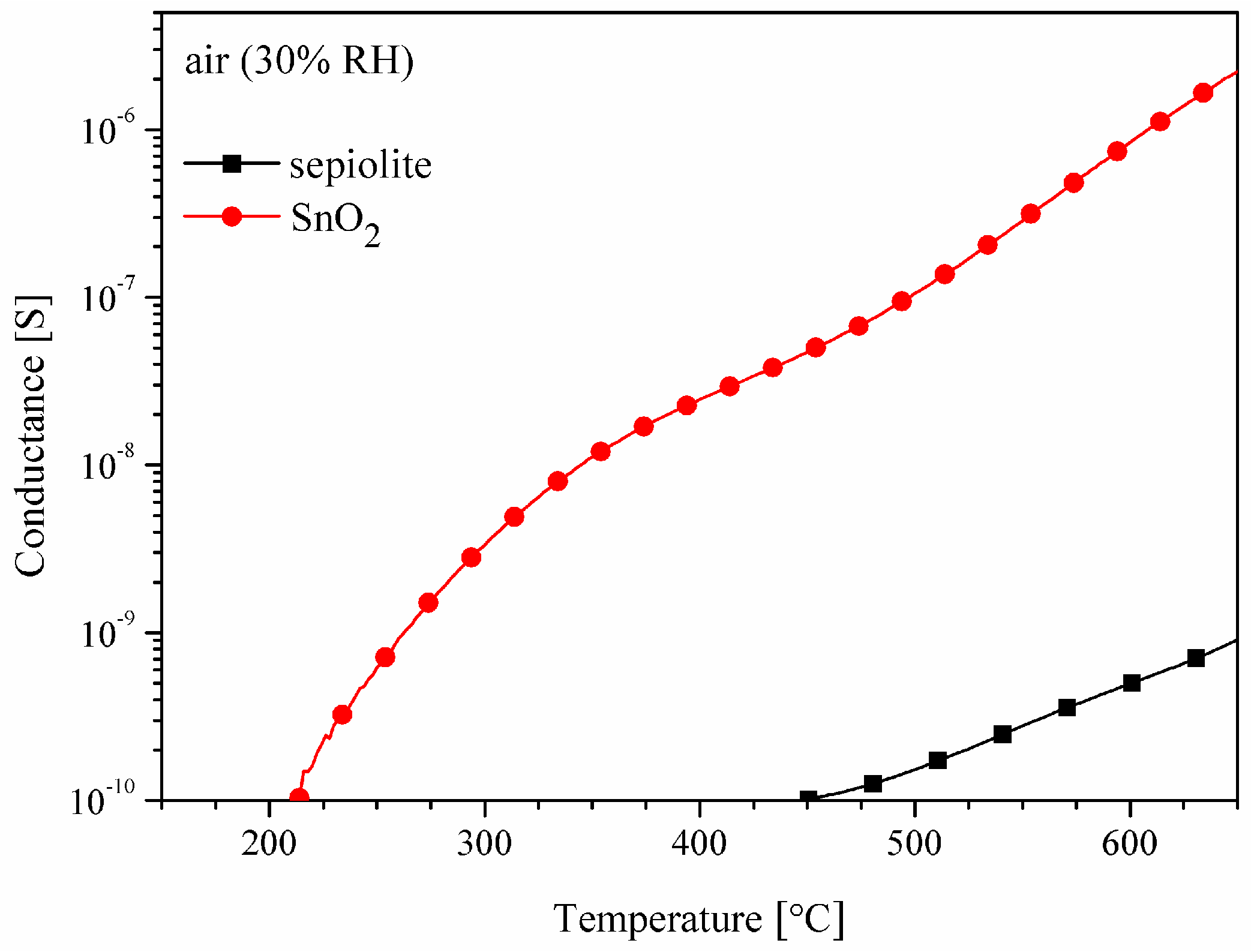
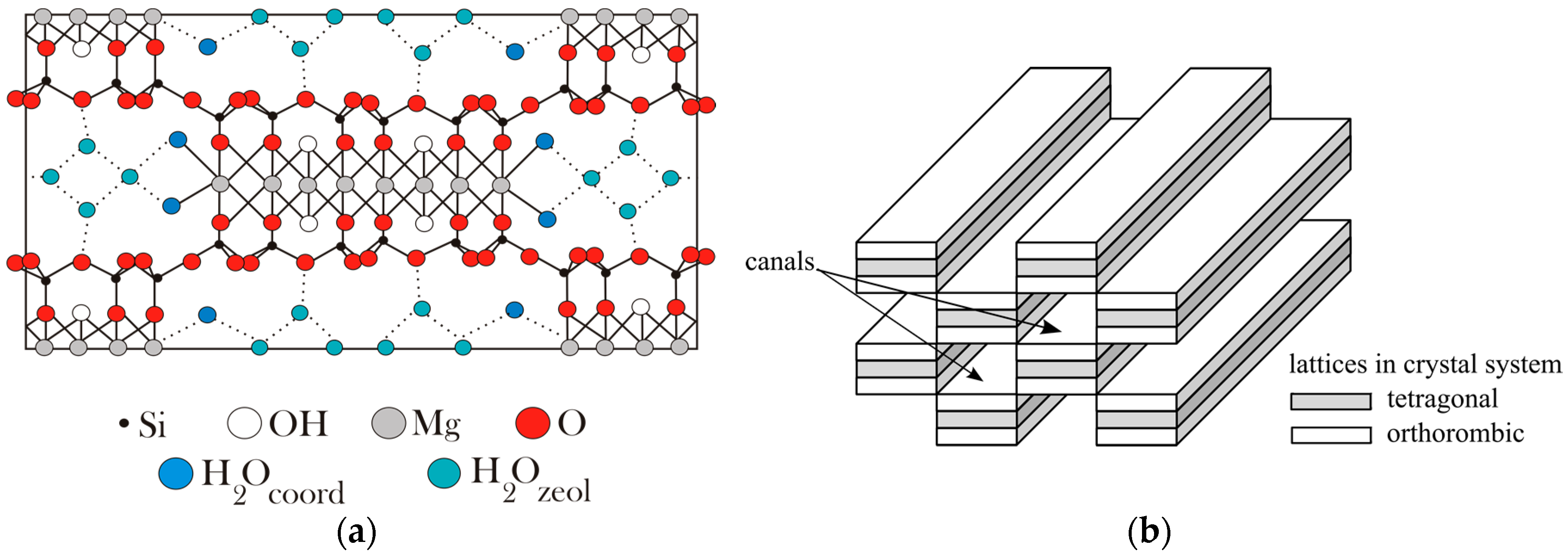
3.1. Properties of the Sepiolite Filter
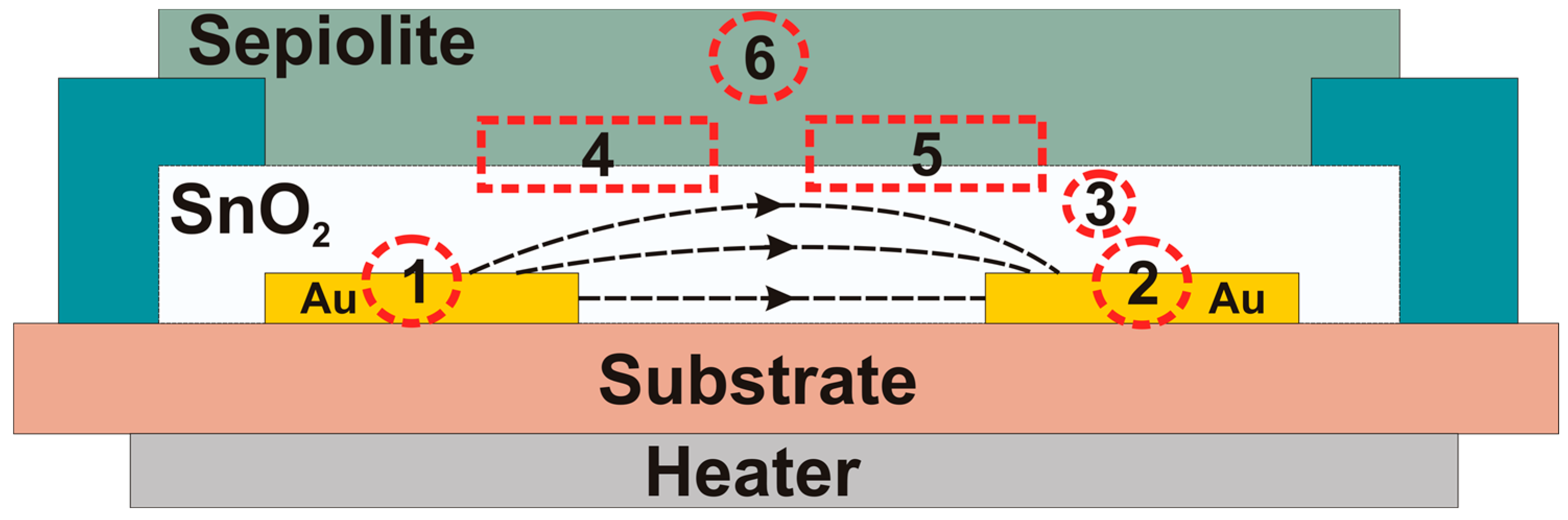
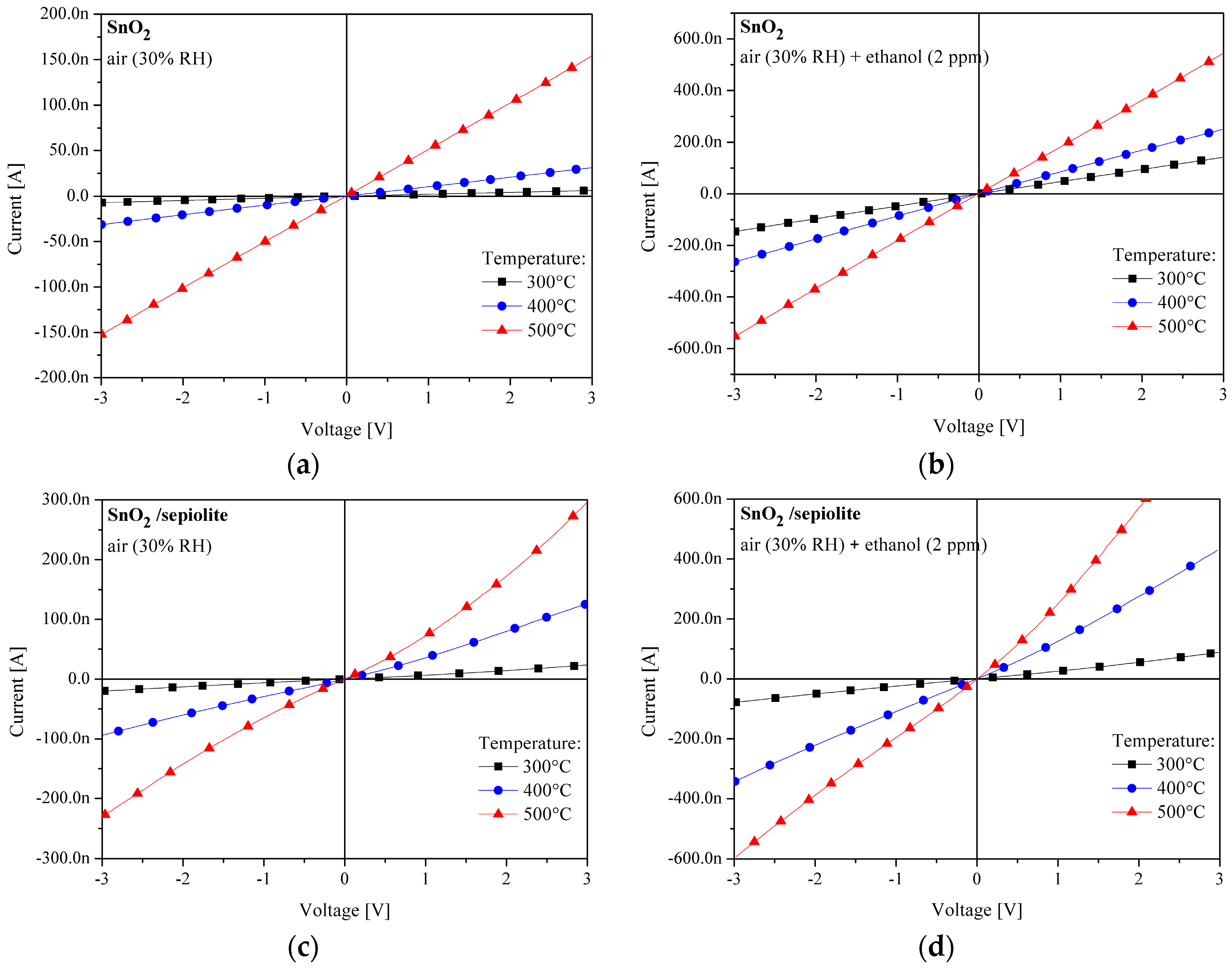
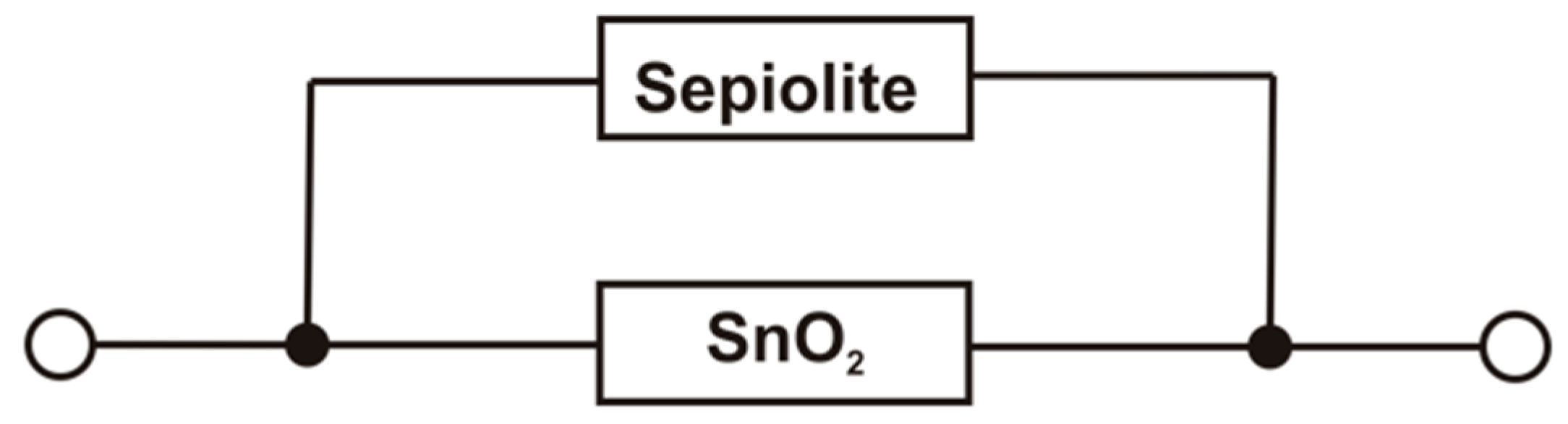
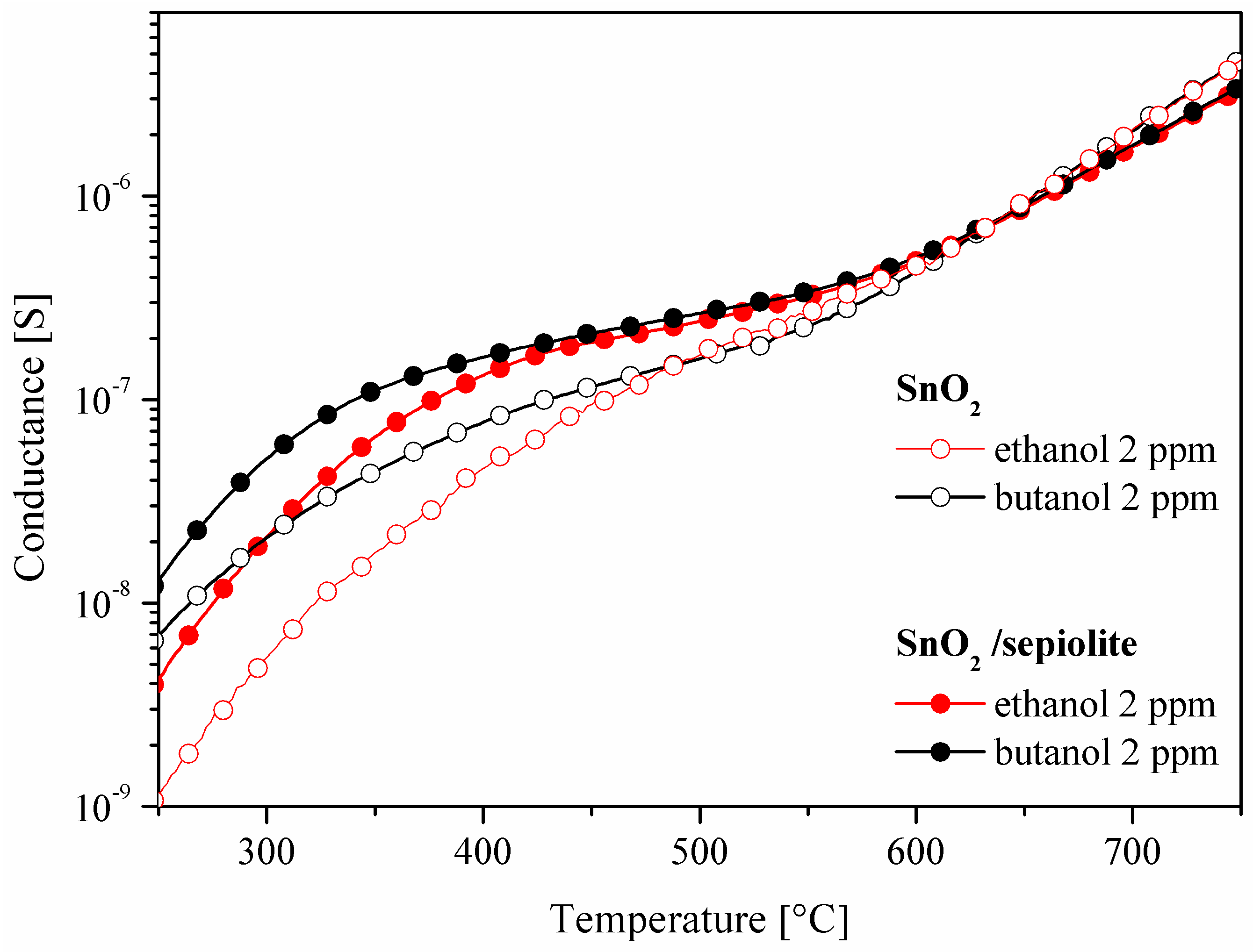
3.2. Current-Voltage Measurements of SnO2 and SnO2/Sepiolite Sensor
- 1
- Metal/metal oxide formed at the phase boundary between the gold electrode and a gas-sensitive material (Au/SnO2—area No. 1), (SnO2/Au—area No. 2),
- 2
