An Assessment of Three Different In Situ Oxygen Sensors for Monitoring Silage Production and Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brief Description of the Tested O2 Sensors
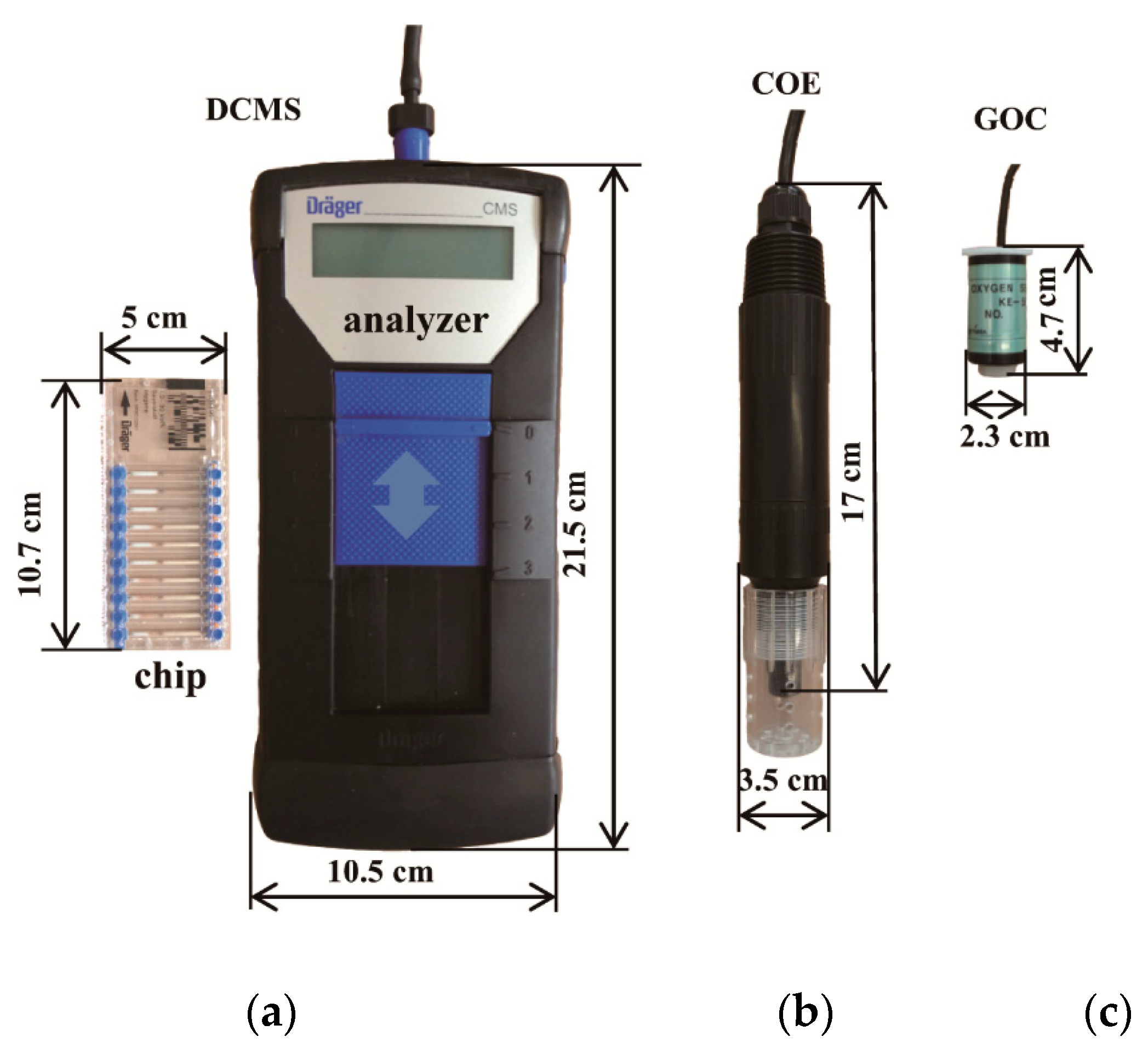
| Sensor | Type | Manufacturer | Output | Accuracy | Reaction | Electrolyte |
|---|---|---|---|---|---|---|
| COE | DO-10 | UNISM Technologies Inc., Beijing, China | 100–800 (mV) | ±0.2 mg/L | 4Ag + 4Cl− → 4AgCl + 4e− (anode) O2 + 2H2O + 4e− → 4OH− (cathode) | KCl |
| GOC | KE-50 | FIGARO Engineering Inc., Osaka, Japan | 0–65.0 (mV) | ±2 vol.% of full scale | 2Pb + 2H2O → 2PbO + 4H+ + 4e− (anode) O2 + 4H+ + 4e− → 2H2O (cathode) | H2SO4 |
| DCMS | 6406490 | Dräger Safety AG & Co. KGaA., Lübeck, Germany | 1–30 vol.% | ±15 vol.% of the measured value | O2 + TiCl3 → TiIVcompound + HCl | None |
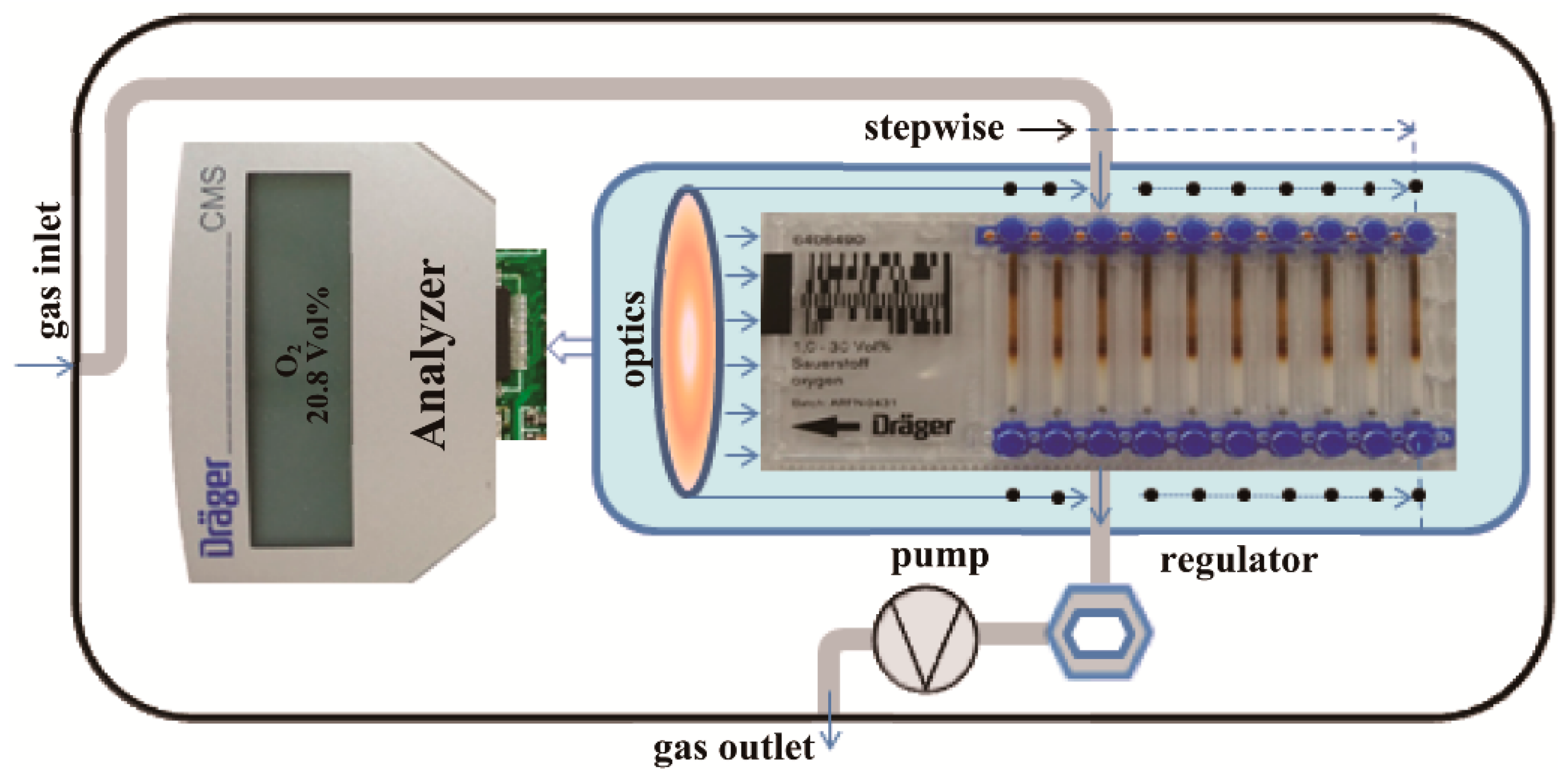
2.2. Cross-Calibration System for O2 Versus CO2

2.3. Cross Calibration of O2 Versus CO2
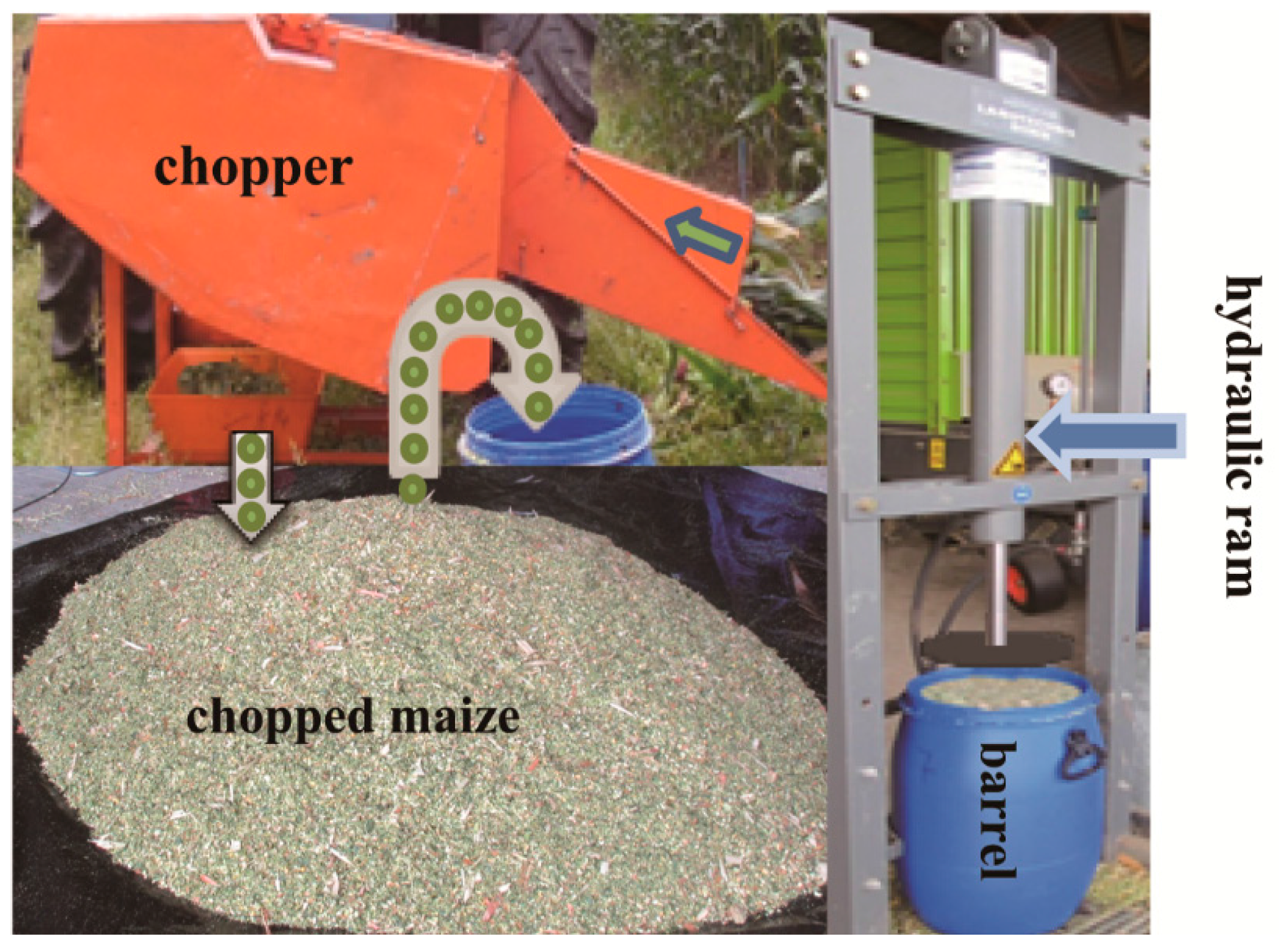
2.4. Silage Material and Experimental Layout
3. Results and Discussion
