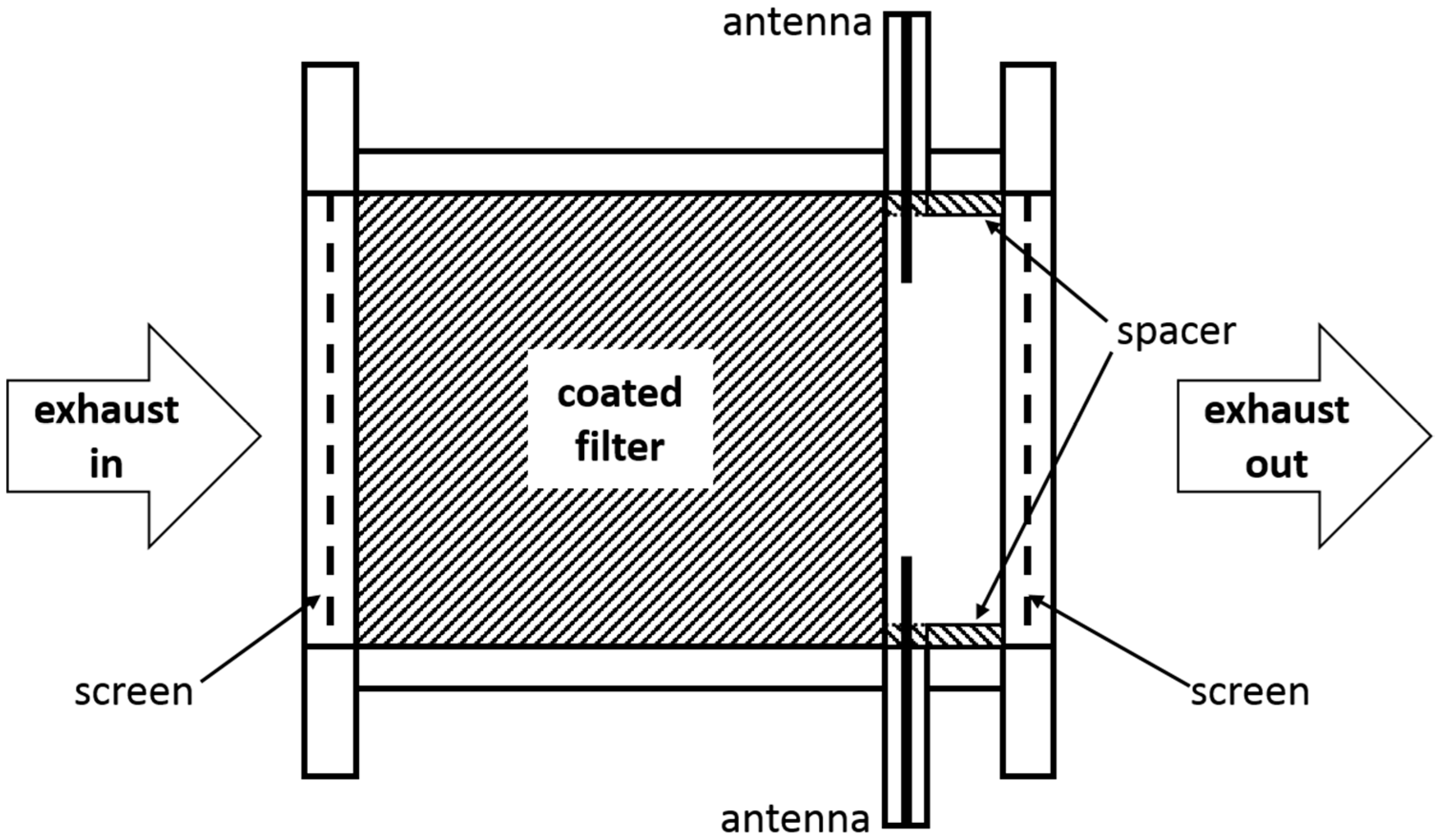
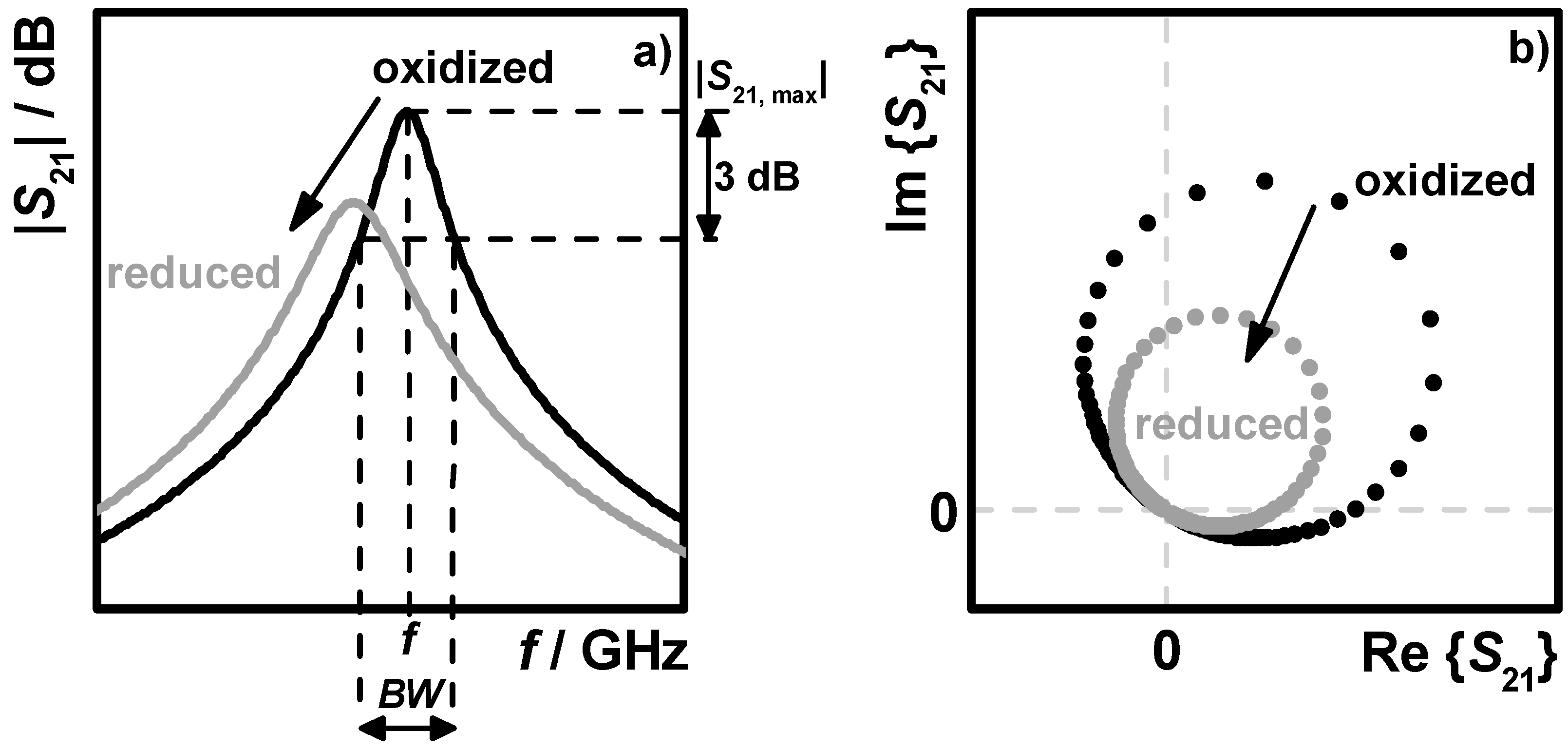
4.1. Oxidation State Determination under Transient Conditions
In former work [
15,
20,
24,
26], the oxidation state determination was conducted under controlled and constant catalyst temperatures. For transient conditions, there are two main effects influencing the cavity perturbation measurements related to temperature. Firstly, there is a geometry change resulting from thermal expansion of the metal canning. This increases the cavity and leads to a lower resonance frequency. Secondly, the material properties of the coated filter depend on temperature. Both, the oxygen storage state (which is the intended measurand) and the conductivity of the coating (which is the physical quantity that is deduced from the measured microwave signal) have a strong temperature dependency, both influencing the resonance frequency and the quality factor. With respect to the non-constant exhaust gas and catalyst temperatures during real-world applications, a temperature compensation is necessary. For TWC coatings, the material effects usually dominate the changes of the resonance frequency, but for other catalyst types with smaller dielectric changes, both effects may be equally large.
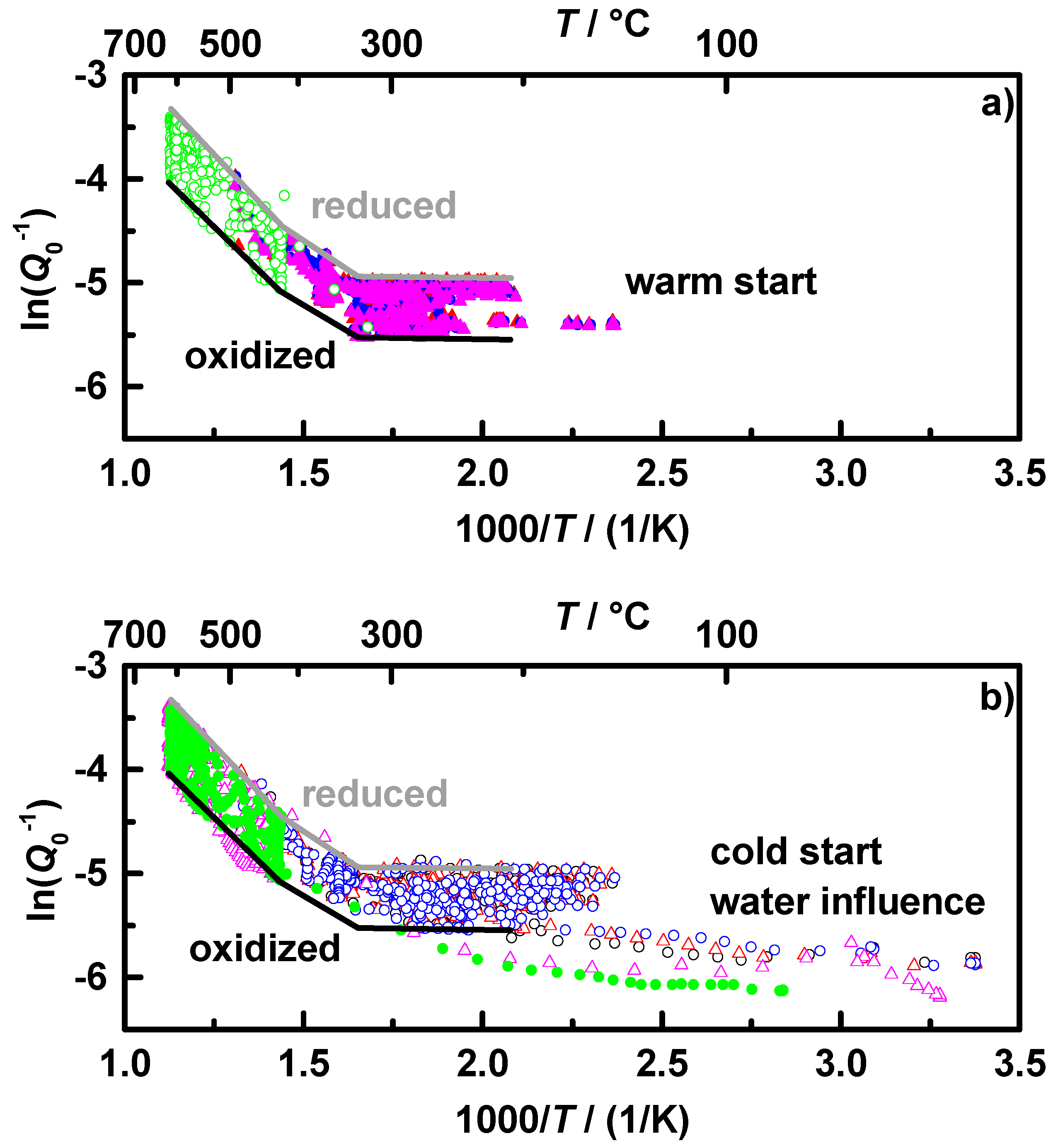
Figure 3 shows the measured resonance frequency in reversed scale (a) and the reciprocal unloaded quality factor (b) as a function of the measured gas temperature downstream of all conducted NEDC and OSC runs with cold and warm start conditions of the coated filter
C1. Each colored symbol type represents one test cycle. The lower temperature region from 25 to 500 °C is mostly represented by the NEDC and the higher temperatures up to 600 °C by the OSC measurements. Based on the theory,
fres shifts to a lower frequency and
Q0−1 increases with increasing complex permittivity of the cavity filling. In the performed transient measurements, the coated filter reached different oxidation states at various temperatures. All data points together offer a characteristic map of the oxidation state as a function of temperature with the upper border representing the fully reduced state and the lower border representing the fully oxidized state of the catalytically coated filters. Between 120 and 300 °C, both,
