Enhanced Dibutyl Phthalate Sensing Performance of a Quartz Crystal Microbalance Coated with Au-Decorated ZnO Porous Microspheres
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of ZnO Porous Microspheres and Au-Decorated ZnO Porous Microspheres
2.2.1. Synthesis of ZnO Porous Microspheres
2.2.2. Preparation of Au-Decorated ZnO Porous Microspheres
2.3. Characterization
2.4. Fabrication of the QCM Sensing Film
2.5. Preparation of Measured Vapors
2.6. Measurement of Gas Sensing Properties
3. Results and Discussion
3.1. XRD Diffraction
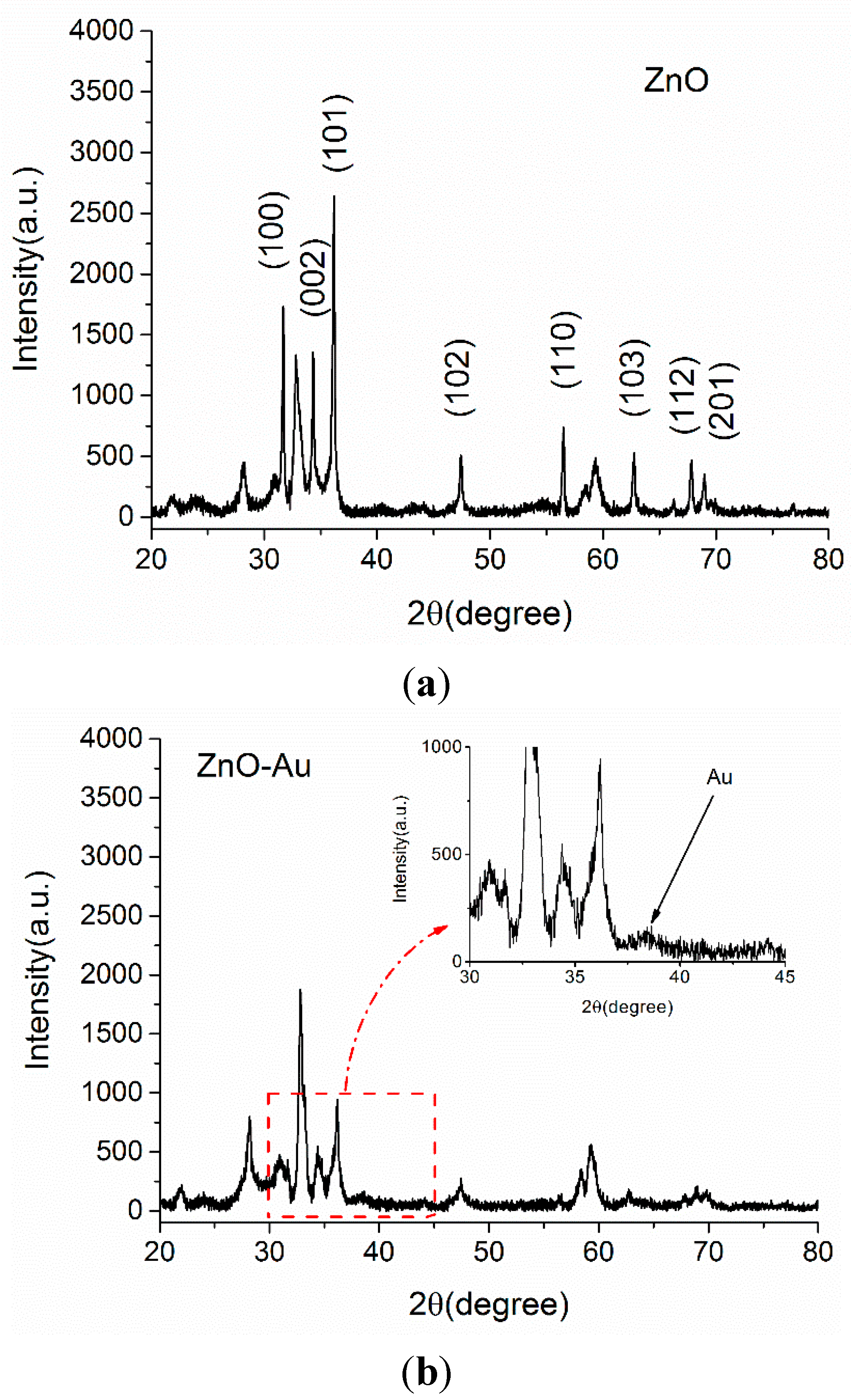
3.2. SEM Morphology

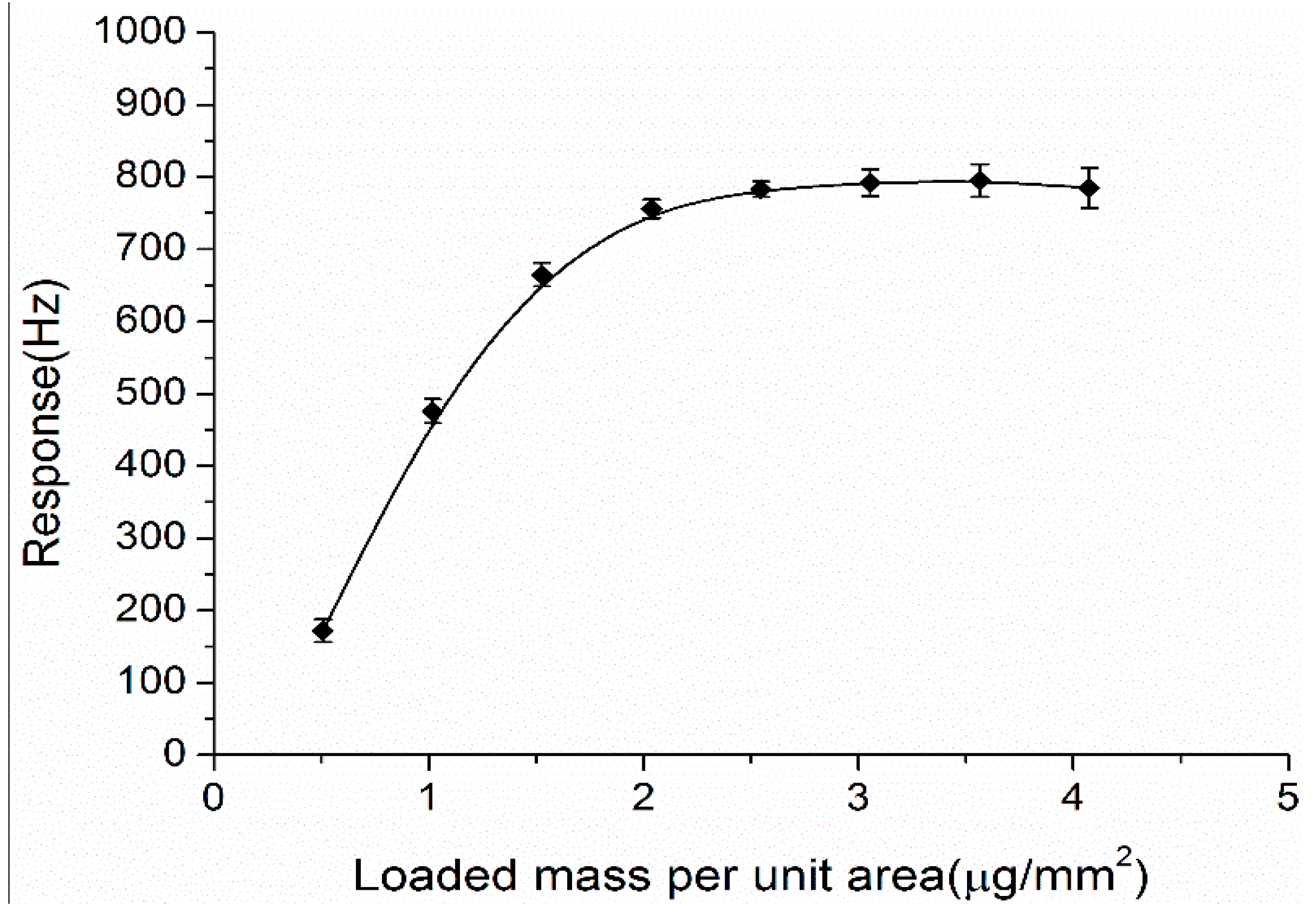
3.3. Optimization of the Coating Load of Nano-Structured Sensing Materials
3.4. Repeatable Response of the ZnO-Au Coated QCM Sensor to DBP

3.5. Sensitivity to Different Concentrations of DBP
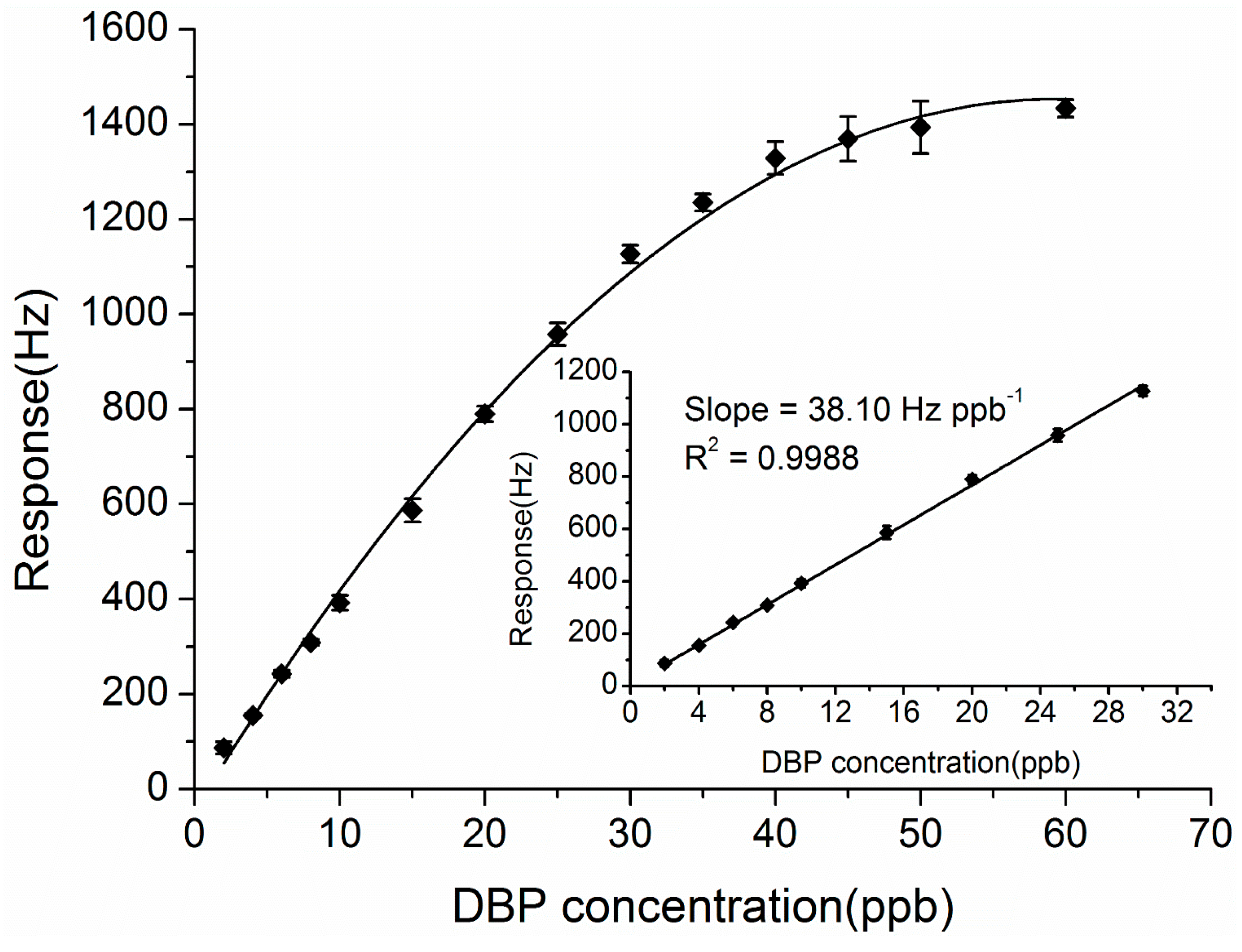
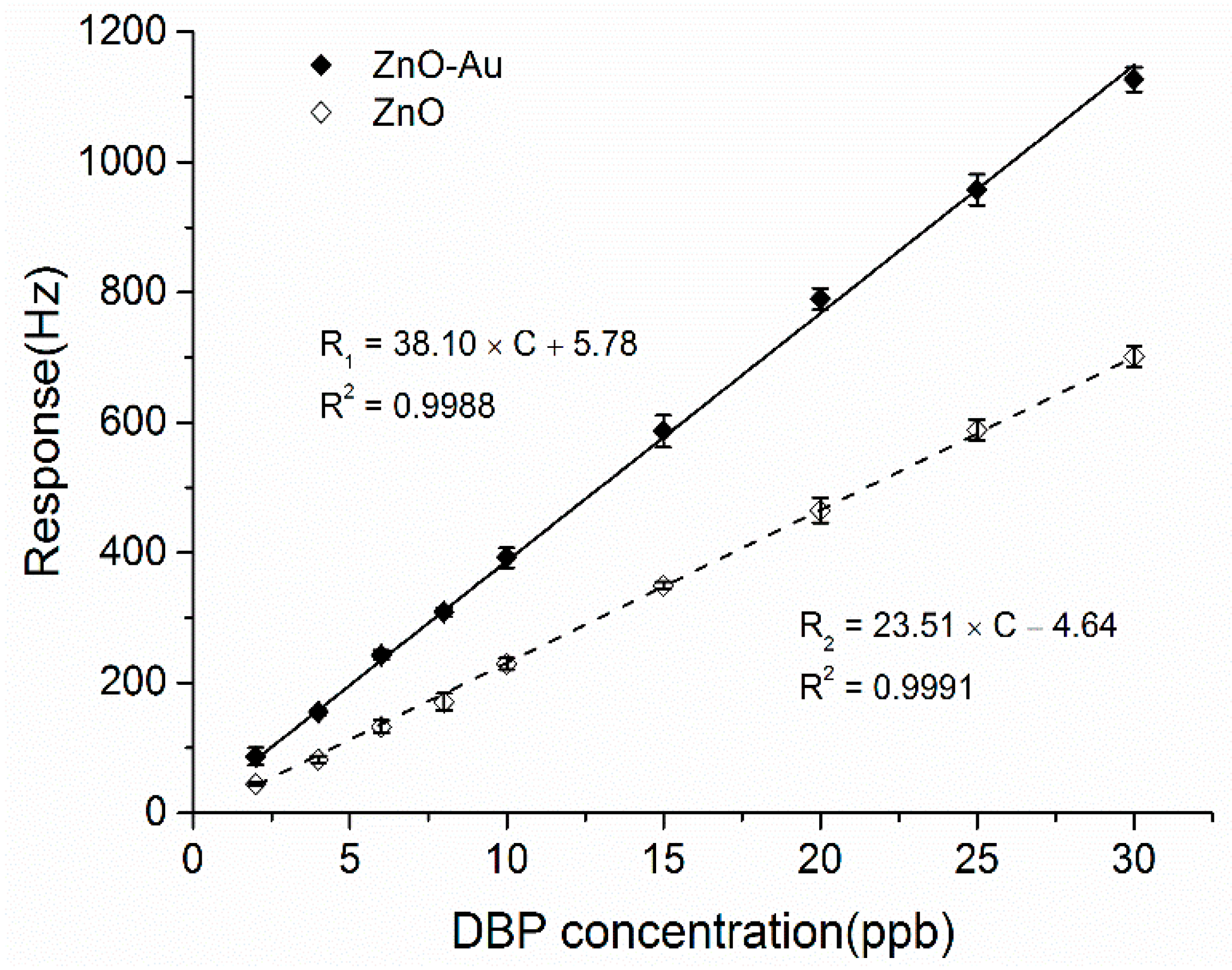
3.6. Selectivity of the ZnO-Au and ZnO Coated QCM Sensors
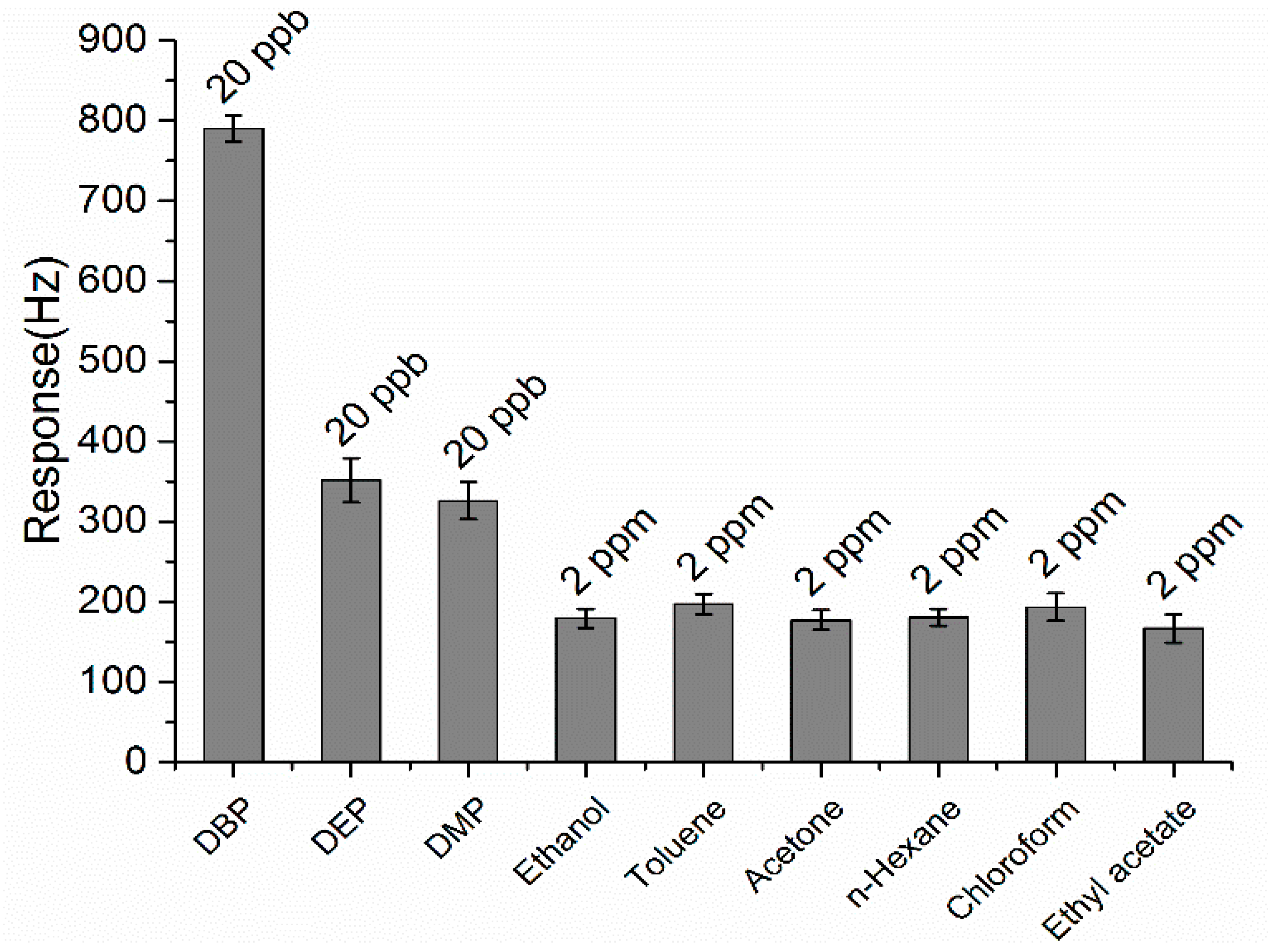
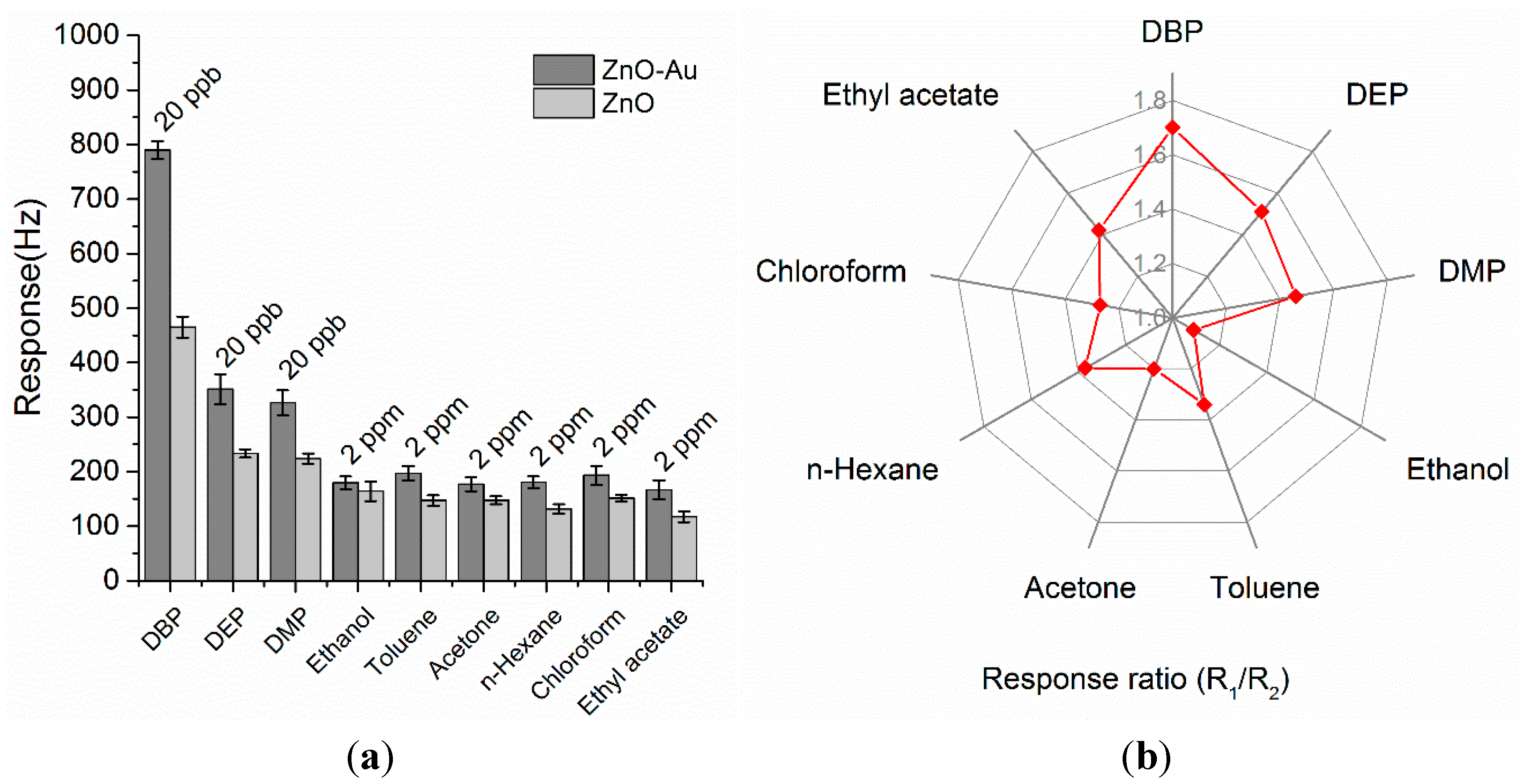
3.7. Long-Term Stability
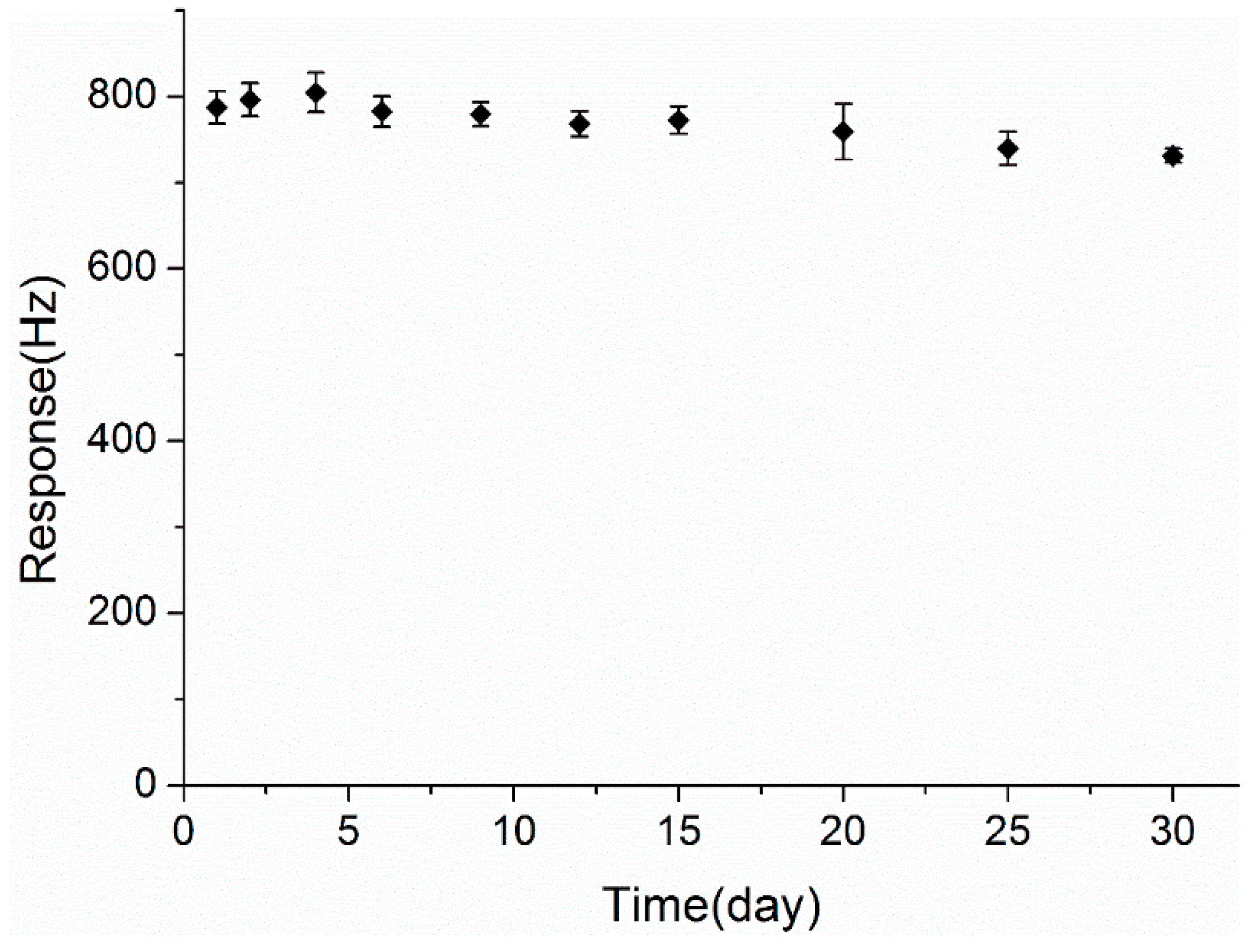
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fromme, H.; Lahrz, T.; Kraft, M.; Fembacher, L.; Dietrich, S.; Sievering, S.; Burghardt, R.; Schuster, R.; Bolte, G.; Volkel, W. Phthalates in german daycare centers: Occurrence in air and dust and the excretion of their metabolites by children (lupe 3). Environ. Int. 2013, 61, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Boberg, J.; Axelstad, M.; Dalgaard, M.; Vinggaard, A.M.; Metzdorff, S.B.; Hass, U. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod. Toxicol. 2010, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; You, L.; Zeng, Q.; Sun, Y.; Huang, Y.H.; Wang, C.; Wang, P.; Cao, W.C.; Yang, P.; Li, Y.F.; et al. Phthalate exposure and human semen quality: Results from an infertility clinic in China. Environ. Res. 2015, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Shukla, M.; Kumar, P.D.; Shukla, Y.; Mathur, N.; Kumar, G.Y.; Saxena, D.K. Correlation of phthalate exposures with semen quality. Toxic. Appl. Pharmacol. 2008, 231, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Lee, H.; Han, S.Y.; Lim, D.S.; Jung, K.K.; Kwack, S.J.; Kim, K.B.; Lee, B.M. An exposure assessment of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) in human semen. J. Toxic. Environ. Health Part A 2009, 72, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, D.; Zhou, Y.; Ma, M.; Li, J.; Wang, Z. Dibutyl phthalate contributes to the thyroid receptor antagonistic activity in drinking water processes. Environ. Sci. Technol. 2010, 44, 6863–6868. [Google Scholar] [CrossRef] [PubMed]
- Weschler, C.J.; Nazaroff, W.W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar] [CrossRef]
- Fromme, H.; Lahrz, T.; Piloty, M.; Gebhart, H.; Oddoy, A.; Rüden, H. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air 2004, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bergh, C.; Torgrip, R.; Emenius, G.; Ostman, C. Organophosphate and phthalate esters in air and settled dust—a multi-location indoor study. Indoor Air 2011, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, H.; Maddalena, R.L.; Singer, B.C.; Hodgson, A.T.; McKone, T.E. Indoor pollutants emitted by office equipment: A review of reported data and information needs. Atmos. Environ. 