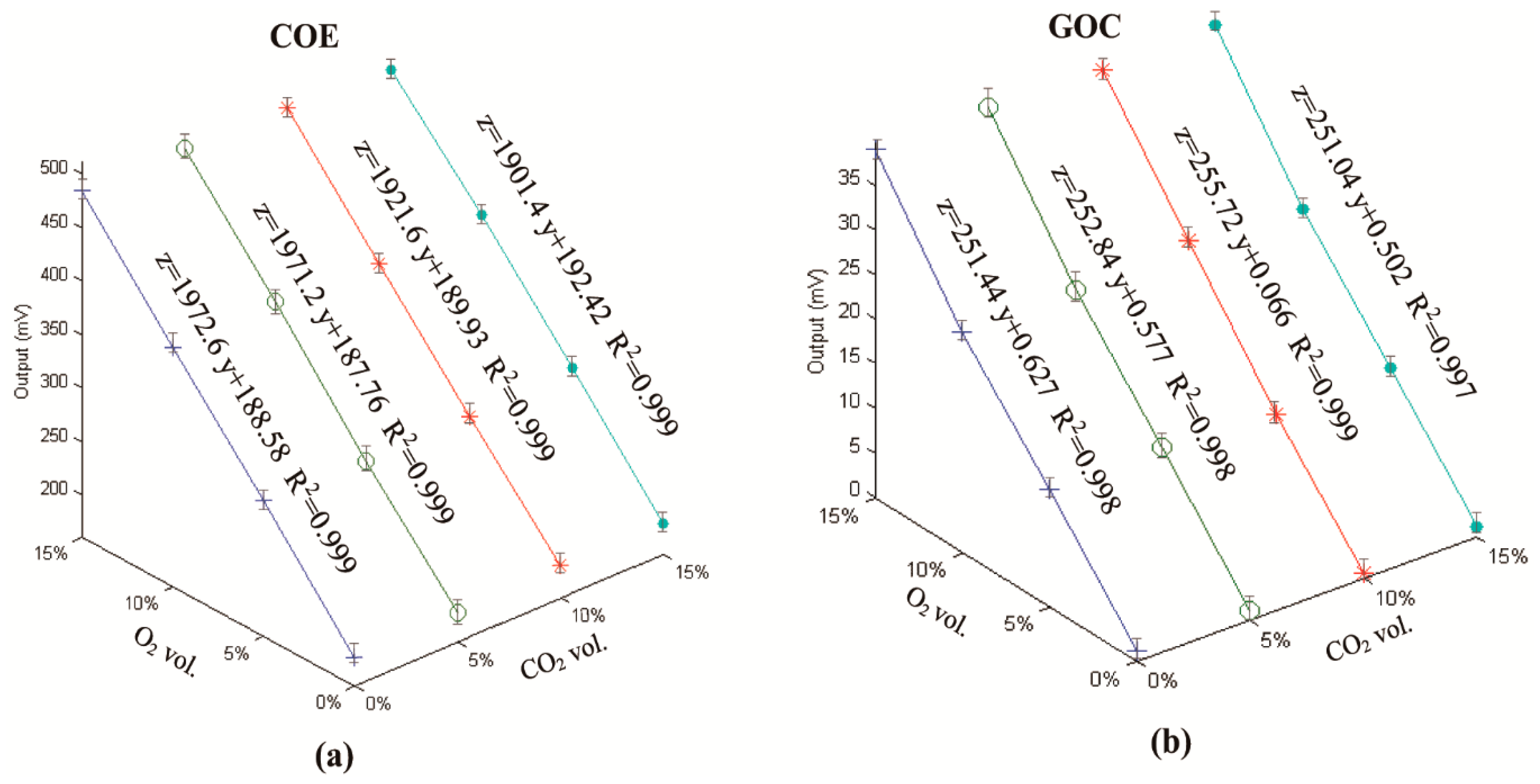
3.1. Results from the Cross-Calibration for the COE and the GOC
3.2. Measurements of the Three O2 Sensors from Test-A
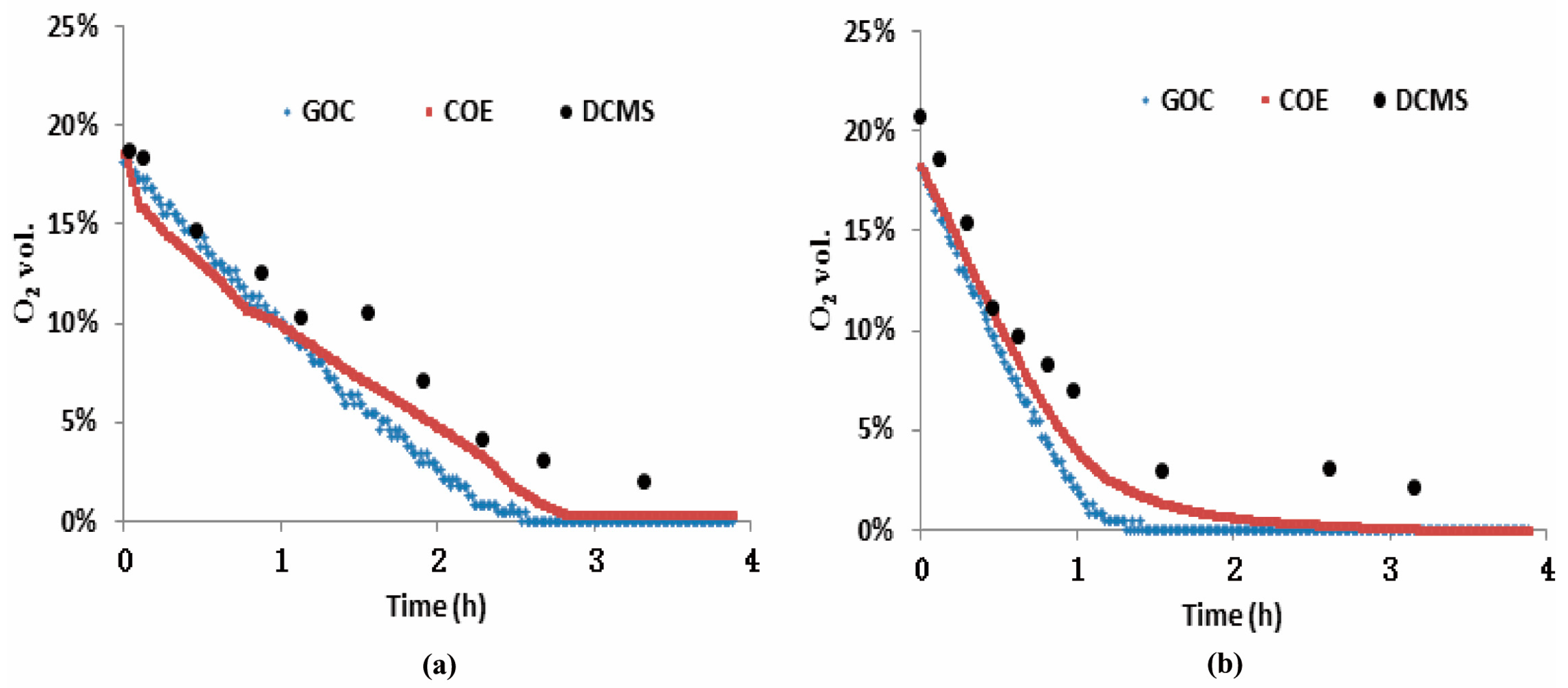

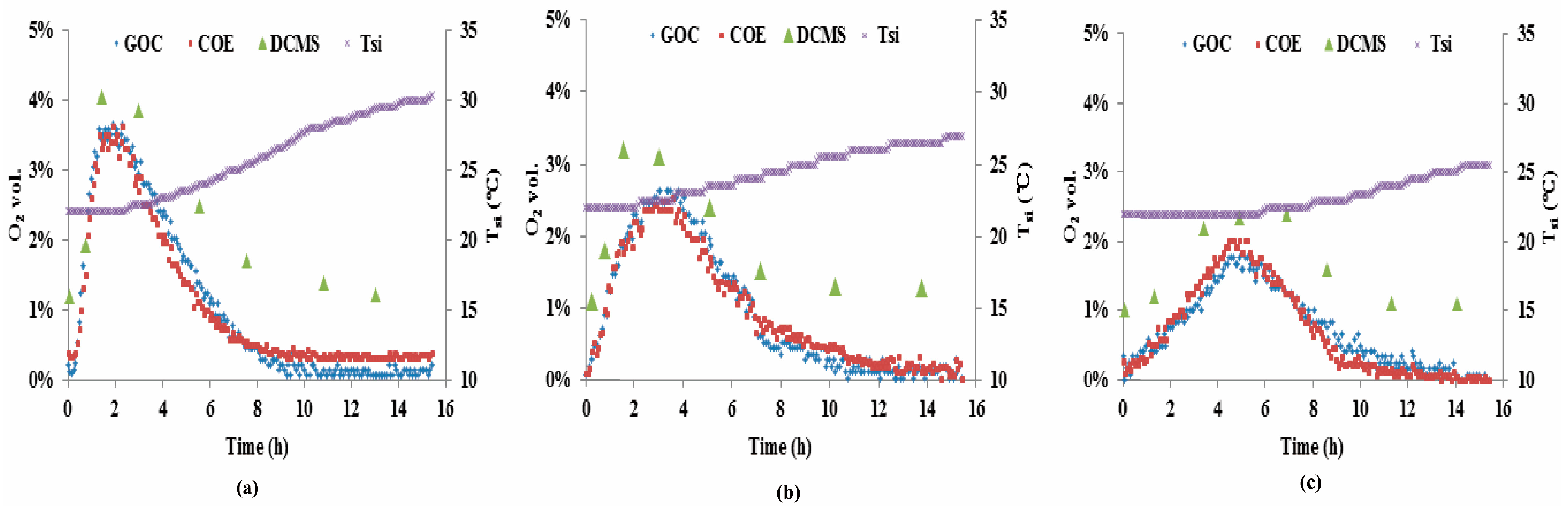
3.3. Measurements of the Three O2 Sensors from Test-B
4. Conclusions
- (i)
- The cross calibration demonstrated that either the COE or the GOC has a linear relationship O2 concentrations and that they were insensitive to CO2 ranging from 0 to 15 vol.%;
- (ii)
- Both the COE and the GOC provided continuous measurements of O2 concentration in maize silage. For the DCMS manual measurement, the general trends in O2 dynamics was captured when the measurement interval was sufficiently short;
- (iii)
- In terms of measurement quality, the calibrated COE and the GOC sensors reported similarly high accuracies. The good-agreement of their measurements from Test-A and -B also confirm that the COE can be used not only for dissolved O2 in liquids, but also for gaseous O2 in maize silage. The reduced accuracy of the DCMS, especially