fres and
Q0−1, form almost horizontal borders. For temperatures over 300 °C, the values and the differences between fully oxidized and fully reduced increase, an observation that coincides fully with [
36].
From
Figure 3 it becomes clear that both analyzed values,
fres and
Q0−1, have mainly similar temperature dependencies, but
fres seems to be additionally influenced by other effects. One of these effects appears to be water, visible in the cold start measurements between 25 and 200 °C, as already observed in lab experiments [
36]. In the same temperature region,
Q0−1 shows a significantly lower sensitivity to water. This behavior could be explained by the correspondence of
fres to ε
1, which is a measure of polarization and the polar nature of water molecules. Additionally,
fres shows no completely reproducible behavior in the continuously performed measurements, for example visible in the pink points compared to the blue and green data points in
Figure 3a. A possible soot accumulation in the NEDCs can be excluded since no change in differential pressure was detected and no irregularity in the changes in
fres over the test cycles occurred. In contrast, the values of
Q0−1 determined in all experimental runs fit together very well and do not show any drift behavior. This again may be explained be the insensitivity of
Q0−1 to the thermal expansion of the canning and by the small influence of water to the losses. The same behavior occurred for the filter with twice the Ceria content
C2. It appears that
Q0−1 is the more appropriate measurand for the oxidation state determination of the TWC coated GPF compared to the resonance frequency,
fres. Hence, we will focus in the following on
Q0−1 to determine the oxidation state during engine operation.
Figure 3.
Temperature dependence of (a) fres in reverse scale and (b) Q0−1, observed during NEDC (max. 500 °C) and OSC runs (max. 620 °C) under cold and warm start conditions for sample C1; each differently colored data set represents one experimental run.
