Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application
Abstract
:1. Introduction
2. Printable Electrochemical DNA Biosensors
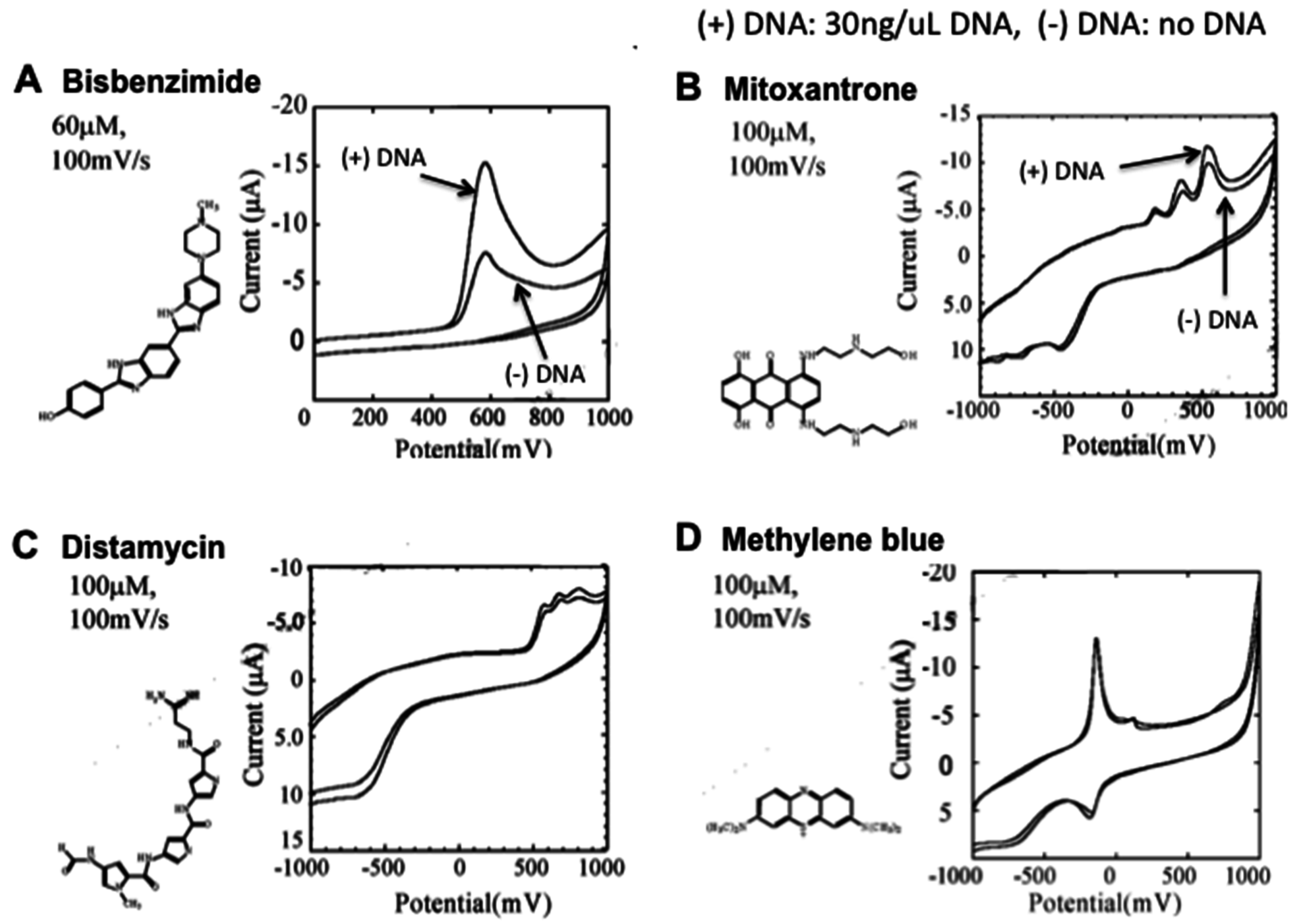
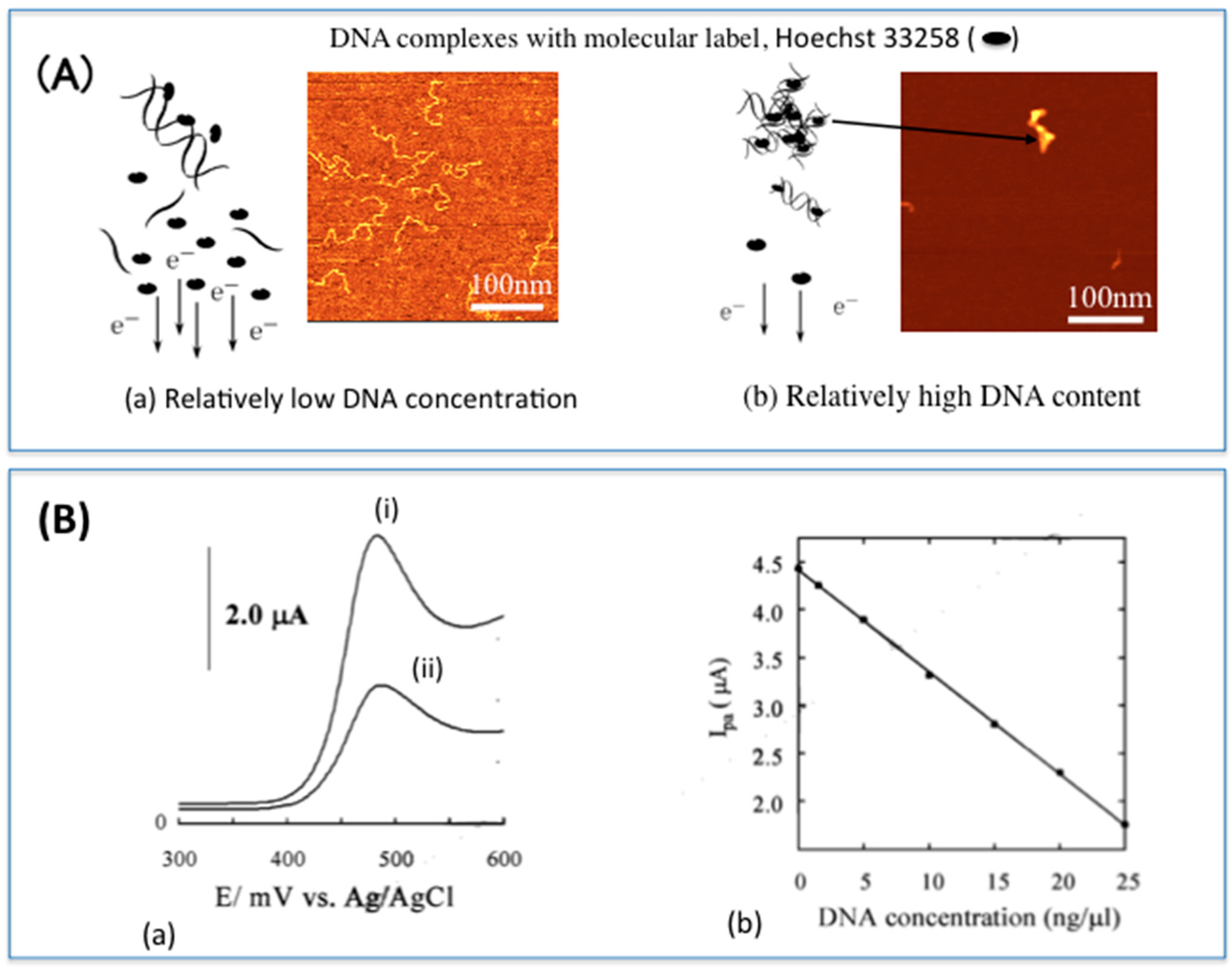
2.1. Electrochemical DNA Biosensors
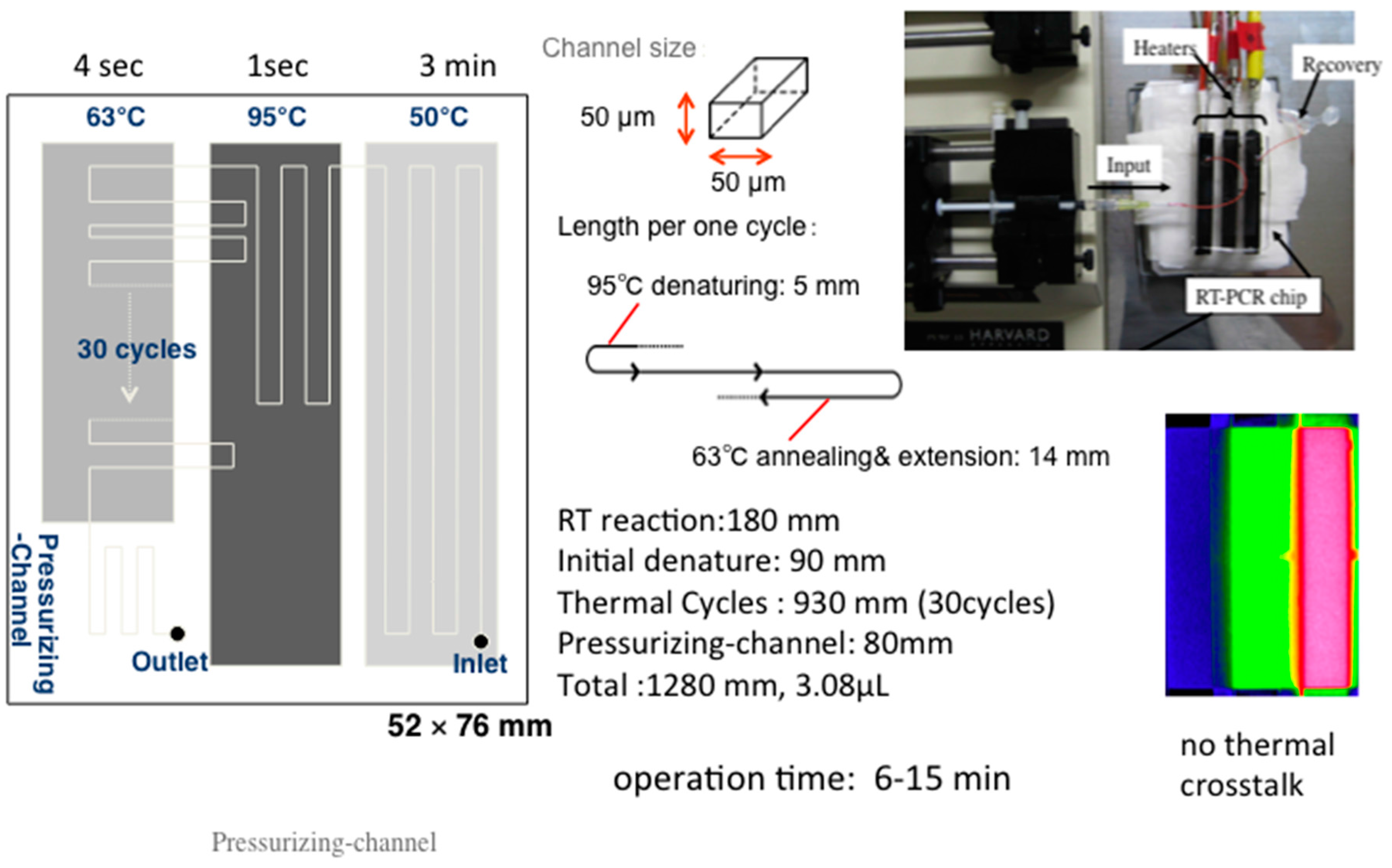
2.2. Integration of DNA Biosensors with Microfluidics RT-PCR
Microfluidic RT-PCR Technique for Detection of Influenza Virus
3. Other Printable Electrochemical Biosensors
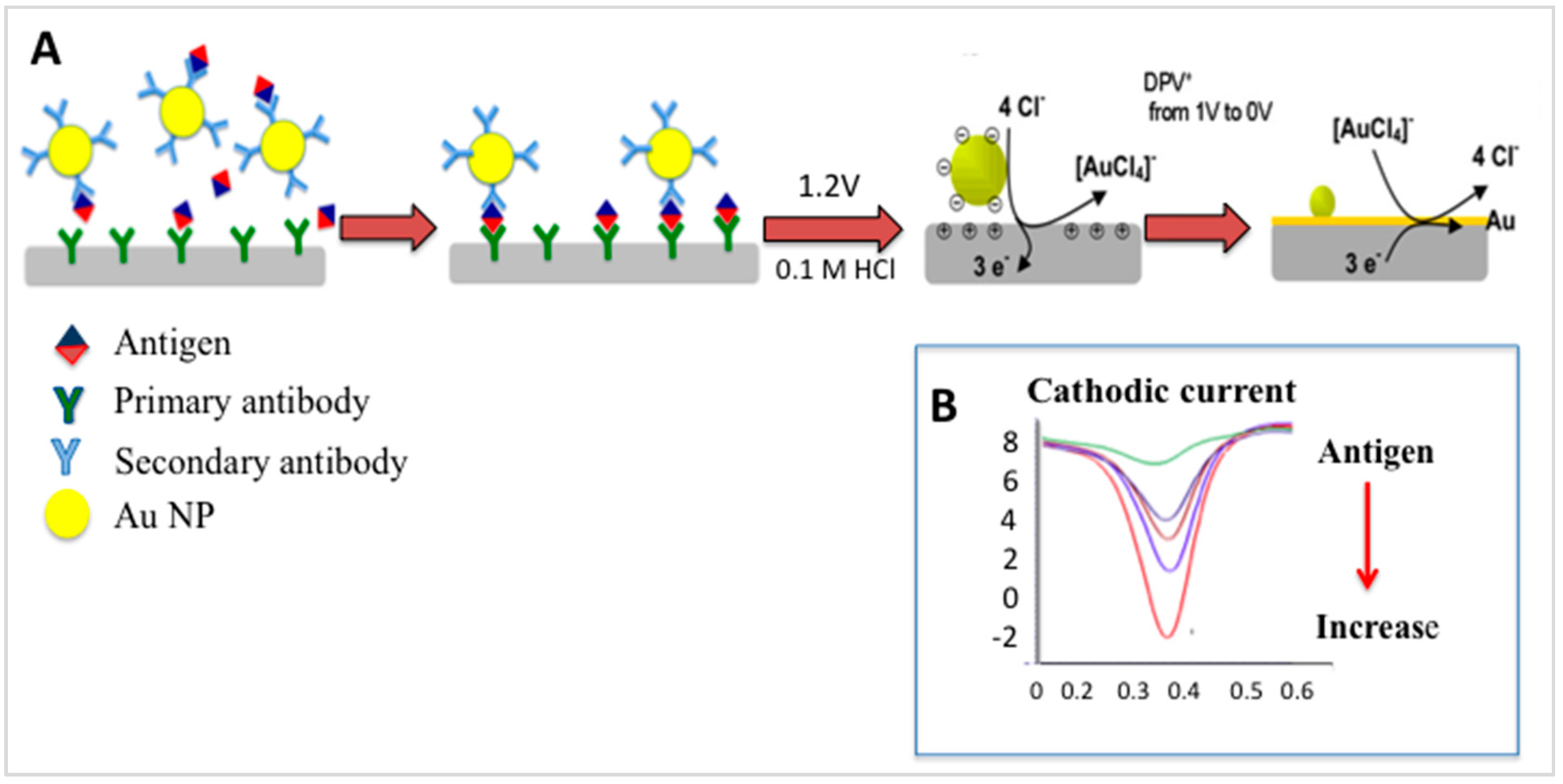
3.1. Electrochemical Immunosensors
Gold-Linked Electrochemical Immuno-Assay (GLEIA)
3.2. Electrochemical Enzyme-Based Biosensors
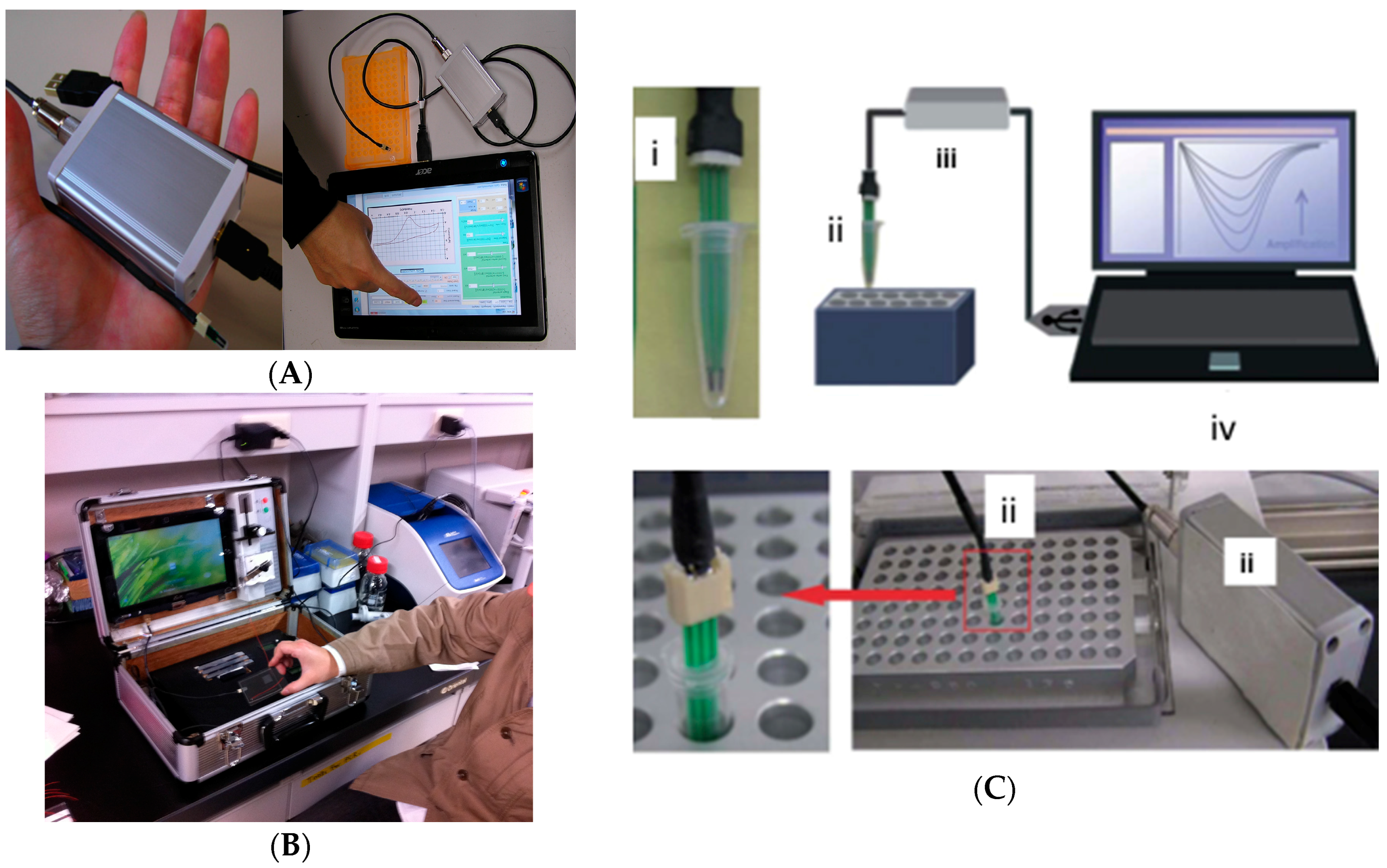
4. Miniaturized Printed and Portable Electrochemical Biosensors
5. Future Outlook and Concluding Remarks
Conflicts of Interest
References
- Rasooly, A.; Herold, K.E. Biosensor Technologies. In Biosensors and Biodetection, Methods and Protocols; Rasooly, A., Herold, K.E., Eds.; Humana Press: New York, NY, USA, 2009. [Google Scholar]
- Andreescu, S.; Sadik, O.A. Trends and challenges in biochemical sensors for clinical and environmental monitoring. Pure Appl. Chem. 2004, 76, 861–878. [Google Scholar] [CrossRef]
- Turner, A. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.C.; Tamiya, E. Introduction to Nanobiosensors and Nanobioanalyses. In Nanobiosensors and Nanobioanalyses; Vestergaard, M.C., Kerman, K., Hsing, I.-M., Tamiya, E., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–20. [Google Scholar]
- Iqbal, S.S.; Mayo, M.W.; Bruno, D.G.; Bronk, B.V. A review of molecular recognition technologies for detection of biological threat agents. Biosens. Bioelectron. 2000, 15, 549–578. [Google Scholar] [CrossRef]
- Turner, A.P.E. Biochemistry: Biosensors—Sense and sensitivity. Science 2000, 290, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Zuzuaregui, A.; Souto, D.; Perez-Lorenzo, E.; Sanches-Gomez, S.; Martinez de Tejara, G.; Brandenburg, K.; Arana, S.; Mujika, M. Novel integrated and portable endotoxin detection system based on an electrochemical biosensor. Analyst 2015, 140, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Cesarino, I.; Moraes, F.C.; Lanza, M.R.V.; Machado, S.A.S. Electrochemical detection of carbamate pesticides in fruit and vegetables with a biosensor based on acetylcholinesterase immobilised on a composite of polyaniline-carbon