at low O2 concentration, likely was caused by the relatively high levels of CO2, from the O2 production in the silage. In addition, the GOC economically seems the least costly option but it is unable to simultaneously measure O2 and Tsi, whereas the COE seems to have a high ratio of performance to cost. Although the DCMS is relatively expensive, the hand-held device readily and conveniently facilitates O2 concentration measurements at multiple points in varied silage treatments.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2012, 68, 1–19. [Google Scholar] [CrossRef]
- Driehuis, F. Silage and safety and quality of dairy foods: A review. J. Sci. Food Agric. 2013, 22, 16–34. [Google Scholar]
- Muck, R.E.; Pitt, R.E. Aerobic deterioration in corn silage relative to silo face. T. ASAE 1994, 37, 735–743. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Drienhuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of Ensiling; Agronomy Publication No. 42; American Society of Agronomy: Madison, WI, USA, 2003; pp. 31–79. [Google Scholar]
- Muck, R.E.; Spoelstra, S.F.; Wikselaar, P.G. Effects of Carbon Dioxide on Fermentation and Aerobic Stability of Maize Silage. J. Sci. Food Agric. 1992, 59, 405–412. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. The relationship of silage temperature with the microbiological status of the face of corn silage bunkers. J. Dairy Sci. 2010, 93, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Bartzanas, T.; Bochtis, D.D.; Green, O.; Sørensen, C.G.; Fidaros, D. Prediction of quality parameters for biomass silage: A CFD approach. Comput. Electron. Agric. 2013, 93, 209–216. [Google Scholar] [CrossRef]
- Green, O.; Nadimi, E.S.; Blanes-Vidal, V.; Rasmus, N.; Jørgensen, R.N.; Drejer Storm, I.M.L.; Sørensen, C.G. Monitoring and modeling temperature variations inside silage stacks using novel wireless sensor networks. Comput. Electron. Agric. 2009, 69, 149–157. [Google Scholar] [CrossRef]
- Sun, Y.R.; Li, M.H.; Cheng, Q.; Jungbluth, K.H.; Buescher, W.; Ma, D.K.; Zhou, H.Y.; Cheng, H. Tracking oxygen and temperature dynamics in maize silage novel application of a Clack oxygen electrode. Biosyst. Eng. 2015, 139, 60–65. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Ashbell, G. Engineering aspects of ensiling. Biosyst. Eng. 2003, 13, 181–188. [Google Scholar] [CrossRef]
- McEniry, J.; Forristal, P.D.; O’Kiely, P. Gas composition of baled grass silage as influenced by the amount, stretch, colour and type of plastic stretch-film used to wrap the bales, and by the frequency of bale handling. Grass Forage Sci. 2011, 66, 277–289. [Google Scholar] [CrossRef]
- Ashbell, G.; Kipnis, T.; Titterton, M.; Hen, Y.; Azrieli, A.; Weinberg, Z.G. Examination of a technology for silage making in plastic bags. Anim. Feed Sci. Technol. 2001, 91, 213–222. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Ashbell, G. Changes in gas composition in corn silages in bunker silos during storage and feedout. Can. Agric. Eng. 1994, 36, 155–158. [Google Scholar]
- Williams, A.G.; Hoxey, R.P.; Lowe, J.F. Changes in temperature and silo composition during ensiling, storage and feeding-out grass silage. Grass Forage Sci. 1997, 52, 176–189. [Google Scholar] [CrossRef]
- Gastóna, A.; Abalone, R.; Bartosik, R.E.; Rodríguez, J.C. Mathematical modeling of heat and moisture transfer of wheat stored in plastic bags (silo bags). Biosyst. Eng. 2009, 104, 72–85. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs and applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Lu, C.C.; Huang, Y.S.; Huang, J.W.; Chang, C.K.; Wu, S.P. A Macroporous TiO2 Oxygen Sensor Fabricated Using Anodic Aluminium Oxide as an Etching Mask. Sensors 2010, 10, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.Q.; Ning, H.S. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Pénicaud, C.; Peyron, S.; Gontard, N.; Guillard, V. Oxygen quantification methods and application to the determination of oxygen diffusion and solubility coefficients in food. Food Rev. Int. 2012, 28, 113–145. [Google Scholar] [CrossRef]
- Diepart, C.; Verrax, J.; Calderon, P.B.; Feron, O.; Jordan, B.F.; Gallez, B. Comparison of methods for measuring oxygen consumption in tumor cells in vitro. Anal. Biochem. 