Figure 3.
Temperature dependence of (a) fres in reverse scale and (b) Q0−1, observed during NEDC (max. 500 °C) and OSC runs (max. 620 °C) under cold and warm start conditions for sample C1; each differently colored data set represents one experimental run.
According to Equation (5),
Q0−1 is proportional to the change of ε
2, which itself is proportional to the conductivity for semiconducting materials in the GHz range by ε
2 =
σ/(2π
f·ε
0) [
37]. Hence, the change in
Q0−1 can be seen as a direct measure of a change in conductivity of the filter coating. As seen in
Figure 3b,
Q0−1 appears to be thermally activated. Therefore, the assumption of an Arrhenius-like behavior of
Q0−1 for a characteristic map of the temperature dependency of the oxidation state is appropriate.
Figure 4a shows the Arrhenius plot of the warm start and
Figure 4b the cold start measurements of the filter
C1. According to the data points of the warm start measurements in
Figure 4a, characteristic lines were fit to the borders for the reduced (grey) and oxidized state (black). It is clearly visible that the data fit has three sections with three different slopes for each state. The temperatures at which the slopes change seem to be the same for the oxidized and the reduced state. The characteristic lines created by the warm start measurements marked into the cold start measurements in
Figure 4b fit very well. However, it seems to be necessary that the exhaust gas (not the TWC coated GPF itself!) has reached temperatures above 200 °C to show negligible water influence. In order to use the created map for oxidation state determination in the NEDCs, the minimum downstream gas temperature for this analysis was set to 150 °C, with assuming almost 200 °C in the coated filter.
Figure 4.
Arrhenius-like representation of
Q0−1 of the conducted NEDC and OSC runs with sample
C1 according to
Figure 3b: (
a) warm start and (
b) cold start conditions, with the characteristic curve of the oxidized (black) and reduced (grey) state; each differently colored data set represents one experimental run. The same labels as shown in
Figure 3 apply.
Figure 4.