nanotubes. Food Chem. 2012, 135, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.N.Y.; Chikae, M.; Ukita, Y.; Takamura, Y. Labelless impedance immunosensor based on polypyrrole-pyrolecarboxylic acid copolymer for hCG detection. Talanta 2011, 85, 2576–2580. [Google Scholar]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Estrela, P. Field-effect transistors: Current advances and challenges in bringing them to point-of-care. In Nanobiosensors and Nanobioanalyses; Vestergaard, M.C., Kerman, K., Hsing, I.-M., Tamiya, E., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–20. [Google Scholar]
- Roberts, G. History’s influence on screen printing’s future. Screen Print. 2006, 96, 22–25. [Google Scholar]
- Hyun, W.J.; Park, O.O.; Chin, B.D. Foldable graphene electronic circuits based on paper substrates. Adv. Mater. 2013, 25, 4729–4734. [Google Scholar] [CrossRef] [PubMed]
- Washe, A.; Lozano-Sanchez, P.; Bejarano-Nosas, D. Facile and versatile approaches to enhancing electrochemical performance of screen printed electrodes. Electrochim. Acta 2013, 91, 166–172. [Google Scholar] [CrossRef]
- Sze, V.W.; Kerman, K. Carbon nanotubes: Advances, integration and application to printable electrochemical biosensors. In Nanobiosensors and Nanobioanalyses; Vestergaard, M.C., Kerman, K., Hsing, I.-M., Tamiya, E., Eds.; Springer: Tokyo, Japan, 2015; pp. 271–289. [Google Scholar]
- Retna, R.; Ohsaka, T. Analytical applications of functionalized self-assembled monolayers on gold electrod: Voltammetric sensing of DOPAC at the physiological level. Electroanalysis 2001, 14, 679–684. [Google Scholar]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent Developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 74, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Andreescu, S.; Marty, J.-L. Design of PEG-aptamer two piece macromolecules as convenient and integrated sensing platform: Application to the label free detection of small size molecules. Biosens. Bioelectron. 2013, 45, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Haider, W.; Rolland, M.; Marty, J.