Multi-Sensor Approach for the Monitoring of Halitosis Treatment via Lactobacillus brevis (CD2)—Containing Lozenges—A Randomized, Double-Blind Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Experimental Section
2.1. Trial Design
2.2. Participants
- (a)
- Adult age (>18 years of age);
- (b)
- Halitosis in active phase;
- (c)
- Informed consent by the patient.
- (a)
- The need to take antibiotics for the presence of signs and/or symptoms of infection;
- (b)
- Use of non-steroidal anti-inflammatory drugs during the 30 days prior to the beginning of the study;
- (c)
- Use of steroid medications during the 30 days prior to the beginning of the study;
- (e)
- Dental care in progress;
- (f)
- Current gingivitis and periodontitis;
- (g)
- Systemic diseases such as: chronic liver disease, chronic renal failure, gastro-esophageal reflux;
- (h)
- Alcoholism and/or drug addiction.
2.3. Study Setting
2.4. Interventions
2.5. Outcomes
- -
- the Breathprint (BP) obtained with the recently introduced technique, called BIONOTE® (at t0, t1 and t2) (this is the “test technique”, described above);
- -
- the Rosenberg score [10], an organoleptic measurement assessed by two calibrated odor judges enrolled for the study, which is, at the actual state of the art, a gold standard, apart from the gas chromatography, in the diagnosis of halitosis;
- -
- the Winkel Tongue Coating Index, anterior and posterior (WTCI, anterior and posterior) [11] an index to evaluate the tongue coating, assessed by two calibrated operators enrolled for the study;
- -
- the gas chromatography score (OralChroma™) [12].
| Outcome Measures | Description of How Outcome Measures Were Assessed |
|---|---|
| Winkel Tongue Coating Index (WTCI), anterior and posterior | The dorsum of the tongue was divided into six areas (three posterior, three anterior) and tongue coating was assessed in each sextant as follows; 0 = no coating, 1 = light coating, 2 = severe coating. The WTCI was obtained by adding all six scores, for a possible range of 0–6 for WTCI anterior and 0–6 for WTCI posterior. |
| The Rosenberg score, organoleptic measurement | The organoleptic measurement depends on a trained examiner that has demonstrated reliability in smelling halitosis. The operator, preferably blindfolded, sniffs the air exhaled from the mouth at a distance of 10 cm. For recording a scale of 5 values is used. The test is considered positive when the “hedonic” value assigned to breath exceeds the number 2. The scale includes the following values: 0 = no odor; 1 = doubtful presence of halitosis; 2 = slight odor but clearly notifiable; 3 = moderate halitosis; 4 = strong halitosis; 5 = very intense halitosis. |
| Gas chromatography score, measured with OralChroma™ | Measure the molecular levels of the three major VSCs (hydrogen sulphide, methyl mercaptan and dimethyl sulfide) in a sample of mouth air. The levels (measured in ppm) are reported in a diagram from low to high level. A cognitive threshold is individuated and levels are individuated as “more than” or “less than” the cognitive threshold. |
| BP (breath print) constructed by BIONOTE® | Individual Breathprint (BP) of a patient is represented with a radar plot; equiangular radii shape each radar plot, where each radius represents one of the 28 sensor responses. The radius length gives magnitude of each sensor response (expressed in Hz, because relative to a resonant frequency shoft of the quartz slice). The radar plot “profile” consists of a line drawn connecting the data values for each radius. |

2.6. Breath Collection
2.7. Randomisation, Allocation and Blinding
2.8. Statistical Methods
2.8.1. Adverse Events
- (a)
- Type of event;
- (b)
- Characteristics of the undesired event;
- (c)
- Date of onset;
- (d)
- Duration (including whether it is expressed in minutes, hours or days);
- (e)
- Maximum intensity reached;
- (f)
- Mode of onset (immediate, gradual or asymptomatic);
- (g)
- Possible therapy of the undesired event;
- (i)
- Report of causality with the product;
- (l)
- Outcome of the undesired event: if it disappeared without modification of the treatment or after stopping the treatment, or if it remained but the study could be continued.