Arrhenius-like representation of
Q0−1 of the conducted NEDC and OSC runs with sample
C1 according to
Figure 3b: (
a) warm start and (
b) cold start conditions, with the characteristic curve of the oxidized (black) and reduced (grey) state; each differently colored data set represents one experimental run. The same labels as shown in
Figure 3 apply.
Before using the characteristic map to analyze the current oxidation state in a test cycle, we will have a short comparison of the two tested coated filters. The above-described procedure for filter
C1 has also been performed for filter
C2. The fitted characteristic oxidation state lines for both filters are displayed in
Figure 5: oxidized (black) and reduced (grey) for
C1 (solid) and
C2 (dashed). Both filters show a very similar behavior. The filter
C2 has always a higher value of
Q0−1 than
C1, according to its higher ceria content and the resulting higher conductivity. The two changes in the slopes are for both samples at almost the same temperatures, at 330 and 430 °C. It seems that the filter coating has three areas with different thermal activation energies. However, resulting of the fact that
Q0−1 has an unknown proportionality to the conductivity, these results do not allow a statement about values of activation energies or even a comparison to other materials. At this point, a recently in [
38] introduced setup using the cavity perturbation method for an exact material characterization of catalysts could be helpful for further investigations of the dielectric properties and conductivity mechanisms of TWC coatings in the GHz range.
Figure 5.
Comparison of the characteristic curves of Q0−1 in an Arrhenius-like representation: the oxidized (black) and reduced (grey) state for the samples C1 (solid) and C2 (dashed) with doubled Ceria content.
Figure 5.
Comparison of the characteristic curves of Q0−1 in an Arrhenius-like representation: the oxidized (black) and reduced (grey) state for the samples C1 (solid) and C2 (dashed) with doubled Ceria content.
Besides the fully oxidized and reduced state, the knowledge about the behavior in the intermediate oxidation states is necessary. Former work already showed a linear relation between the oxidation state and the resonance frequency [
24]. To investigate the relation between oxidation state and microwave data,