-L. Electrochemical grafting of long spacer arms of hexamethyldiamine on a screen printed carbon electrode surface: Application in target induced ochratoxin A electrochemical aptasensor. Analyst 2013, 138, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.N.T.; Takamura, Y.; Tamiya, E.; Vestergaard, M.C. Modified screen printed electrode for development of a highly sensitive label-free impedimetric immunosensor to detect amyloid beta peptides. Anal. Chim. Acta 2015, 892, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Gorski, W.; Garcia, C.D. Nanomolar Detection of Glutamate at a Biosensor Based on Screen-Printed Electrodes Modified with Carbon Nanotubes. Electroanalysis 2011, 10, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Simon, B.; Macanas, J.; Munoz, M.; Fabregas, E. Evaluation of different mediator-modified screen-printed electrodes used in a flow system as amperometric sensors for NADH. Talanta 2007, 71, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Simon, B.; Fabregas, E. New redox mediator-modified polysulfone composite films for the development of dehydrogenase-based biosensors. Biosens. Bioelectron. 2006, 22, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Millan, K.M.; Mikkelsen, S.R. Sequence-selective biosensor for DNA based on electroactive hybridization indicators. Anal. Chem. 1993, 65, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Z.; Cai, Q.; Peng, F.-Y.; Huang, X.-X.; Jia, Y.-L. Screen-printed electrochemical biosensor for detection of DNA hybridization. Chin. J. Anal. Chem. 2012, 40, 1194–1200. [Google Scholar] [CrossRef]
- Dai, L.; Hai, B.; Van Hieu, N.; Vinh, H. Electrochemical detection of short HIV sequences on chitosan/Fe3O4 nanoparticle based screen printed electrodes. Mater. Sci. Eng. C 2011, 31, 477–485. [Google Scholar]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Odenthal, K.L.; Gooding, J.J. An introduction to electrochemical DNA biosensors. Analyst 2007, 132, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, A.A.; Buzzeo, M.C.; Barton, J.K. DNA-mediated electrochemistry. Bioconj. Chem. 2008, 19, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.J.; Wan, Y.; Yowanto, H.; Li, J.; Tao, C.; James, M.D.; Tan, C.L.; Blackburn, G.F.; Meade, T.J. Electronic Detection of Single-Base Mismatches in DNA with Ferrocene-Modified Probes. J. Am. Chem. Soc. 2001, 123, 11155–11161. [Google Scholar] [CrossRef] [PubMed]