2008, 42, 1371–1388. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.L.; Guo, Y.; Wu, Q.; Yang, M.; Wu, M.H.; Kannan, K. Phthalate diesters in airborne PM(2.5) and PM(10) in a suburban area of Shanghai: Seasonal distribution and risk assessment. Sci. Total Environ. 2014, 467–498. [Google Scholar] [CrossRef] [PubMed]
- Rakkestad, K.E.; Dye, C.J.; Yttri, K.E.; Holme, J.A.; Hongslo, J.K.; Schwarze, P.E.; Becher, R. Phthalate levels in norwegian indoor air related to particle size fraction. J. Environ. Monit. 2007, 9, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Tirendi, S.; Barrero-Moreno, J.; Kotzias, D. Investigation of volatile organic compounds and phthalates present in the cabin air of used private cars. Environ. Int. 2009, 35, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yan, C.; Yu, H.; Chen, L.; Dong, F. One-step fabrication of ammonia sensor by electrospinning PS-b-PMA nanofibers on quartz crystal microbalance. Sens. Actuators B Chem. 2014, 203, 459–464. [Google Scholar] [CrossRef]
- Sauerbrey, G.Z. The use of quartz oscillators for weighing layers and for microweighing. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Erol, A.; Okur, S.; Yağmurcukardeş, N.; Arıkan, M.Ç. Humidity-sensing properties of a ZnO nanowire film as measured with a QCM. Sens. Actuators B Chem. 2011, 152, 115–120. [Google Scholar] [CrossRef]