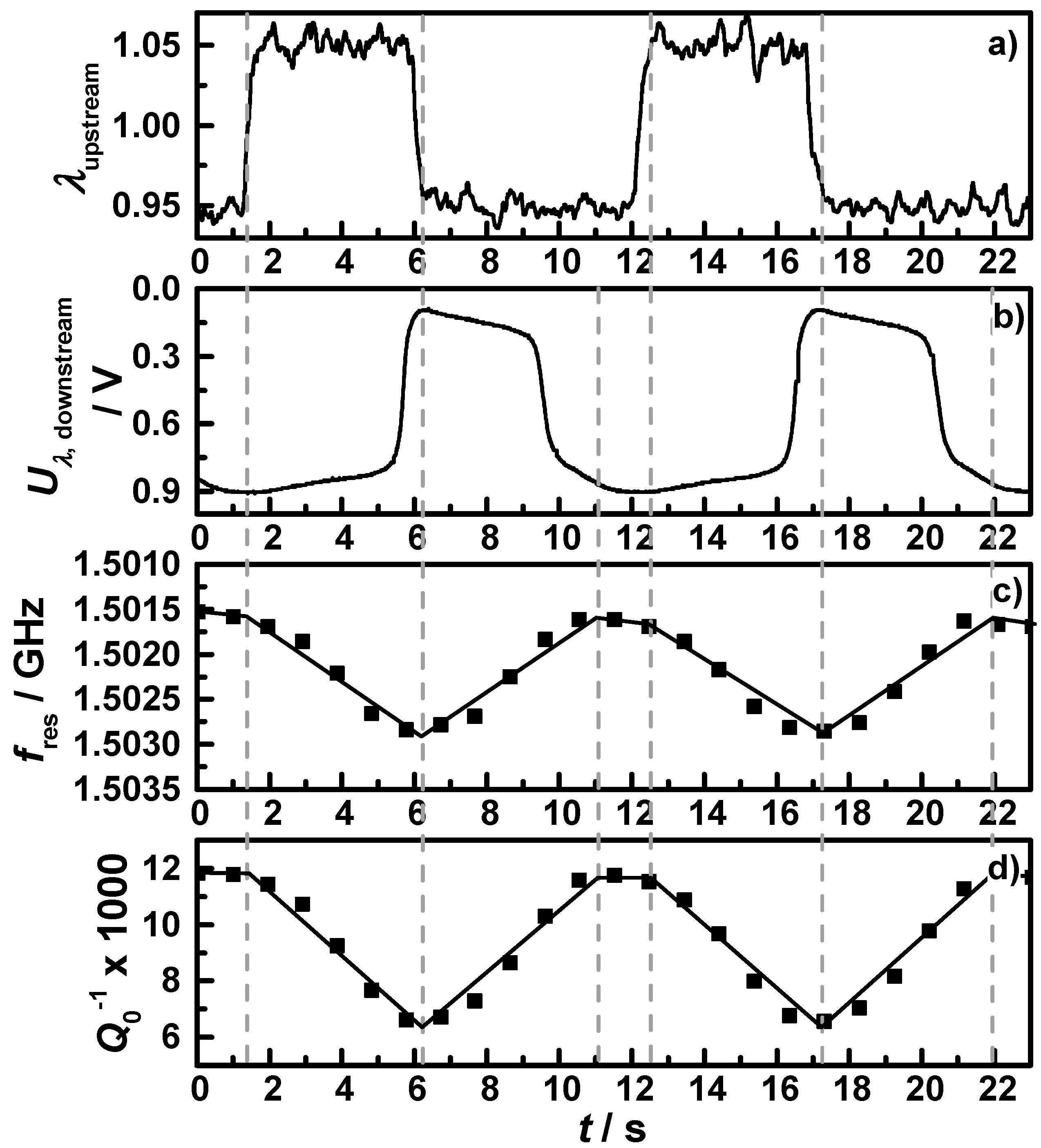
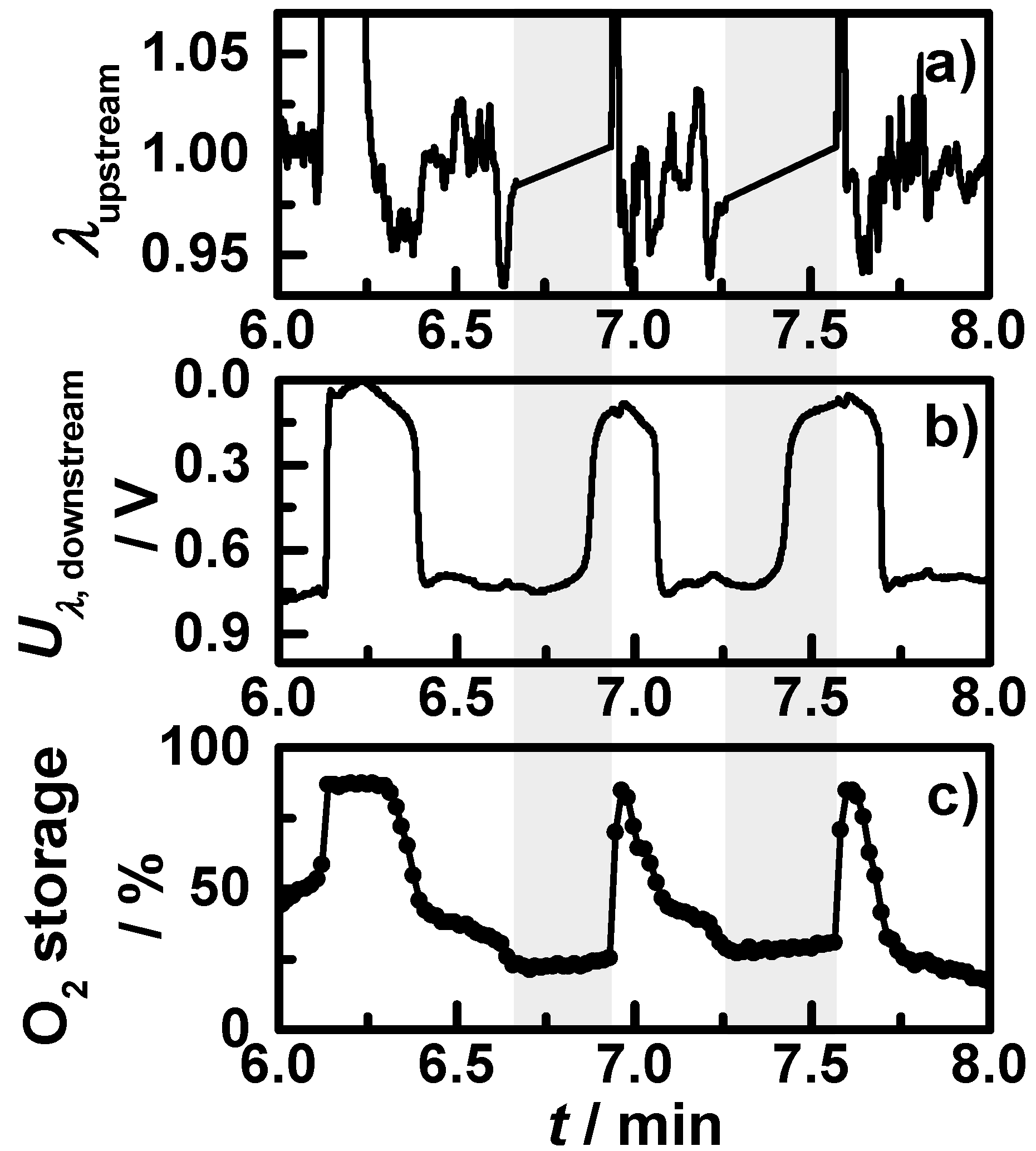
Figure 6 shows exemplarily a short section of one OSC measurement at the operation point at 425 °C, with (a) the air-fuel ratio λ as derived by the upstream broadband lambda probe; (b) the downstream lambda probe signal; (c) the resonance frequency in reverse scale; and (d) the reciprocal quality factor. Vertical grey dashed lines indicate the point in time when either the upstream broadband lambda probe or the downstream lambda probe switched from lean to rich or
vice versa. According to the changes of the lambda probe signals and the measured data points, black lines are drawn into the two microwave signals representing the idealized signal that would mirror the oxidation state of the coated filter. One can clearly see that the assumption of an almost linear relation between the oxidation state and both the resonance frequency and the reciprocal quality factor is appropriate. The small changes in the slope and the resulting deviations between the drawn lines and the measured data points are related to the locally distributed electric field strength of the TE
111 mode, which has a maximum in the middle of the cavity and decreases to the front and the end of the catalyst. Nevertheless, it correlates well and this relation was observed in all conducted OSC measurements at all operation points. Since we focused on the quality factor, the oxidation state can be calculated with the knowledge of the fully oxidized and reduced state with Equation (6) for transient conditions with an estimated maximum error less than 10%:
The application of the characteristic map of the oxidation state of
Figure 4 and
Figure 5 for filter
C1 on an NEDC with warm start conditions is displayed in
Figure 7. It shows (a) the theoretical vehicle speed; (b) the downstream exhaust gas temperature; (c) the air-fuel ratio λ measured by the upstream broadband lambda probe; (d) the downstream lambda probe signal; (e) the resonance frequency in reverse scale; (f) the reciprocal unloaded quality factor with the lines for the exhaust gas temperature dependent oxidized (
Qox−1(
T), red dashed) and reduced state (
Qred−1(
T), blue dashed); and (g) the calculated oxygen storage degree, using Equation (6).