- Metal oxide/metal oxide occurring inside the gas-sensitive layer between the individual grains (SnO2/SnO2—area No. 3),
- 3
- Metal oxide/sepiolite, created as a result of the imposition of a passive filter layer (SnO2/sepiolite—area No. 4), (sepiolite/SnO2—area No. 5),
- 4
- Sepiolite/sepiolite, occurring between the fibers of the filter (area No. 6).
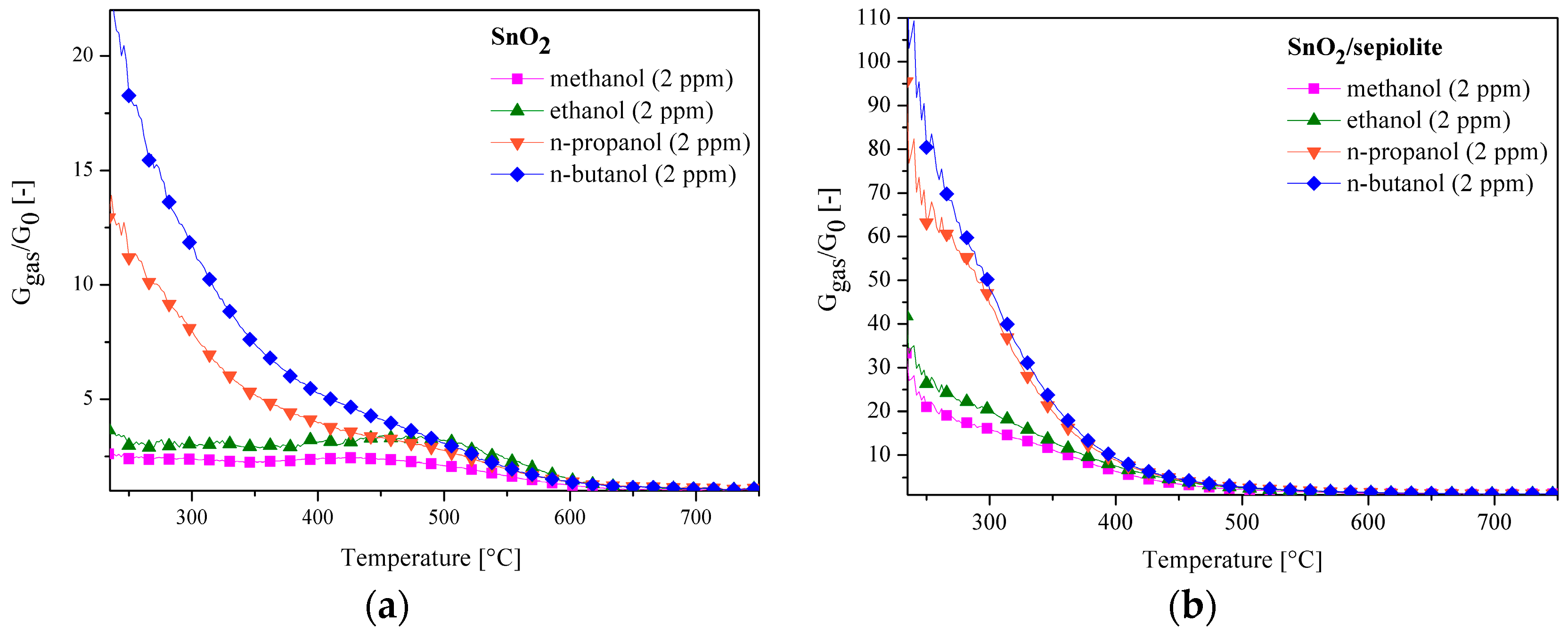
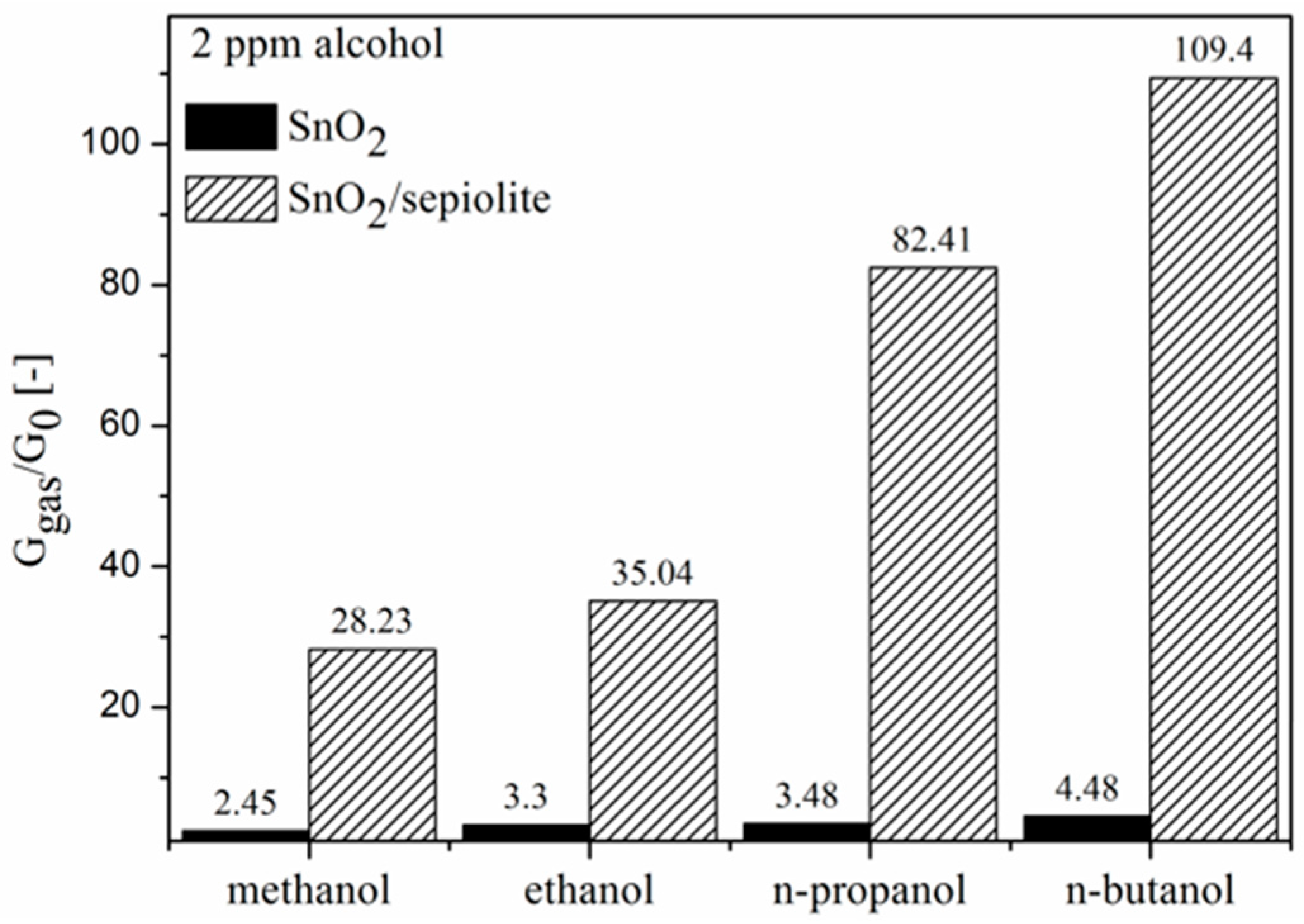
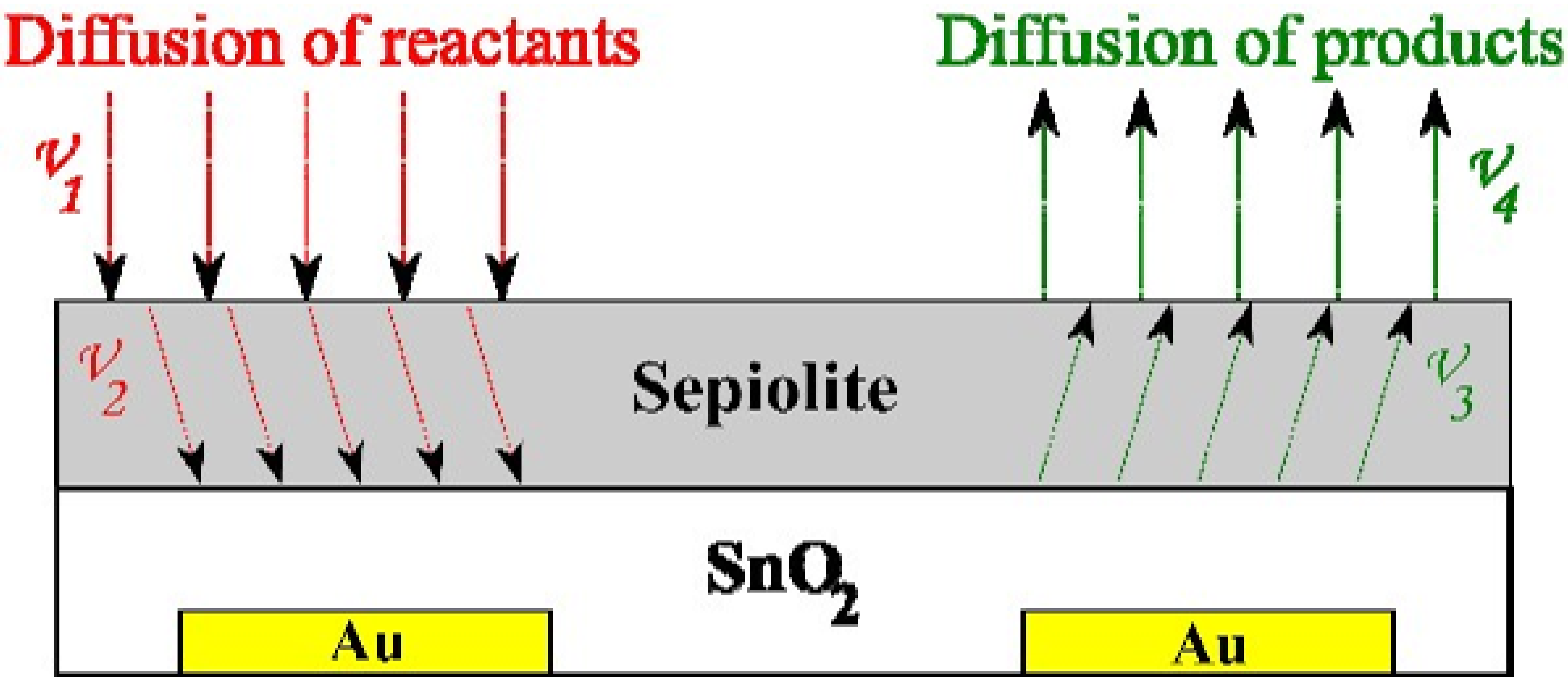
3.3. C1–C4 Alcohol Detection Using SnO2 and SnO2/Sepiolite Sensor
- is the conductance of the active layer,
- is the conductance of the sepiolite layer.