- Khot, L.R.; Panigrahi, S.; Lin, D. Development and evaluation of piezoelectric-polymer thin film sensors for low concentration detection of volatile organic compounds related to food safety applications. Sens. Actuators B Chem. 2011, 153, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, P.; Hu, R.; Zhang, J.; Ma, X.; Luo, Z.; Li, G. A dibutyl phthalate sensor based on a nanofiber polyaniline coated quartz crystal monitor. Sensors 2013, 13, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, K.; Fan, G.; Luo, Z.; Li, G. Development of a high-sensitivity plasticizer sensor based on a quartz crystal microbalance modified with a nanostructured nickel hydroxide film. Meas. Sci. Technol. 2015, 26. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Yang, M.; Gao, S.; Zuo, G.; Yan, C.; Cheng, Z. Single crystal WO(3) nanoflakes as quartz crystal microbalance sensing layer for ultrafast detection of trace sarin simulant. Anal. Chim. Acta 2009, 654, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Lu, C.J.; Tian, W.C.; Sheen, H.J. A vapor response mechanism study of surface-modified single-walled carbon nanotubes coated chemiresistors and quartz crystal microbalance sensor arrays. Talanta 2015, 131, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mañoso, E.S.; Herrera-Basurto, R.; Simonet, B.M.; Valcárcel, M. A quartz crystal microbalance modified with carbon nanotubes as a sensor for volatile organic compounds. Sens. Actuators B Chem. 2013, 186, 811–816. [Google Scholar] [CrossRef]
- Zheng, J.; Li, G.; Ma, X.; Wang, Y.; Wu, G.; Cheng, Y. Polyaniline–TiO2 nano-composite-based trimethylamine QCM sensor and its thermal behavior studies. Sens. Actuators B Chem. 2008, 133, 374–380. [Google Scholar] [CrossRef]
- Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. NO2 sensing properties of flame-made mnox-loaded ZnO-nanoparticle thick film. Sens. Actuators B Chem. 2014, 204, 239–249. [Google Scholar] [CrossRef]
- Pei, Z.; Ma, X.; Ding, P.; Zhang, W.; Luo, Z.; Li, G. Study of a QCM dimethyl methylphosphonate sensor based on a ZnO-modified nanowire-structured manganese dioxide film. Sensors 2010, 10, 8275–8290. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhao, C.; Zhang, J.; Peng, Y.; Xie, E. Enhanced gas sensing performance of electrospun Pt-functionalized NiO nanotubes with chemical and electronic sensitization. ACS Appl. Mater. Interfaces 2013, 5, 7410–7416. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Nagaraju, G.; Lee, S.H.; Yu, J.S. PDMS-based triboelectric and transparent nanogenerators with ZnO nanorod arrays. ACS Appl. Mater. Interfaces 2014, 6, 6631–6637. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, H.; An, T.K.; Park, C.E.; Yong, K. Hybrid-type quantum-dot cosensitized ZnO nanowire solar cell with enhanced visible-light harvesting. ACS Appl. Mater. Interfaces 2013, 5, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Splendid one-dimensional nanostructures of zinc oxide: A new nanomaterial family for nanotechnology. ACS Nano 2008, 2, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Shewale, P.S.; Agawane, G.L.; Shin, S.W.; Moholkar, A.V.; Lee, J.Y.; Kim, J.H.; Uplane, M.D. Thickness dependent H2S sensing properties of nanocrystalline ZnO thin films derived by advanced spray pyrolysis. Sens. Actuators B Chem. 2013, 177, 695–702. [Google Scholar] [CrossRef]