- Ozsoz, M.; Erdem, A.; Kerman, K.; Ozkan, D.; Tugrul, B.; Topcouglu, N.; Ekren, H.; Taylan, M. Electrochemical genosensor based on colloidal gold nanoparticles for the detection of Factor V Leiden mutation using disposable pencil graphite electrodes. Anal. Chem. 2003, 75, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Paredes, G.; González-García, M.B.; Costa-García, A. Genosensor for detection of four pneumoniae bacteria using gold nanostructured screen-printed carbon electrodes as transducers. Sens. Actuators B Chem. 2010, 149, 329–335. [Google Scholar] [CrossRef]
- Galandová, J.; Ovádeková, R.; Ferancová, A.; Labuda, J. Disposable DNA biosensor with the carbon nanotubes-polyethyleneimine interface at a screen-printed carbon electrode for tests of DNA layer damage by quinazolines. Anal. Bioanal. Chem. 2009, 394, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Vestergaard, M.; Nagatani, N.; Takamura, Y.; Tamiya, E. Electrochemical Genosensor Based on Peptide Nucleic Acid-Mediated PCR and Asymmetric PCR Techniques: Electrostatic Interactions with a Metal Cation. Anal. Chem. 2006, 78, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Congur, G.; Eksin, E. Multi channel screen printed array of electrodes for enzyme-linked voltammetric detection of MicroRNAs. Sens. Actuators B Chem. 2013, 188, 1089–1095. [Google Scholar] [CrossRef]
- LaGier, M.J.; Scholin, C.A.; Fell, J.W.; Wang, J.; Goodwin, K.D. An electrochemical RNA hybridization assay for detection of the fecal indicator bacterium Escherichia coli. Mar. Pollut. Bull. 2005, 50, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, H.-H.; Heller, A. Enzyme-Amplified Amperometric Detection of 3000 Copies of DNA in a 10-μL Droplet at 0.5 fM Concentration. Anal. Chem. 2003, 75, 3267–3269. [Google Scholar] [CrossRef] [PubMed]
- Won, B.Y.; Shin, S.; Baek, S.; Jung, Y.L.; Li, T.; Shin, S.C.; Cho, D.Y.; Lee, S.B.; Park, H.G. Investigation of the signaling mechanism and verification of the performance of an electrochemical real-time PCR system based on the interaction of methylene blue with DNA. Analyst 2011, 136, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Yuan, H.Y.; Li, J.; Yu, R.Q. Surface-modified cobalt-based sensor as a phosphate-sensitive electrode. Anal. Chem. 1995, 67, 288–291. [Google Scholar] [CrossRef]
- Meruva, R.K.; Meyerhoff, M.E. Mixed potential response mechanism of cobalt electrodes toward inorganic phosphate. Anal. Chem. 1996, 68, 2022–2026. [Google Scholar]