2.8.2. Effectiveness of the Treatment
- (1)
- Treatment efficacy assessment via each single technique:
- By ANOVA test for panellist analysis: Rosenberg score, WTCI (anterior) and WTCI (posterior) (t0, t1 and t2 assessments)
- By partial least square—discriminate analysis (PLS-DA) for OralChroma™
- By PLS-DA for BIONOTE®
- (2)
- Treatment efficacy assessment via a data fusion of the three techniques’ outputs using PLS-DA
- (3)
- Correlation study between the three techniques
3. Results and Discussion
3.1. Results
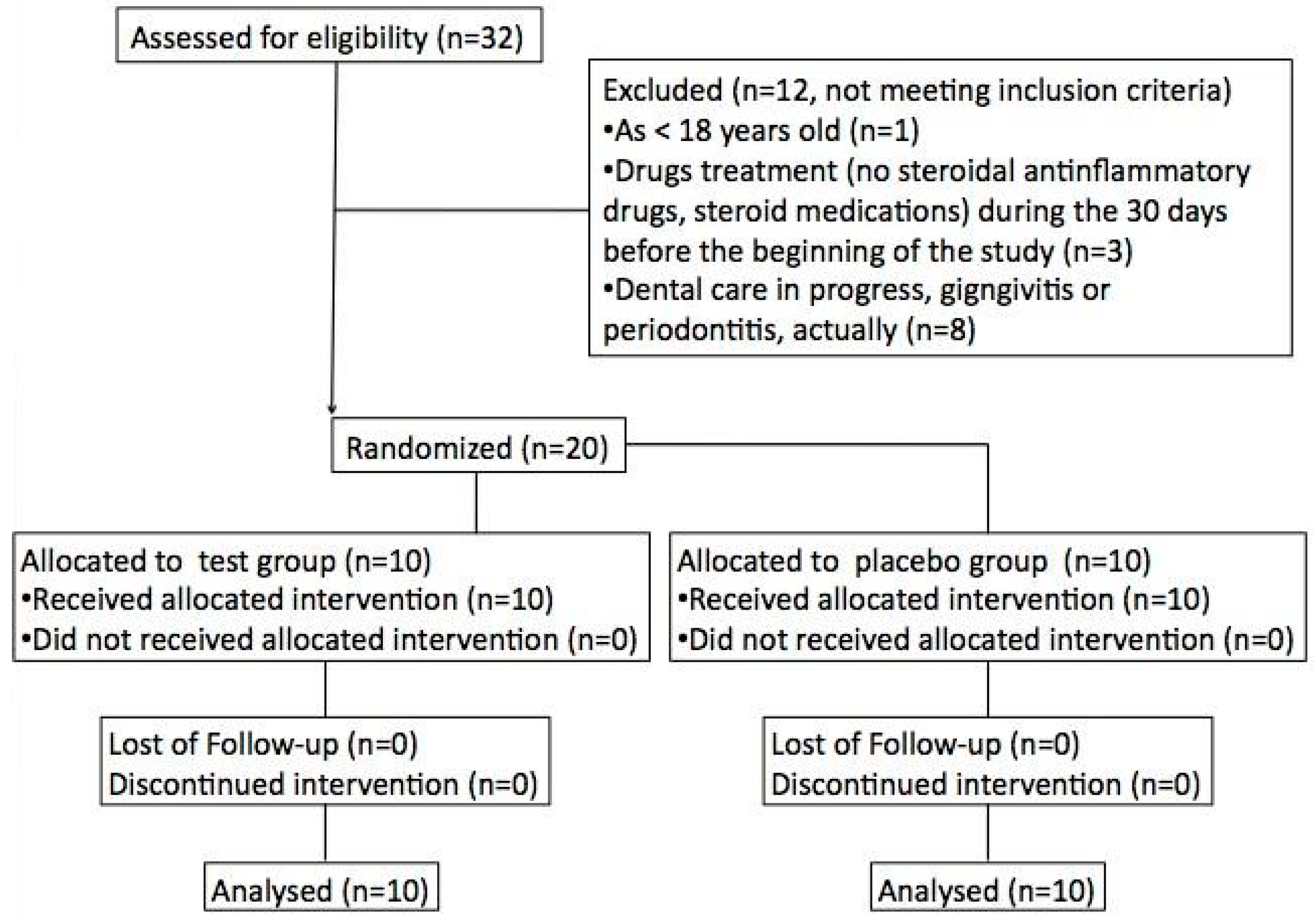
3.1.1. Participant Flow
| Lactobacillus brevis (CD2)—Containing Lozenges (n = 10) | Placebo (n = 10) | |
|---|---|---|
| Age (mean years ± SD) | 33 ± 9 | 36 ± 7 |
| Sex (males) | 12 | 11 |
3.1.2. Outcomes and Estimation
- (a)