Figure 6.
Section of a performed OSC measurement of filter C1 with continuous lean-rich changes at 425 °C: (a) the air-fuel ratio λ measured by the upstream broadband lambda probe; (b) downstream lambda probe signal; (c) the resonance frequency in reverse scale; and (d) the reciprocal quality factor.
Figure 6.
Section of a performed OSC measurement of filter C1 with continuous lean-rich changes at 425 °C: (a) the air-fuel ratio λ measured by the upstream broadband lambda probe; (b) downstream lambda probe signal; (c) the resonance frequency in reverse scale; and (d) the reciprocal quality factor.
The start-stop phases with engine shut-off are marked by grey shades. The NEDC is divided into urban cycles with start stop intervals (until minute 13.5) and an extra-urban cycle with highway speed. The additionally displayed resonance frequency and the reciprocal quality factor have a similar behavior, but fres appears less affected by the changing catalyst state.
After the start, the upstream lambda probe shows for the first three minutes almost no control activity. In this time span, there are two short accelerations (t1, t2) resulting in small downstream lambda drops. In the microwave-derived oxygen storage degree, these two drops are clearly visible. After the third minute, the upstream lambda probe shows more control activity.
The oxygen storage degree and the downstream lambda signal decrease correspondingly; e.g., the stepwise downstream lambda probe change at
t3 goes along with slowly decreasing oxygen storage degree. At several points in time (
t4,
t5,
t6,
t7), the engine brake is used for deceleration, with the result of a fuel-cut leading to both a temperature drop and a prompt fully oxidation of the catalyst. This is mirrored by both lambda probe signals as well as by the oxygen storage degree. After the sixth minute, the start-stop phases with engine shut-off occur in the cycle. The first two start-stop phases are shown in a close-up view in
Figure 8, with (a) the air-fuel ratio λ measured by the upstream broadband lambda probe; (b) the downstream lambda probe signal; and (c) the measured oxygen storage degree. One can see: when the engine is shut off, no air-fuel ratio λ signal can be obtained of the upstream broadband lambda probe and the signal of the downstream lambda probe increases; most likely an effect of oxygen diffusing from the end of the exhaust pipe to the lambda probe. In this time, the measured oxygen storage degree remains on an almost constant level.
Figure 7.
NEDC measurement with warm start conditions for sample C1: (a) vehicle speed; (b) downstream exhaust gas temperature; (c) the air-fuel ratio λ measured by the upstream broadband lambda probe; (d) downstream lambda probe signal; (e) resonance frequency in reverse scale; (f) reciprocal quality factor with characteristic lines for temperature dependent reduced (blue) and oxidized (red) state and (g) the oxygen storage degree calculated by the quality factor and the characteristic lines. Start-stop phases with engine shut off are shaded grey.
Figure 7.
NEDC measurement with warm start conditions for sample C1: (a) vehicle speed; (b) downstream exhaust gas temperature; (c) the air-fuel ratio λ measured by the upstream broadband lambda probe; (d) downstream lambda probe signal; (e) resonance frequency in reverse scale; (f) reciprocal quality factor with characteristic lines for temperature dependent reduced (blue) and oxidized (red) state and (g) the oxygen storage degree calculated by the quality factor and the characteristic lines. Start-stop phases with engine shut off are shaded grey.
Figure 8.
Section of the NEDC measurement with warm start conditions from
Figure 7 showing the first two start-stop phases (shaded grey): (
a) the air-fuel ratio λ measured by the upstream broadband lambda probe; (
b) downstream lambda probe signal; and (
c) the microwave-derived oxygen storage degree.
Figure 8.
Section of the NEDC measurement with warm start conditions from
Figure 7 showing the first two start-stop phases (shaded grey): (
a) the air-fuel ratio λ measured by the upstream broadband lambda probe; (
b) downstream lambda probe signal; and (
c) the microwave-derived oxygen storage degree.
When the engine starts again, the TWC coating gets oxidized by a short lean pulse. In all phases of the urban cycles following to a full oxidation, the oxygen storage degree decreases slowly until the next start-stop or engine brake phase occurs. After the last start-stop interval (around minute 13.5), the last part of the NEDC with the extra-urban cycle starts. From t7 to t8, the oxygen storage degree changes continuously with different slopes corresponding to accelerations, always mirrored by the lambda probe signals.
In the end of each NEDC (after
t8), the engine control has to test the oxygen storage capacity (OSC) for on-board diagnosis (OBD), by short lean-rich-lean jumps and analyzing the time a full reduction requires. A closer look on this OSC step is displayed in
Figure 9, with (a) the upstream broadband lambda probe; (b) the downstream lambda probe and (c) the microwave-derived oxygen storage degree. In the beginning, the upstream air-fuel ratio is hold almost constant around one, then the engine changes to lean conditions until the downstream lambda probe signal indicates a lean atmosphere. Then the TWC coating is fully oxidized. The microwave-derived oxygen storage degree increases corresponding to the lambda probes signals from 40% to 100%. Then the engine control switches to rich conditions until the downstream lambda probe signal indicates rich. The oxygen storage degree decreases and reaches exactly 0%. After a lean pulse and stoichiometric conditions, the oxygen storage degree remains at 30% in the end. The measured oxygen storage degree of the OSC step in the NEDC fits perfectly to the lambda probe signals. This demonstrates that the microwave-based technique is more accurate for the oxygen storage capacity determination than the approach utilizing the downstream lambda probe signal. Additionally, this technique could be able to determine the OSC by analyzing the slope of the oxidation state change without the need to fully oxidize or reduce the catalyst to ensure conversion and avoid unnecessary emissions in the OBD step.