- Defever, T.; Druet, M.; Rochelet-Dequaire, M.; Joannes, M. Real-Time Electrochemical Monitoring of the Polymerase Chain Reaction by Mediated Redox Catalysis. J. Am. Chem. Soc. 2009, 131, 11433–11441. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.W.; Lee, T.M.H.; Hsing, I.M. Electrochemical Real-Time Polymerase Chain Reaction. J. Am. Chem. Soc. 2006, 128, 13374–13375. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.W.; Lee, T.M.H.; Hsing, I.M. Electrochemistry-based real-time PCR on a microchip. Anal. Chem. 2008, 80, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Vestergaard, M.; Tamiya, E. Electrochemical DNA biosensors: Protocols for intercalator-based detection of hybridization in solution and at the surface. Methods Mol. Biol. 2009, 504, 99–113. [Google Scholar] [PubMed]
- Fang, T.H.; Ramalingam, N.; Dong, X.D.; Ngin, T.S.; Xianting, Z.; Lai, K.A.T.; Peng, H.T.Y.; Hai-Qing, G. Real-time PCR microfluidic devices with concurrent electrochemical detection. Biosens. Bioelectron. 2009, 24, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Saito, M.; Kondoh, K.; Hossain, M.M.; Koketsu, R.; Sasaki, T.; Nagatani, N.; Ikuta, K.; Tamiya, E. Rapid detection for primary screening of influenza A virus: Microfluidic RT-PCR chip and electrochemical DNA sensor. Analyst 2011, 136, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Defever, T.; Druet, M.; Evard, D.; Marchal, D.; Limoges, B. Real-time electrochemical PCR with a DNA intercalating redox probe. Anal. Chem. 2011, 83, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Mizukami, T.; Morita, Y.; Murakami, Y.; Yokoyama, K.; Tamiya, E. Electrochemical gene detection using microelectrode array on DNA chip. Electrochemistry 2001, 69, 1013–1016. [Google Scholar]
- Yamanaka, K.; Sekine, S.; Uenoyama, T.; Wada, M.; Ikeuchi, T.; Saito, M.; Yamaguchi, Y.; Tamiya, E. Quantitative Detection for Porphyromonas gingivalis in Tooth Pocket and Saliva by Portable Electrochemical DNA Sensor Linked with PCR. Electroanalysis 2014, 26, 2686–2692. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kusakawa, T.; Saito, M.; Kaji, S.; Oomura, M.; Iwabuchi, S.; Morita, Y.; Hasan, Q.; Tamiya, E. Electrochemical DNA quantification based on aggregation induced by Hoechst 33258. Electrochem. Commun. 2004, 6, 337–343. [Google Scholar] [CrossRef]
- Yamanaka, K.; Ikeuchi, T.; Saito, M.; Nagatani, N.; Tamiya, E. Electrochemical detection of specific DNA and respiratory activity of Escherichia coli. Electrochim. Acta 2012, 82, 132–136. [Google Scholar] [CrossRef]