- Treatment efficacy assessment with the different scores
- -
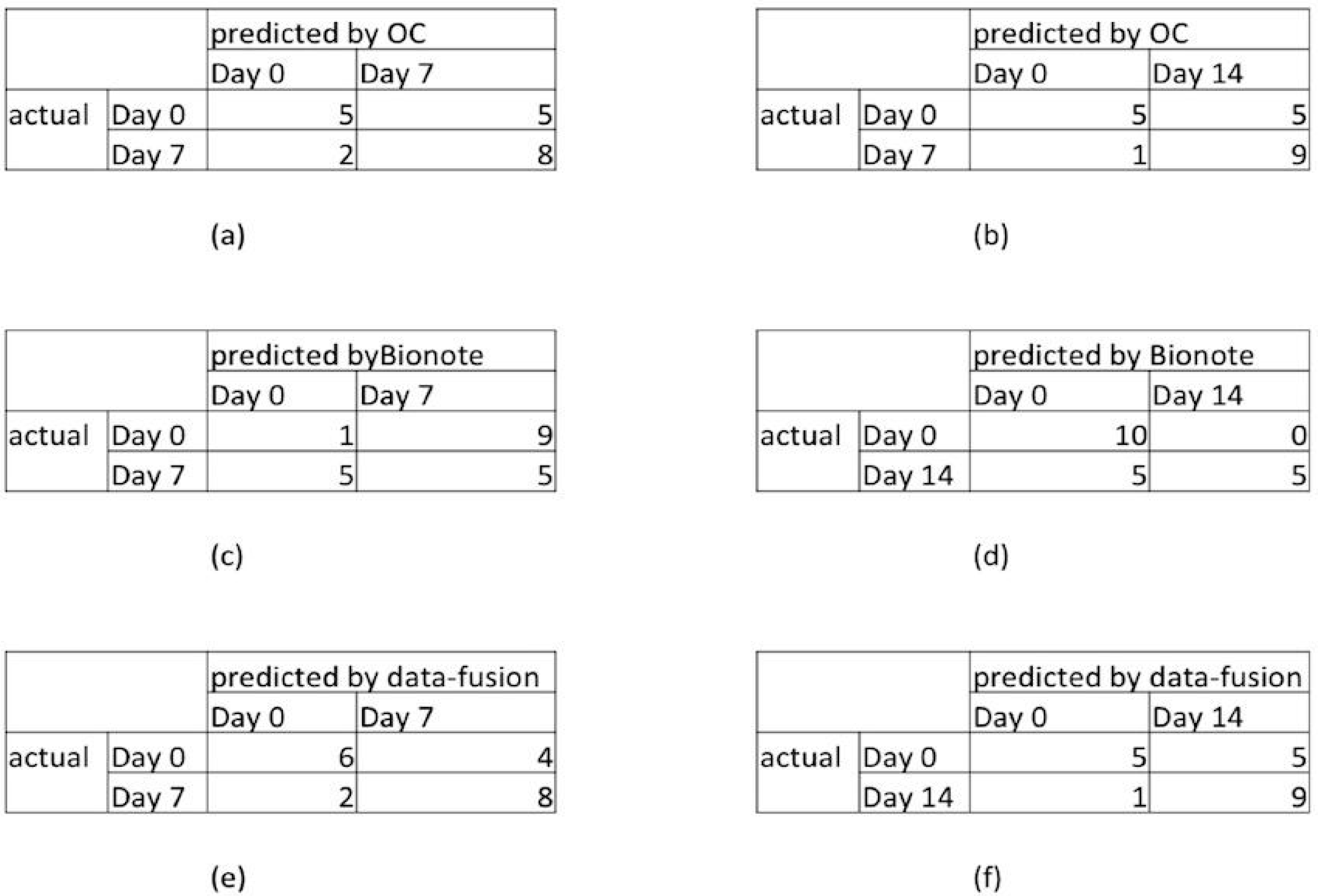
- Binary classification (0–7): correct classification of 50% of day 0 and of 80% of day 7 (see the confusion matrix reported in Figure 3a).
- -
- Binary classification (0–14): correct classification of 50% of day 0 and of 90% of day 14 (see the confusion matrix reported in Figure 3b).
- -
- Binary classification (7–14): non-significant results.
- -
- Binary classification (0–7): correct classification of 90% of day 0 and of 50% of day 7 (see the confusion matrix reported in Figure 3c).
- -
- Binary classification (0–14): correct classification of 100% of day 0, and of 50% of day 14 (see confusion matrix reported in Figure 3d).
- -
- Binary classification (7–14): not significant results.