- Paukku, Y.; Michalkova, A.; Leszczynski, J. Quantum-chemical comprehensive study of the organophosphorus compounds adsorption on zinc oxide surfaces. J. Phys. Chem. C 2009, 113, 1474–1485. [Google Scholar] [CrossRef]
- Sahay, P.P.; Nath, R.K. Al-doped ZnO thin films as methanol sensors. Sens. Actuators B Chem. 2008, 134, 654–659. [Google Scholar] [CrossRef]
- Song, H.; Yang, H.; Ma, X. A comparative study of porous ZnO nanostructures synthesized from different zinc salts as gas sensor materials. J. Alloys Compd. 2013, 578, 272–278. [Google Scholar] [CrossRef]
- Minh, V.A.; Tuan, L.A.; Huy, T.Q.; Hung, V.N.; Quy, N.V. Enhanced NH3 gas sensing properties of a QCM sensor by increasing the length of vertically orientated ZnO nanorods. Appl. Surf. Sci. 2013, 265, 458–464. [Google Scholar] [CrossRef]
- Della Gaspera, E.; Guglielmi, M.; Martucci, A.; Giancaterini, L.; Cantalini, C. Enhanced optical and electrical gas sensing response of sol-gel based NiO-Au and ZnO-Au nanostructured thin films. Sens. Actuators B Chem. 2012, 164, 54–63. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Fei, T.; Zhang, T. Enhanced acetone sensing performances of hierarchical hollow Au-loaded NiO hybrid structures. Sens. Actuators B Chem. 2012, 161, 178–183. [Google Scholar] [CrossRef]
- Xia, W.; Mei, C.; Zeng, X.; Fan, G.; Lu, J.; Meng, X.; Shen, X. Nanoplate-built ZnO hollow microspheres decorated with gold nanoparticles and their enhanced photocatalytic and gas-sensing properties. ACS Appl. Mater. Interfaces 2015, 7, 11824–11832. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.-S.; Kim, D.H.; Jeong, H.Y.; Kim, Y.H.; Nahm, S.H.; Kang, C.-Y.; Kim, J.-S.; Lee, W.; Jang, H.W. Utilization of both-side metal decoration in close-packed SnO2 nanodome arrays for ultrasensitive gas sensing. Sens. Actuators B Chem. 2015, 213, 314–321. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, Y.; Fang, Y.; Wan, Y.; Xia, W.; Sha, J. Understanding the origin of ferromagnetism in ZnO porous microspheres by systematic investigations of the thermal decomposition of Zn5(OH)8Ac2·2H2O to ZnO. J. Phys. Chem. C 2011, 115, 14576–14582. [Google Scholar] [CrossRef]
- Shen, X.; Chen, L.; Li, D.; Zhu, L.; Wang, H.; Liu, C.; Wang, Y.; Xiong, Q.; Chen, H. Assembly of colloidal nanoparticles directed by the microstructures of polycrystalline ice. ACS Nano 2011, 5, 8426–8433. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ghosh, A.; Tudu, B.; Bhuyan, L.P.; Tamuly, P.; Bhattacharyya, N.; Bandyopadhyay, R.; Chatterjee, A. Detection of linalool in black tea using a quartz crystal microbalance sensor. Sens. Actuators B Chem. 2014, 190, 318–325. [Google Scholar] [CrossRef]
- Banimuslem, H.; Hassan, A.; Basova, T.; Esenpınar, A.A.; Tuncel, S.; Durmuş, M.; Gürek, A.G.; Ahsen, V. Dye-modified carbon nanotubes for the optical detection of amines vapours. Sens. Actuators B Chem. 2015, 207, 224–234. [Google Scholar] [CrossRef]
- Donovan, S.F. New method for estimating vapor pressure by the use of gas chromatography. J. Chromatogr. A 1996, 749, 123–129. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Xu, H.; Chen, Y.; Shen, X.; Xu, Z. Photochemical deposition of Ag nanocrystals on hierarchical ZnO microspheres and their enhanced gas-sensing properties. CrystEngComm 2012, 14, 719–725. [Google Scholar] [CrossRef]