- Carter, M.T.; Rodriguez, M.; Bard, A.J. Voltammetric studies of the interaction of metal chelates with DNA. 2. Tris-chelated complexes of cobalt(III) and iron(II) with 1,10-phenanthroline and 2,2′-bipyridine. J. Am. Chem. Soc. 1989, 111, 8901–8911. [Google Scholar] [CrossRef]
- Wang, S.; Peng, T.; Yang, C.F.J. Investigation on the interaction of DNA and electroactive ligands using a rapid electrochemical method. Biochem. Biophys. Methods 2003, 55, 191–204. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic paper-based analytical devices (μPADs) and micro total analysis systems (μTAS): Development, applications and future trends. Chromatographia 2013, 76, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Schneegass, I.; Brautigam, R.; Kohler, J.M. Miniaturized flow-through PCR with different template types in a silicon chip thermocycler. Lab Chip 2001, 1, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Ferguson, B.S.; Eisenstein, M.; Plaxco, K.W.; Soh, H.T. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 2015, 48, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.S.; Heithoff, D.M.; Ferguson, B.S.; Soh, H.T.; Mahan, M.J.; Plaxco, K.W. Microfluidic chip-based detection and intraspecies strain discrimination of Salmonella serovars derived from whole blood of septic mice. Appl. Environ. Microbiol. 2013, 79, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, S.; Kim, Y.T.; Jung, J.H.; Jim, D.H.; Seo, T.S. A fully integrated microdevice for biobarcode assay based biological agent detection. Lab Chip 2015, 15, 2744–2748. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.U.; Mello, A.J.; Manz, A. Chemical Amplification: Continuous-Flow PCR on a Chip. Science 1998, 280, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zuo, X.; Li, Z.; Deng, W.; Shi, J.; Zhang, G.; Huang, Q.; Song, S.; Fan, C. A Bubble-Mediated Intelligent Microscale Electrochemical Device for Single-Step Quantitative Bioassays. Adv. Mater. 2014, 26, 4671–4676. [Google Scholar] [CrossRef] [PubMed]
- WHO Information for Laboratory Diagnosis of Pandemic (H1N1) 2009 Virus in Humans-Revised. Available online: http://www.who.int/csr/resources/publications/swineflu/WHO_Diagnostic_RecommendationsH1N1_20090521.pdf (accessed on 3 March 2010).