- oxygen to the surface of tin dioxide,
- the determined compounds to the surface of the sensor material,
- the reaction products (7)–(10) from the surface of the sensor material.
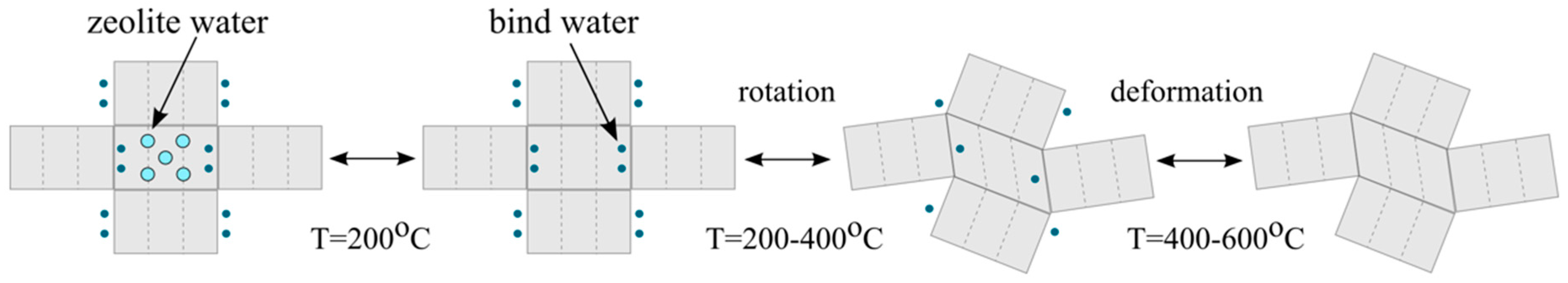
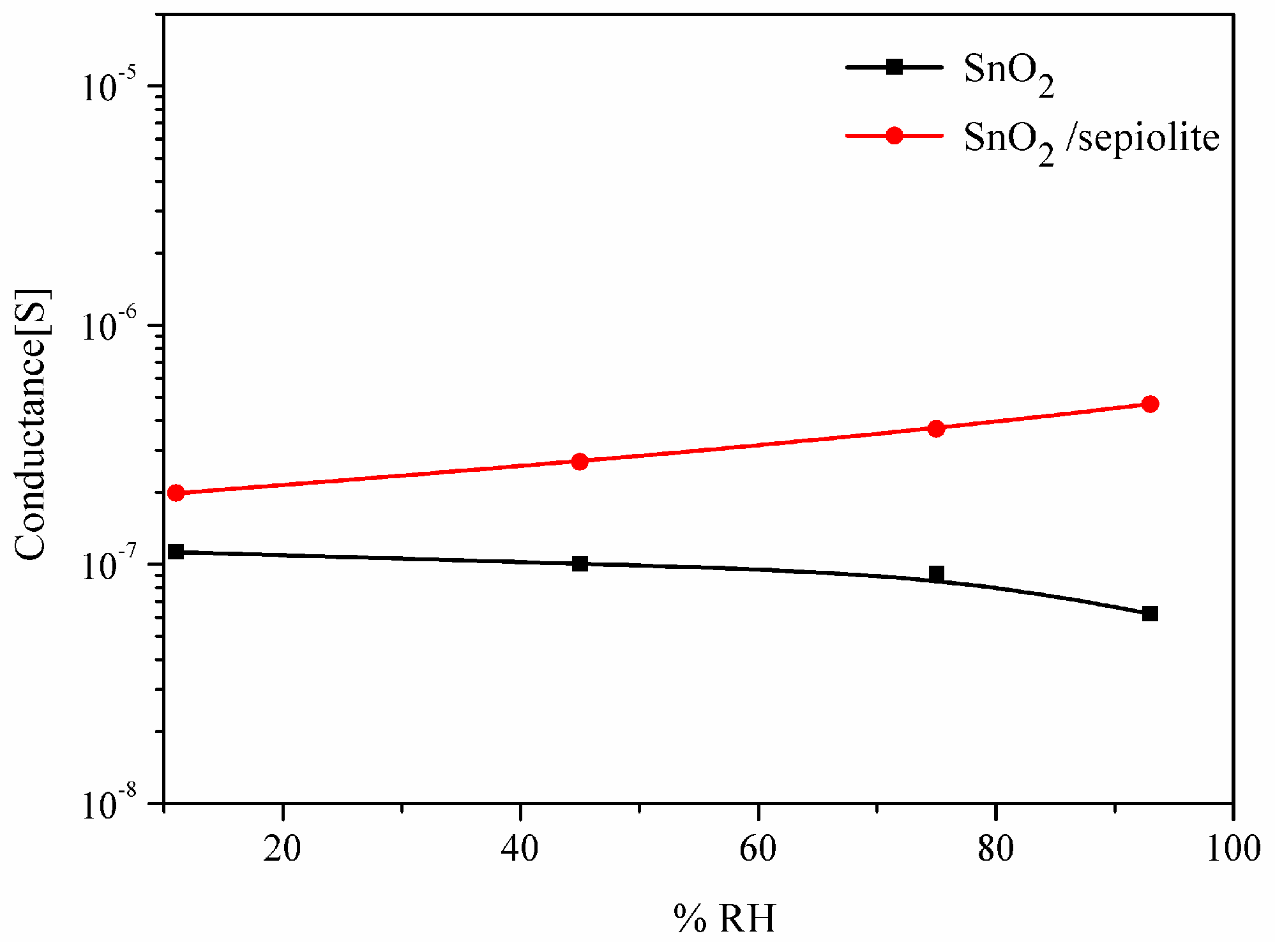
3.4. The Impact of Humidity on the Sensor
- hygroscopic, formed as a result of the adsorption of H2O molecules on the sepiolite surface, with the amount of water depending on the humidity of the air,
- zeolitic, located in the channels of the sepiolite;