2010, 396, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Warburton, P.R.; Sawtelle, R.S.; Watson, A.; Wang, A.Q. Failure prediction for a galvanic oxygen sensor. Sens. Actuators B Chem. 2001, 72, 197–203. [Google Scholar] [CrossRef]
- Lübeck. Dräger-Tubes & CMS Handbook, 17th ed.; Dräger Safety AG & Co. KGaA: Lübeck, Germany, 2015; pp. 63–399. [Google Scholar]
- Trettnak, W.; Gruber, W.; Reininger, F.; Leary, P.O.; Klimant, I. New instrumentation for optical measuring of oxygen in gas or dissolved in liquids. Adv. Space Res. 1996, 18, 139–148. [Google Scholar] [CrossRef]
- Lee, J.H.; Lima, T.S.; Seo, Y.; Bishop, P.L.; Papautsky, I. Needle-type dissolved oxygen microelectrode array sensors for in situ measurements. Sens. Actuators B Chem. 2007, 128, 179–185. [Google Scholar] [CrossRef]
- Sosna, M.; Denuault, G.; Pascal, R.W.; Prien, R.D.; Mowlem, M. Development of a reliable microelectrode dissolved oxygen sensor. Sens. Actuators B Chem. 2007, 123, 344–351. [Google Scholar] [CrossRef]