Figure 9.
Section of the NEDC measurement with warm start conditions from
Figure 7 showing the OSC step: (
a) the air-fuel ratio λ measured by the upstream broadband lambda probe; (
b) downstream lambda probe signal; and (
c) the microwave-derived oxygen storage degree.
Figure 9.
Section of the NEDC measurement with warm start conditions from
Figure 7 showing the OSC step: (
a) the air-fuel ratio λ measured by the upstream broadband lambda probe; (
b) downstream lambda probe signal; and (
c) the microwave-derived oxygen storage degree.
4.2. High Soot Loading Experiments
During the conducted NEDCs no notable soot accumulation was observed, neither by the differential pressure sensor nor by the microwave-derived data. Besides the known low raw soot emission of the used homogeneous gasoline engine, the catalytic filter coating could be the reason, since all accumulated soot oxidizes instantaneously under normal driving conditions [
7]. To investigate the influence of fully non-realistic high soot loadings, the engine was forced for six hours to produce high amounts of soot and to prevent an unwanted soot oxidation by an operation under constant extreme rich conditions (λ = 0.85).
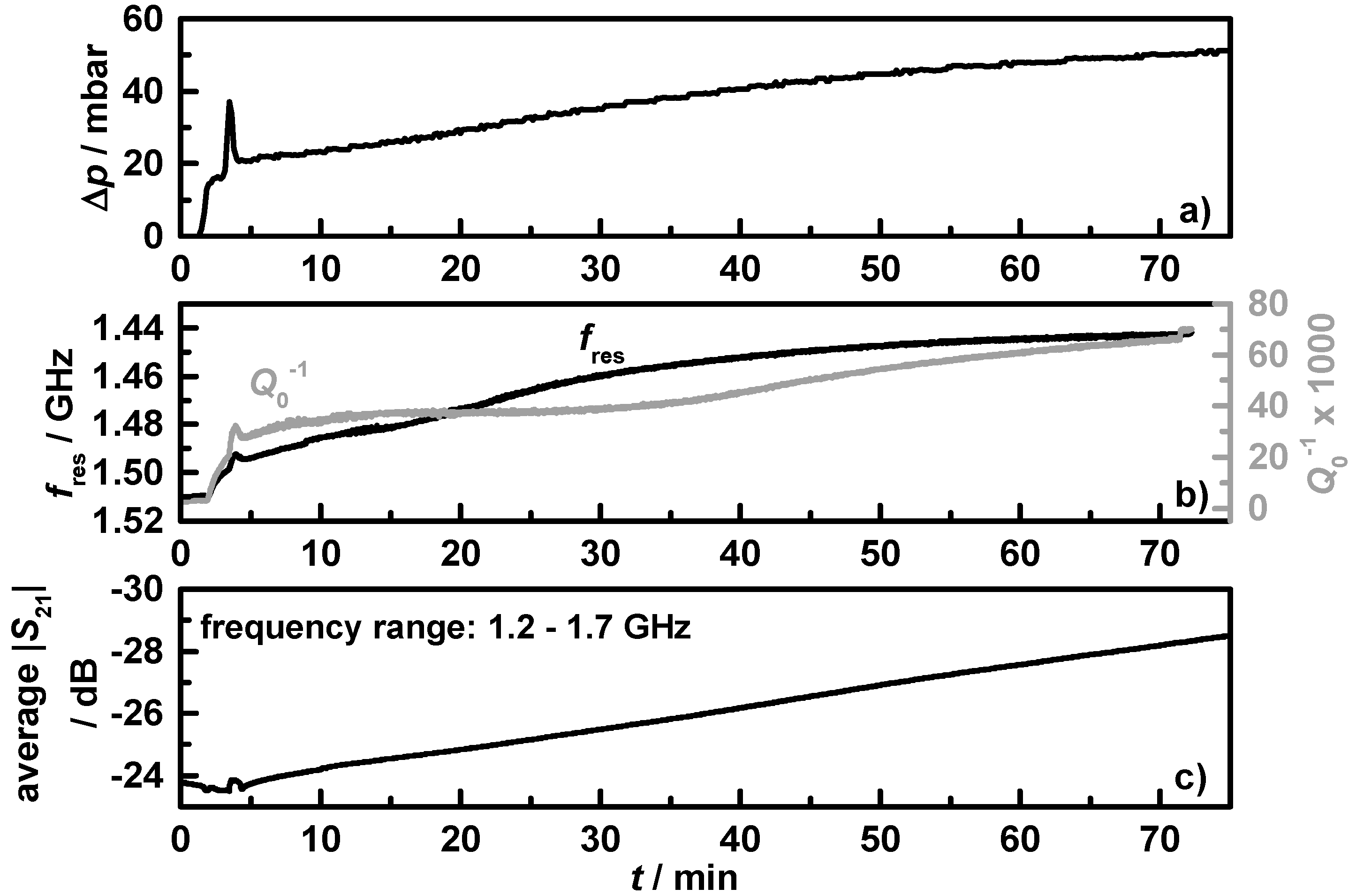
Figure 10 shows the results of the first 75 min of the soot loading experiment for filter
C1, with (a) the differential pressure, Δ
p; (b) the resonance frequency (black) and the reciprocal quality factor (grey); and (c) the averaged transmission parameter in the frequency range from 1.2 to 1.7 GHz. Under the used extreme rich conditions, the engine produced high amounts of soot, mirrored in the instantaneous differential pressure increase. In the first minutes of soot loading, Δ
p increases fast and then slower with a constant slope. This behavior is known from DPF, where the pores inside the ceramic walls get filled first [
39]. With filled pores the filter efficiency and the differential pressure is higher and the soot accumulates mostly on and no more in the filter walls. The differential pressure peak around minute four resulted from a short change in operating conditions with higher exhaust gas flow and temperature.