- Nafisi, S.; Saboury, A.A.; Keramat, N.; Neault, J.F.; Tajimir-Riahi, H.A. Stability and structural features of DNA intercalation with ethidium bromide, acridine orange and methylene blue. J. Mol. Struct. 2007, 827, 35–43. [Google Scholar] [CrossRef]
- Zhao, G.-C.; Zhu, J.-J.; Chen, H.-Y. Spectroscopic studies of the interactive model of methylene blue with DNA by means of β-cyclodextrin. Spectrochim. Acta Part A 1999, 55, 1109–1117. [Google Scholar] [CrossRef]
- Yang, W.; Ozsoz, M.; Hibbert, D.B.; Gooding, J.J. Evidence for the direct interaction between methylene blue and guanine bases using DNA-modified carbon paste electrodes. Electroanalysis 2002, 14, 1299–1302. [Google Scholar] [CrossRef]
- Tani, A.; Thomson, A.J.; Butt, J.N. Methylene blue as an electrochemical discriminator of single- and double-stranded oligonucleotides immobilised on gold substrates. Analyst 2001, 126, 1756–1759. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Ozkan, D.; Kara, P.; Meric, B.; Gooding, J.J.; Oszos, M. Voltammetric determination of DNA hybridization using methylene blue and self-assembled alkanethiol monolayer on gold electrodes. Anal. Chim. Acta 2002, 462, 39–47. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Idegami, K.; Chikae, M.; Kerman, K.; Chaumpluk, P.; Shimamura, S.; Tamiya, E. Electrochemical DNA biosensor using a disposable electrochemical printed (DEP) chip for the detection of SNPs from unpurified PCR amplicons. Analyst 2007, 132, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Hasan, Q.; Hossain, M.M.; Saito, M.; Tamiya, E. Meat species identification based on the loop mediated isothermal amplification and electrochemical DNA sensor. Food Control 2010, 21, 599–605. [Google Scholar] [CrossRef]
- Yalow, R.S.; Berson, S.A. Assay of plasma insulin in human subjects by immunological methods. Lett. Nat. 1959, 184, 1648–1649. [Google Scholar] [CrossRef]
- Dou, W.; Tang, W.; Zhao, G. A disposable electrochemical immunosensor arrays using 4-channel screen-printed carbon electrode for simultaneous detection of Escherichia coli O157:H7 and Enterobacter sakazakii. Electrochim. Acta 2013, 37, 79–85. [Google Scholar] [CrossRef]
- San, L.; Zeng, D.; Song, S.; Zuo, X.; Zhang, H.; Wang, C.; Wu, J.; Mi, X. An electrochemical immunosensor for quantitative detection of ficolin-3. Nanotechnology 2016, 27, 254003. [Google Scholar] [CrossRef] [PubMed]
- Vig, A.; Muñoz-Berbel, X.; Radoi, A.; Cortina-Puig, M.; Marty, J.-L. Characterization of the gold-catalyzed deposition of silver on graphite screen-printed electrodes and their application to the development of impedimetric immunosensors. Talanta 2009, 80, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.E.; Vermeer, A.W.P.; Norde, W. Adsorption of immunoglobulin G on core-shell latex particles precoated with chaps. J. Colloid Interf. Sci. 2000, 231, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Charelier, R.C.; Gengenbach, T.R.; Griesser, H.J.; Brigham-Burke, M.; O’Shannessy, D.J. A general method to recondition and reuse BIAcore sensor chips fouled with covalently immobilized protein/peptide. Anal. Biochem. 1995, 229, 112–118. [Google Scholar] [CrossRef]
- Charles, P.T.; Goldman, E.R.; Rangasammy, J.G.; Schauer, C.L.; Chen, M.S.; Taitt, C.R. Fabrication and characterization of 3D hydrogel microarrays to measure antigenicity and antibody functionality for biosensor application. Biosens. Bioelectron. 2004, 20, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Palma, R.; Borghs, G.; Declerck, P.; Goddeeris, B. Comparison of random and oriented immobilization of antibody fragments on mixed self-assembled monolayers. J. Immunol. Methods 2006, 312, 167–181. [Google Scholar]
- Xu, Z.; Yin, H.; Tian, Z.; Zhou, Y.; Ai, S. Electrochemical immunoassays for the detection of the activity of DNA methyltransferase by using the rolling circle amplification technique. Microchim. Acta 2014, 181, 471–477. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, M.B.; Fernandez-Sanchez, C.; Costa-Garcia, A. Colloidal gold as an electrochemical label of streptavidin-biotin interaction. Biosens. Bioelectron. 2000, 15, 315–321. [Google Scholar] [CrossRef]
- Dequaire, M.; Degrand, C.; Limoges, B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal. Chem. 2000, 72, 5521–5528. [Google Scholar] [CrossRef] [PubMed]
- Chikae, M.; Idegami, K.; Kerman, K.; Nagatani, N.; Ishikawa, M.; Takamura, Y.; Tamiya, E. Direct fabrication of catalytic metal nanoparticles onto the surface of a screen-printed carbon electrode. Electrochem. Commun. 2006, 8, 1375–1380. [Google Scholar] [CrossRef]
- Benson, J.; Fung, C.M.; Lioyd, J.S.; Deganello, D.; Smith, N.A.; Teng, K.S. Direct patterning of gold nanoparticles using flexographic printing for biosensing applications. Nanoscale Res. Lett. 2015, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Gómez, V.; Hernández-Santos, D.; González-García, M.B.; Pingarron-Carrazon, J.M.; Costa-Garcia, A. Simultaneous detection of free and total prostate specific antigen on a screen-printed electrochemical dual sensor. Biosens. Bioelectron. 2009, 24, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Idegami, K.; Chikae, M.; Kerman, K.; Nagatani, N.; Yuhi, T.; Tamiya, E. Gold Nanoparticle-Based Redox Signal Enhancement for Sensitive Detection of Human Chorionic Gonadotropin Hormone. Electroanalysis 2008, 20, 14–21. [Google Scholar] [CrossRef]