- Wittkampf, M.; Chemnitius, G.C.; Cammann, K.; Rospert, M.; Mokwa, W. Silicon thin film sensor for measurement of dissolved oxygen. Sens. Actuators B Chem. 1997, 43, 40–44. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, G.; Sun, Y.; Li, M.; Jungbluth, K.H.; Maack, C.; Buescher, W.; Schütt, K.-B.; Boeker, P.; Schulze Lammers, P.; Zhou, H.; et al. An Assessment of Three Different In Situ Oxygen Sensors for Monitoring Silage Production and Storage. Sensors 2016, 16, 91. https://doi.org/10.3390/s16010091
Shan G, Sun Y, Li M, Jungbluth KH, Maack C, Buescher W, Schütt K-B, Boeker P, Schulze Lammers P, Zhou H, et al. An Assessment of Three Different In Situ Oxygen Sensors for Monitoring Silage Production and Storage. Sensors. 2016; 16(1):91. https://doi.org/10.3390/s16010091
Chicago/Turabian StyleShan, Guilin, Yurui Sun, Menghua Li, Kerstin H. Jungbluth, Christian Maack, Wolfgang Buescher, Kai-Benjamin Schütt, Peter Boeker, Peter Schulze Lammers, Haiyang Zhou, and et al. 2016. "An Assessment of Three Different In Situ Oxygen Sensors for Monitoring Silage Production and Storage" Sensors 16, no. 1: 91. https://doi.org/10.3390/s16010091