- Lin, H.-B.; Shih, J.-S. Fullerene C60-cryptand coated surface acoustic wave quartz crystal sensor for organic vapors. Sens. Actuators B Chem. 2003, 92, 243–254. [Google Scholar] [CrossRef]
- Baik, J.M.; Zielke, M.; Kim, M.H.; Turner, K.L.; Wodtke, A.M.; Moskovits, M. Tin-oxide-nanowire-based electronic nose using heterogeneous catalysis as a functionalization strategy. ACS Nano 2010, 4, 3117–3122. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Fan, G.; Hu, R.; Li, G. Enhanced Dibutyl Phthalate Sensing Performance of a Quartz Crystal Microbalance Coated with Au-Decorated ZnO Porous Microspheres. Sensors 2015, 15, 21153-21168. https://doi.org/10.3390/s150921153
Zhang K, Fan G, Hu R, Li G. Enhanced Dibutyl Phthalate Sensing Performance of a Quartz Crystal Microbalance Coated with Au-Decorated ZnO Porous Microspheres. Sensors. 2015; 15(9):21153-21168. https://doi.org/10.3390/s150921153
Chicago/Turabian StyleZhang, Kaihuan, Guokang Fan, Ruifen Hu, and Guang Li. 2015. "Enhanced Dibutyl Phthalate Sensing Performance of a Quartz Crystal Microbalance Coated with Au-Decorated ZnO Porous Microspheres" Sensors 15, no. 9: 21153-21168. https://doi.org/10.3390/s150921153
APA StyleZhang, K., Fan, G., Hu, R., & Li, G. (2015). Enhanced Dibutyl Phthalate Sensing Performance of a Quartz Crystal Microbalance Coated with Au-Decorated ZnO Porous Microspheres. Sensors, 15(9), 21153-21168. https://doi.org/10.3390/s150921153