- the molecules bound on the boundaries of the layers that have the octahedral system of network nodes;
- structural, bound in the form of hydroxyl groups in octahedral layers.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cabot, A.; Arbiol, J.; Cornet, A.; Morante, J.R.; Chen, F.; Liu, M. Mesoporous catalytic filters for semiconductor gas sensors. Thin Solid Films 2003, 436, 64–69. [Google Scholar] [CrossRef]
- Oliaee, S.N.; Khodadadi, A.; Mortazavi, Y.; Alipour, S. Highly selective Pt/SnO2 sensor to propane or methane in presence of CO and ethanol, using gold nanoparticles on Fe2O3 catalytic filter. Sens. Actuators B Chem. 2010, 147, 400–405. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Weh, T.; Fleischer, M.; Meixner, H. Optimization of physical filtering for selective high temperature H2 sensors. Sens. Actuators B Chem. 2000, 68, 146–150. [Google Scholar] [CrossRef]
- Licznerski, B.W.; Nitsch, K.; Teterycz, H.; Sobański, T.; Wiśniewski, K. Characterisation of electrical parameters for multilayer SnO2 gas sensors. Sens. Actuators B Chem. 2004, 103, 69–75. [Google Scholar] [CrossRef]
- Tournier, G.; Pijolat, C. Selective filter for SnO2-based gas sensor: Application to hydrogen trace detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Vilaseca, M.; Coronas, J.; Cirera, A.; Cornet, A.; Morante, J.R.; Santamaria, J. Gas detection with SnO2 sensors by zeolite films. Sens. Actuators B Chem. 2007, 124, 99–110. [Google Scholar] [CrossRef]
- Balci, S. Thermal decomposition of sepiolite and variations in pore structure with and without acid pre-treatment. J. Chem. Technol. Biotechnol. 1996, 66, 95–104. [Google Scholar] [CrossRef]
- Suchorska-Woźniak, P.; Nawrot, W.; Rac, O.; Fiedot, M.; Teterycz, H. Improving the sensitivity of the ZnO gas sensor to dimethyl sulfide. In Proceedings of the 39th International Microelectronics and Packaging IMAPS Poland Conference, Gdansk, Poland, 20–23 September 2015.
- Suchorska-Woźniak, P.; Halek, G.; Halek, P.; Teterycz, H. The influence of sepiolite filter on the conductance of resistive gas sensor based on SnO2. In Proceedings of the 2012 International Students and Young Scientists Workshop “Photonics and Microsystems”: International Optoelectronics Workshop, Szklarska Poręba, Poland, 6–8 July 2012.