- (b)
- Treatment efficacy assessment via a data fusion of the three technique
- -
- Binary classification (t0–t1): correlated classification in 60% of cases at t0, and 80% at t1 (see the confusion matrix reported in Figure 3e);
- -
- Binary classification (t0–t2): correlated classification in 50% of cases at t0, and 90% at t2 (see the confusion matrix reported in Figure 3f);
- -
- Binary classification (t1–t2): non-significant results.
3.1.3. Correlation Study between the Three Techniques
| RMSEC LV1 | RMSEC LV2 | RMSECV LV1 | RMSECV LV2 | #LV | LV1 PRESS | LV2 PRESS | |
|---|---|---|---|---|---|---|---|
| Binary classification (0–7) | 0.45 | 0.43 | 0.49 | 0.48 | 2 | 4.88 | 4.74 |
| Binary classification (0–14) | 0.46 | 0.45 | 0.542 | 0.541 | 2 | 5.89 | 5.87 |
| Binary classification (7–14) | 0.44 | 0.43 | 0.47 | 0.49 | 2 | 6.7 | 7.2 |
| RMSEC LV1 | RMSEC LV2 | RMSECV LV1 | RMSECV LV2 | #LV | LV1 PRESS | LV2 PRESS | |
|---|---|---|---|---|---|---|---|
| Binary classification (0–7) | 0.43 | 0.41 | 0.45 | 0.44 | 2 | 4.75 | 4.66 |
| Binary classification (0–14) | 0.44 | 0.43 | 0.47 | 0.46 | 2 | 4.89 | 4.75 |
| Binary classification (7–14) | 0.56 | 0.54 | 0.51 | 0.54 | 2 | 6.4 | 7.1 |
| RMSEC LV1 | RMSEC LV2 | RMSECV LV1 | RMSECV LV2 | #LV | LV1 PRESS | LV2 PRESS | |
|---|---|---|---|---|---|---|---|
| Binary classification (0–7) | 0.61 | 0.54 | 0.50 | 0.51 | 2 | 8.77 | 6.43 |
| Binary classification (0–14) | 0.60 | 0.58 | 0.66 | 0.59 | 2 | 8.79 | 7.11 |
| Binary classification (7–14) | 0.53 | 0.50 | 0.55 | 0.53 | 2 | 8.32 | 8.58 |
3.2. Discussion
- (a)
- The evaluation of the accuracy of a recently developed system—the BIONOTE®—in the assessment of treatment outcomes
- (b)
- Efficacy of Lactobacillus brevis (CD2)—containing lozenges in the treatment of halitosis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scarlata, S.; Pennazza, G.; Santonico, M.; Pedone, C.; Antonelli Incalzi, R. Exhaled breath analysis by electronic nose in respiratory disease. Expert Rev. Mol. Diagn. 2015, 15, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.D.; Pleil, J.D. Simply breath-taking? Developing a strategy for consistent breath sampling. J. Breath Res. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Seesaard, T.; Lorwongtragool, P.; Kerdcharoen, T. Development of fabric-based chemical gas sensors for use as wearable electronic noses. Sensors 2015, 15, 1885–1902. [Google Scholar] [CrossRef] [PubMed]
- Pennazza, G.; Santonico, M.; Finazzi Agrò, A. Narrowing the gap between breathprinting and disease diagnosis, a sensor perspective. Sens. Actuators B Chem. 2013, 179, 270–275. [Google Scholar] [CrossRef]
- Salako, N.O.; Philip, L. Comparison of the use of the Halimeter and the Oral Chroma in the assessment of the ability of common cultivable oral anaerobic bacteria to produce malodorous volatile sulfur compounds from cysteine and methionine. Med. Princ. Pract. 2011, 20, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Riccia, D.N.; Bizzini, F.; Perilli, M.G.; Polimeni, A.; Trinchieri, V.; Amicosante, G.; Cifone, M.G. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007, 4, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.L.; Murty, V.L.N.; Piotrowski, J. Salivary Mucins in Oral Mucosal Defense. Gen. Pharmacol. 1996, 27, 761–771. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Slomiany, A. Porphyromonas gingivalis lipopolysaccharide interferes with salivary mucin synthesis through inducible nitric oxide synthase activation by ERK and p38 kinase. Biochem. Biophys. Res. Commun. 2002, 297, 1149–1153. [Google Scholar] [CrossRef]
- Casiano-Colon, A.; Marquis, R.E. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl. Environ. Microbiol. 1988, 54, 1318–1324. [Google Scholar] [PubMed]