- Viet, N.X.; Chikae, M.; Ukita, Y.; Maehashi, K.; Matsumoto, K.; Tamiya, E.; Hung, P.; Takamura, Y. Gold-linked electrochemical immunoassay on single-walled carbon nanotube for highly sensitive detection of human chorionic gonadotropin hormone. Biosens. Bioelectron. 2013, 42, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardivascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Rahman, M.N.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Ispas, C.; Crivat, G.; Andreescu, S. Review: Recent developments in enzyme-based biosensors for biomedical analysis. Anal. Lett. 2012, 45, 168–186. [Google Scholar] [CrossRef]
- Dounin, V.; Veloso, A.J.; Schulze, H.; Backmann, T.T.; Kerman, K. Disposable electrochemical printed gold chips for the analysis of acetylcholinesterase inhibition. Anal. Chim. Acta 2010, 669, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lomillo, M.A.; Dominguez-Renedo, O.; Ferreira-Goncalves, L.; Arcos-Martinez, M.J. Sensitive enzyme-biosensor based on screen-printed electrodes for Ochratoxin A. Bionsens. Bioelectron. 2010, 25, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lomillo, M.A.; Dominguez-Renedo, O.; Hernandez-Martin, A.; Arcos-Martinez, M.J. Electrochemical determination of levetiracetam by screen-printed based biosensors. Bioelectrochemisyry 2009, 74, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lomillo, M.A.; Dominguez-Renedo, O.; Matos, P.; Arcos-Martinez, M.J. Disposable biosensors for determination of biogenic amines. Anal. Chim. Acta 2010, 665, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.A.; Messina, G.A.; Bertolino, F.A.; Salinas, E.; Raba, J. Screen-printed enzymatic biosensor modified with carbon nanotube for the methimazole determination in pharmaceuticals formulations. Sens. Actuators B Chem. 2008, 133, 256–262. [Google Scholar] [CrossRef]
- Chikae, M.; Kerman, K.; Nagatani, N.; Takamuya, Y.; Tamiya, E. An electrochemical on-field sensor forsystem for the detection of compost maturity. Anal. Chim. Acta 2007, 581, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Karuppiah, C.; Chen, S.-M.; Periakaruppan, P. A highly sensitive and selective enzymatic biosensor based on direct electrochemistry of haemoglobin at zinc oxide nanoparticles modified activated screen printed carbon electrode. Electroanalysis 2014, 26, 1984–1993. [Google Scholar] [CrossRef]
- Sanches, S.; Pumera, M.; Cabruja, E.; Fabregas, E. Carbon nanotube/polysulfone composite screen-printed electrochemical enzyme biosensors. Analyst 2007, 132, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Zhang, L.; Li, D.-W.; Karpuzov, D.; Long, Y.-T. Electrocatalytic oxidation of NADH on graphene oxide and reduces graphene oxide modified screen-printed electrode. Int. J. Electrochem. Sci. 2011, 6, 819–829. [Google Scholar]
- Ali, T.A.; Mohamed, G.G.; Azzam, E.M.S.; Abd-eaal, A.A. Thiol surfactant assembled on gold nanoparticles ion exchanger for screen-printed electrode fabrication. Potentiometric determination of Ce(III) in environmental polluted samples. Sens. Actuators B Chem. 2014, 191, 192–203. [Google Scholar] [CrossRef]
- Song, W.; Zhang, L.; Shi, L.; Li, D.-W.; Li, Y.; Long, Y.-T. Simulatneous determination of cadminum (II), lead (II) and copper (II) by using a screen-printed electrode modified with mercury nano-droplets. Microchim. Acta 2010, 169, 321–326. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensiing: A review (2008–2013). Mcrochim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Halder, A.; Zhang, M.; Chi, Q. Electroactive and biocompartiblefunctionalization of graphenefor the development of biosensing platforms. Biosens. Bioelectron. 2016, 87, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Kuralay, F.; Campuzano, S.; Haake, D.A.; Wang, J. Highly sensitive disposable nucleic acid biosensors for direct bioelectronic detection in raw biological samples. Talanta 2011, 85, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Baraket, A.; Lee, M.; Zine, N.; Sagaud, M.; Bausells, J.; Errachid, A. A fully integrated electrochemical biosensor platform fabrication process, for cytokins detection. Biosens. Bioelectron. 2014, 87, 377–379. [Google Scholar]
- Guanovat, T.; Valdes-Ramirez, G.; Windmiller, J.R.; Andrade, F.J.; Wang, J. Bandage-based wearable potentiometric sensorfor monitoring wound pH. Electroanalysis 2014, 26, 1345–1353. [Google Scholar]
- Jia, W.; Bandodkar, A.J.; Valdez-Ramirez, G.; Windmiller, J.R.; Yang, Z.; Ramirez, J.; Cha, G.; Wnag, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Bandokar, A.J.; Jia, W.; Yardimci, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, K.; Vestergaard, M.C.; Tamiya, E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016, 16, 1761. https://doi.org/10.3390/s16101761
Yamanaka K, Vestergaard MC, Tamiya E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors. 2016; 16(10):1761. https://doi.org/10.3390/s16101761
Chicago/Turabian StyleYamanaka, Keiichiro, Mun’delanji C. Vestergaard, and Eiichi Tamiya. 2016. "Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application" Sensors 16, no. 10: 1761. https://doi.org/10.3390/s16101761