- Sen, S.; Chakraborty, N.; Rana, P.; Narjinary, M.; Mursalin, Sk.d.; Tripathy, S.; Pradhan, D.K.; Sen, A. Nanocrystalline gallium ferrite: A novel material for sensing very low concentration of alcohol vapour. Ceram. Int. 2015, 41, 10110–10115. [Google Scholar] [CrossRef]
- Wang, W.; Tian, Y.; Li, X.; Wang, X.; He, H.; Xu, Y.; He, C. Enhanced ethanol sensing properties of Zn-doped SnO2 porous hollow microspheres. Appl. Surf. Sci. 2012, 261, 890–895. [Google Scholar] [CrossRef]
- Mathew, T.L.; Pownraj, P.; Abdulla, S.; Pullithadathil, B. Technologies for clinical diagnosis using expired human breath analysis. Diagnostics 2015, 5, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Licznerski, B.W.; Nitsch, K.; Teterycz, H.; Szecówka, P.M.; Wiśniewski, K. Humidity insensitive thick-film methane sensor based on SnO2/Pt. Sens. Actuators B Chem. 1999, 57, 192–196. [Google Scholar] [CrossRef]
- Suchorska-Woźniak, P.; Rac, O.; Fiedot, M.; Teterycz, H. Analysis of SnO2|WO3 heterocontact properties during the detection of hydrogen sulphide. Sensors 2014, 14, 20480–20499. [Google Scholar] [CrossRef] [PubMed]
- Alkan, M.; Benlikaya, R. Poly(vinyl alcohol) nanocomposites with sepiolite and heat-treated sepiolites. J. Appl. Polym. Sci. 2009, 112, 3764–3774. [Google Scholar] [CrossRef]
- Esmer, K.; Yeniyol, M. Current–voltage characteristics and aging of sepiolite oriented by magnetic field. Mater. Lett. 1999, 38, 445–449. [Google Scholar] [CrossRef]
- Lokanatha, S.; Bhattacherjee, S. Variation of electrical properties during dehydration. Bull. Mater. Sci. 1985, 7, 111–115. [Google Scholar] [CrossRef]
- D’Espinose de la Caillerie, J.-B.; Fripiat, J.J. Al modified sepiolite as catalyst or catalyst support. Catal. Today 1992, 14, 125–140. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Teterycz, H. Thick-Film Chemical Gas Sensors on the Basis of Tin Dioxide; Oficyna Wydawnicza Politechniki Wrocławskiej: Wroclaw, Poland, 2005. [Google Scholar]
- Chang, S.C. Sensing Mechanisms in Thin Film Tin Oxide. In Chemical Sensors; Seiyama, T., Fueki, K., Shiokawa, J., Suzuki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Martin, D.; Kaur, P.; Duprez, D.; Gaigneaux, E.F.; Ruiz, P.; Delmon, B. Impact of surface mobility in selective oxidation. Isotopic exchange of 18O2 with 16O2 on various oxides: MoO3, SnO2 and Sb2O4. Effect of reducer gas. Catal. Today 1996, 32, 329–336. [Google Scholar] [CrossRef]
- McAleer, J.F.; Moseley, P.T.; Norris, J.O.W.; Williams, D.E. Tin Dioxide gas sensors. Part 1—Aspects of the surface chemistry revealed by electrical conductance variations. J. Chem. Soc. Faraday Trans. 1987, 83, 1323–1346. [Google Scholar] [CrossRef]
- Yener, N.; Önal, M.; Üstünışık, G.; Sarıkaya, Y. Thermal behavior of a mineral mixture of sepiolite and dolomite. J. Therm. Anal. Calorim. 2007, 88, 813–817. [Google Scholar] [CrossRef]
- Teterycz, H.; Klimkiewicz, R.; Licznerski, B.W. The behaviour of gas sensitive materials in the atmosphere with oxygen shortage. In Proceedings of the 26th International Spring Seminar of Electronics Technology, High Tatras, Slovakia, 8–11 May 2003.
- Vivaldi, J.L.M.; Hach-Ali, P.F. Differential Thermal Analysis; Academic Press: London, UK, 1969. [Google Scholar]
- Bailey, S.W. Structures of layer silicates. In Crystal Structures of Clay Minerals and Their X-ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society: London, UK, 1980; pp. 2–123. [Google Scholar]

| Compound | Chemical Formula | μ [D] | εr [-] | Sensitivity | |
|---|---|---|---|---|---|
| without Filter | with Filter | ||||
| Methanol | CH3OH | 1.70 | 33.00 | 2.45 | 28.23 |
| Ethanol | CH3CH2OH | 1.69 | 24.30 | 3.30 | 35.04 |
| n-propanol | CH3CH2CH2OH | 1.68 | 20.10 | 3.48 | 82.41 |
| n-butanol | CH3CH2CH2CH2OH | 1.66 | 17.80 | 4.48 | 109.40 |
| Process No. | Material | Temp. [°C] | Physical and Chemical Changes | References |
|---|---|---|---|---|
| (1) | Sepiolite | >50 °C | desorption of from surface of sepiolite | [16] |
| (2) | SnO2 | <130 °C | desorption of from surface of SnO2 | [24] |
| (3) | SnO2 | 150 °C | desorption of and transformation of begins | [20] |
| (4) | Sepiolite | 246 °C | release of zeolitic water | [16] |
| (5) | SnO2 | 280 °C | decrease the amount of water | [24] |
| (6) | SnO2 | 400 °C | desorption of water formed from | [20] |
| (7) | Sepiolite | 450 and 494 °C | release of water molecules located in nodes of the octahedral system | [25] |
| (8) | SnO2 | 520 °C | desorption of | [20] |
| (9) | SnO2 | >550 °C | thermal dissociation of SnO2 | [26] |
| (10) | Sepiolite | 780–833 °C | removal of of sepiolite | [25] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchorska-Woźniak, P.; Rac, O.; Fiedot, M.; Teterycz, H. The Impact of Sepiolite on Sensor Parameters during the Detection of Low Concentrations of Alcohols. Sensors 2016, 16, 1881. https://doi.org/10.3390/s16111881
Suchorska-Woźniak P, Rac O, Fiedot M, Teterycz H. The Impact of Sepiolite on Sensor Parameters during the Detection of Low Concentrations of Alcohols. Sensors. 2016; 16(11):1881. https://doi.org/10.3390/s16111881
Chicago/Turabian StyleSuchorska-Woźniak, Patrycja, Olga Rac, Marta Fiedot, and Helena Teterycz. 2016. "The Impact of Sepiolite on Sensor Parameters during the Detection of Low Concentrations of Alcohols" Sensors 16, no. 11: 1881. https://doi.org/10.3390/s16111881
APA StyleSuchorska-Woźniak, P., Rac, O., Fiedot, M., & Teterycz, H. (2016). The Impact of Sepiolite on Sensor Parameters during the Detection of Low Concentrations of Alcohols. Sensors, 16(11), 1881. https://doi.org/10.3390/s16111881