- Rosenberg, M.; McCulloch, C.A. Measurement of oral malodor: Current methods and future prospects. J. Periodontol. 1992, 63, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Winkel, E.G.; Roldan, S.; van Winkelhoff, A.J.; Herrera, D.; Sanz, M. Clinical effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc-lactate on oral halitosis. J. Clin. Periodontol. 2003, 30, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Tangerman, A.; Winkel, E.G. The portable gas chromatograph OralChroma™: A method of choice to detect oral and extra-oral halitosis. J. Breath Res. 2008, 2. [Google Scholar] [CrossRef] [PubMed]
- Santonico, M.; Pennazza, G.; Grasso, S.; D’Amico, A.; Bizzarri, M. Design and test of a biosensor-based multisensorial system: A proof of concept study. Sensors 2013, 13, 16625–16640. [Google Scholar] [CrossRef] [PubMed]
- Antonelli Incalzi, R.; Pennazza, G.; Scarlata, S.; Santonico, M.; Vernile, C.; Cortese, L.; Frezzotti, E.; Pedone, C.; D’Amico, A. Comorbidity modulates non invasive ventilation-induced changes in breath print of obstructive sleep apnea syndrome patients. Sleep Breath. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Pennazza, G.; Santonico, M.; Antonelli Incalzi, R.; Scarlata, S.; Chiurco, D.; Vernilea, C.; D’Amico, A. Measure chain for exhaled breath collection and analysis: A novel approach suitable for frail respiratory patients. Sens. Actuators B Chem. 2014, 204, 578–587. [Google Scholar] [CrossRef]
- Pennazza, G.; Santonico, M.; D’Amico, A.; Antonelli Incalzi, R.; Petriaggi, M. Pneumopipe—Auxiliary Device for Collection and Sampling of Exhaled Air. Domanda EU 12425057.2, 25 September 2013. [Google Scholar]
- Tenax® GR Adsorbent Resin for Trapping Volatiles. Scientific Instrument Services, Inc. Available online: http://www.sisweb.com/index/referenc/ tenaxgrm.htm (accessed on 20 May 2011).
- Kapatral, V.; Anderson, I.; Ivanova, N.; Reznik, G.; Los, T.; Lykidis, A.; Bhattacharyya, A.; Bartman, A.; Gardner, W.; Grechkin, G.; et al. Genome Sequence and Analysis of Oral Bacterium Fusobacterium nucleatum Strain ATCC 25586. J. Bacteriol. 2002, 184, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Zilm, P.S.; Gully, N.J.; Rogers, A.H. Growth pH and transient increases in amino acid availability influence polyglucose synthesis by Fusobacterium nucleatum grown in continuous culture. FEMS Microbiol. Lett. 2002, 215, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem. J. 2003, 376, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Packer, L. S-Adenosylmethionine: Molecular, biological, and clinical aspects—An introduction. Am. J. Clin. Nutr. 2002, 76, 1148S–1150S. [Google Scholar] [PubMed]
- Yerlikaya, A. Polyamines and S-Adenosylmethionine Decarboxylase. Turk. J. Biochem. 2004, 29, 208–214. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, E.; Tecco, S.; Santonico, M.; Vernile, C.; Ciciarelli, D.; Tarantino, E.; Marzo, G.; Pennazza, G. Multi-Sensor Approach for the Monitoring of Halitosis Treatment via Lactobacillus brevis (CD2)—Containing Lozenges—A Randomized, Double-Blind Placebo-Controlled Clinical Trial. Sensors 2015, 15, 19583-19596. https://doi.org/10.3390/s150819583
Marchetti E, Tecco S, Santonico M, Vernile C, Ciciarelli D, Tarantino E, Marzo G, Pennazza G. Multi-Sensor Approach for the Monitoring of Halitosis Treatment via Lactobacillus brevis (CD2)—Containing Lozenges—A Randomized, Double-Blind Placebo-Controlled Clinical Trial. Sensors. 2015; 15(8):19583-19596. https://doi.org/10.3390/s150819583
Chicago/Turabian StyleMarchetti, Enrico, Simona Tecco, Marco Santonico, Chiara Vernile, Daniele Ciciarelli, Ester Tarantino, Giuseppe Marzo, and Giorgio Pennazza. 2015. "Multi-Sensor Approach for the Monitoring of Halitosis Treatment via Lactobacillus brevis (CD2)—Containing Lozenges—A Randomized, Double-Blind Placebo-Controlled Clinical Trial" Sensors 15, no. 8: 19583-19596. https://doi.org/10.3390/s150819583