Figure 10 gives clear evidence that the resonance frequency and the reciprocal quality factor mirror the differential pressure signal. The already mentioned peak is also visible as an effect of the short increase in temperature in the microwave data (but is much smaller, since the mass flow does not affect the resonance behavior [
40]). Due to the high losses of the accumulated soot, the resonance is only analyzable for the first 70 min. Therefore, the averaged transmission parameter was used for higher soot loading determination, and shows an almost linear decrease over the first 75 min from −24 to −29 dB. Such a procedure has been suggested for microwave-based DPF loading estimation by several authors [
17,
35,
40].
Figure 10.
Beginning of the 6 h soot loading experiment on filter C1 with (a) the differential pressure; (b) the resonance frequency (black) and reciprocal quality factor (grey); and (c) the averaged transmission parameter in the frequency range from 1.2 to 1.7 GHz.
Figure 10.
Beginning of the 6 h soot loading experiment on filter C1 with (a) the differential pressure; (b) the resonance frequency (black) and reciprocal quality factor (grey); and (c) the averaged transmission parameter in the frequency range from 1.2 to 1.7 GHz.
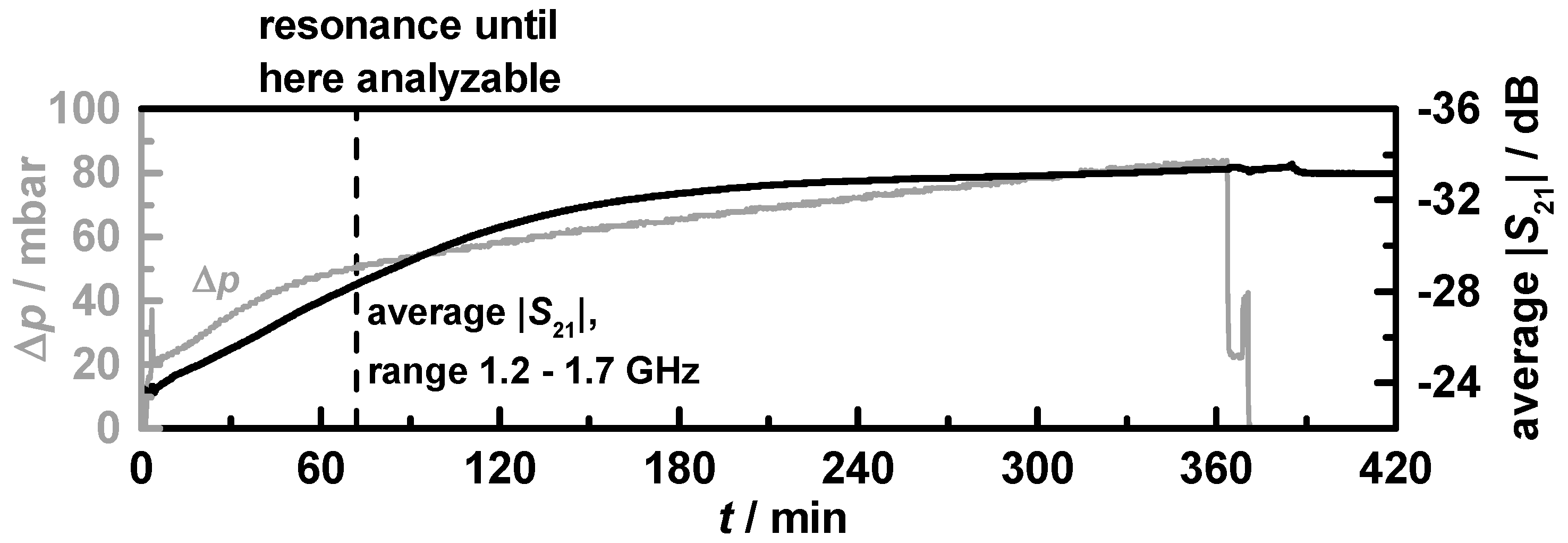
The whole six-hour lasting soot loading run for filter
C1 is shown in
Figure 11. The solid grey curve stands for the differential pressure and the averaged transmission parameter is indicated by the black curve.
Figure 11.
Entire 6 h-lasting soot loading run (filter C1). Differential pressure (grey) and averaged transmission parameter in the frequency range from 1.2 to 1.7 GHz (black) agree well. After ca. 70 min (black dashed line), the losses were too high and the resonance peak could not be evaluated anymore.
Figure 11.
Entire 6 h-lasting soot loading run (filter C1). Differential pressure (grey) and averaged transmission parameter in the frequency range from 1.2 to 1.7 GHz (black) agree well. After ca. 70 min (black dashed line), the losses were too high and the resonance peak could not be evaluated anymore.
The point in time when fres and Q0−1 were no appropriate means anymore to measure the soot loading is marked with a black dashed line. It becomes obvious that the averaged transmission, which changes from −24 to −34 dB during the six-hour-lasting loading period, correlates well with the soot loading as indicated by the differential pressure. After the engine was stopped, the signal remained on its level. The filter with doubled Ceria content C2 showed almost identical results.
Ash loading of filters is an issue which has not been considered here. It is clear, for a long-term investigation, one may expect that ash, which cannot be removed by oxidation, accumulates in the coated GPF and affects the resonance frequency and perhaps also the quality factor. Effects on ash loading have been described recently described for DPF by Sappok and Bromberg [
41]. Whether the strategies suggested there can be applied for GPF as well, may be investigated in the future.