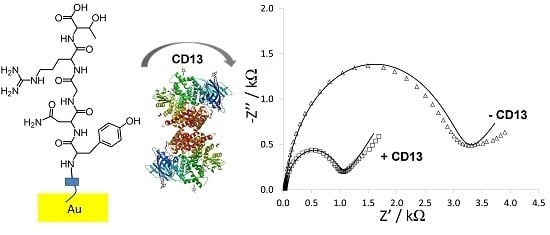
Electrochemical Characterization of Protein Adsorption onto YNGRT-Au and VLGXE-Au Surfaces
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Peptide-Au Films
2.2. Electrochemical Measurements
3. Results and Discussion
3.1. Preparation and Characterization of Peptide-Au Surfaces


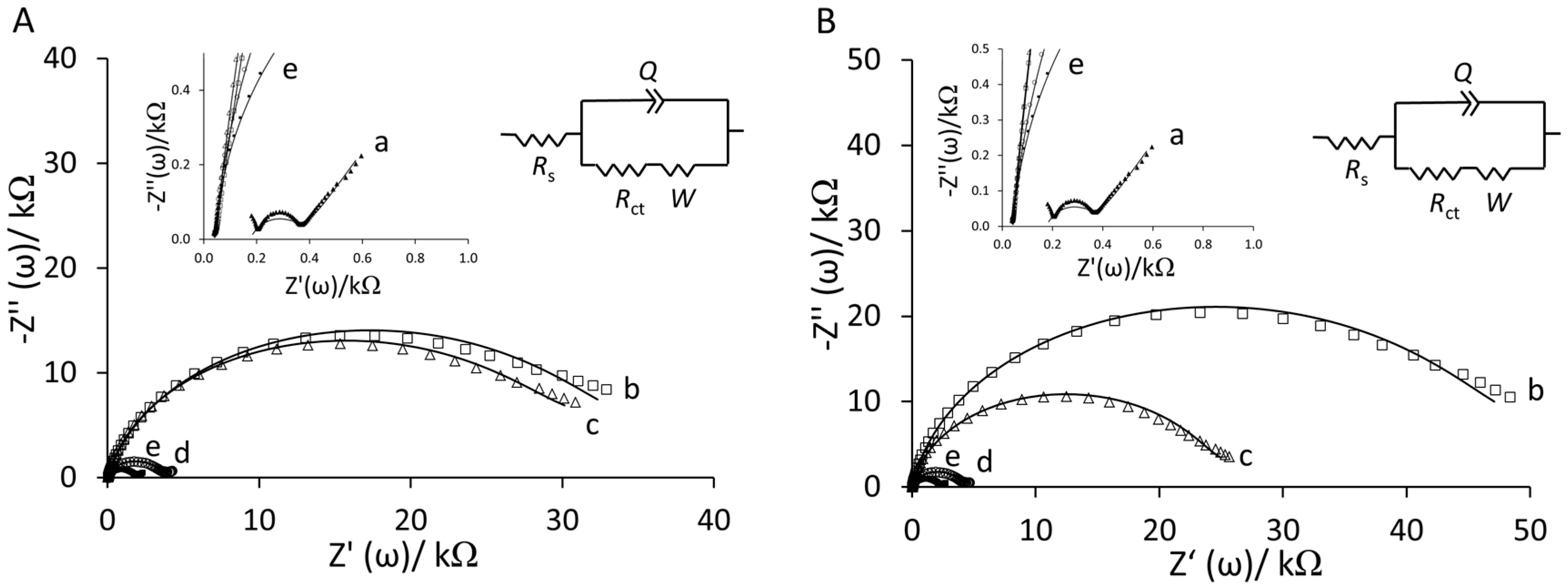

| RS (kΩ) | CPE (µΩ−1·sn) | n | Rct (kΩ) | W (µΩ−1·s0.5) | |
|---|---|---|---|---|---|
| YNGRT-Au | 0.04 ± 0.01 | 1.10 ± 0.13 | 0.94 ± 0.01 | 2.46 ± 0.69 | 523 ± 86.2 |
| VLGXE-Au | 0.04 ± 0.01 | 1.33 ± 0.23 | 0.93 ± 0.01 | 1.82 ± 0.35 | 570 ± 88.9 |
3.2. Protein Binding to Peptide-Au Surfaces
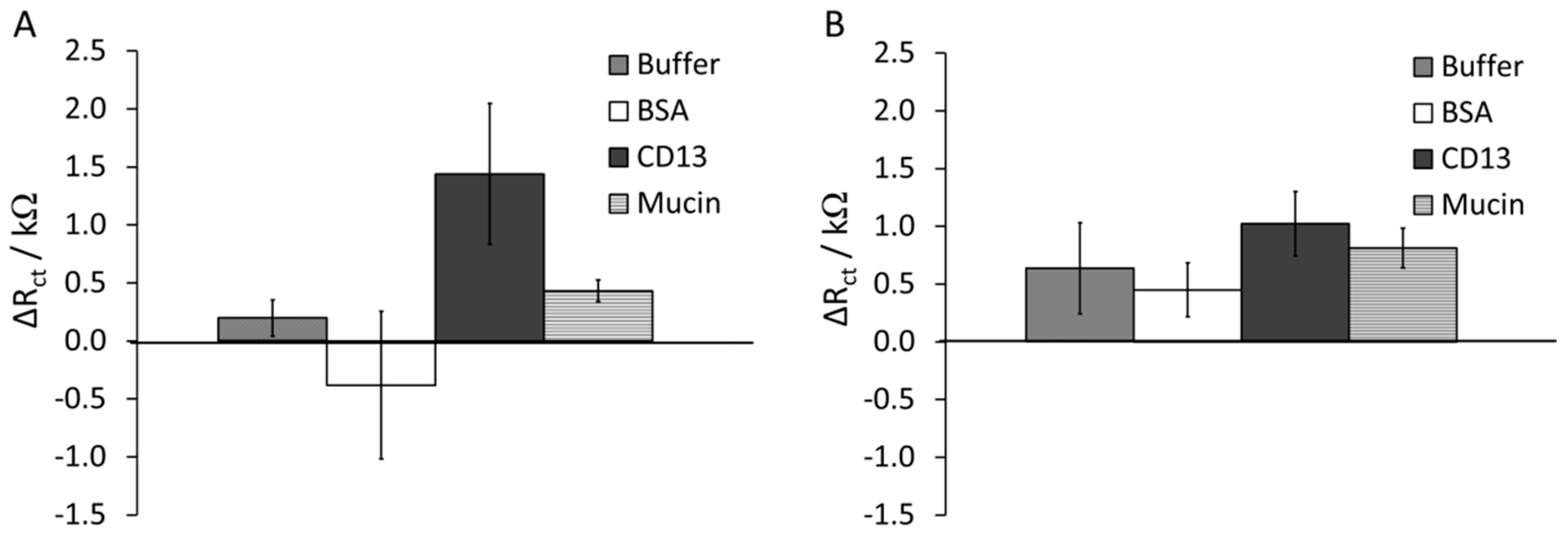
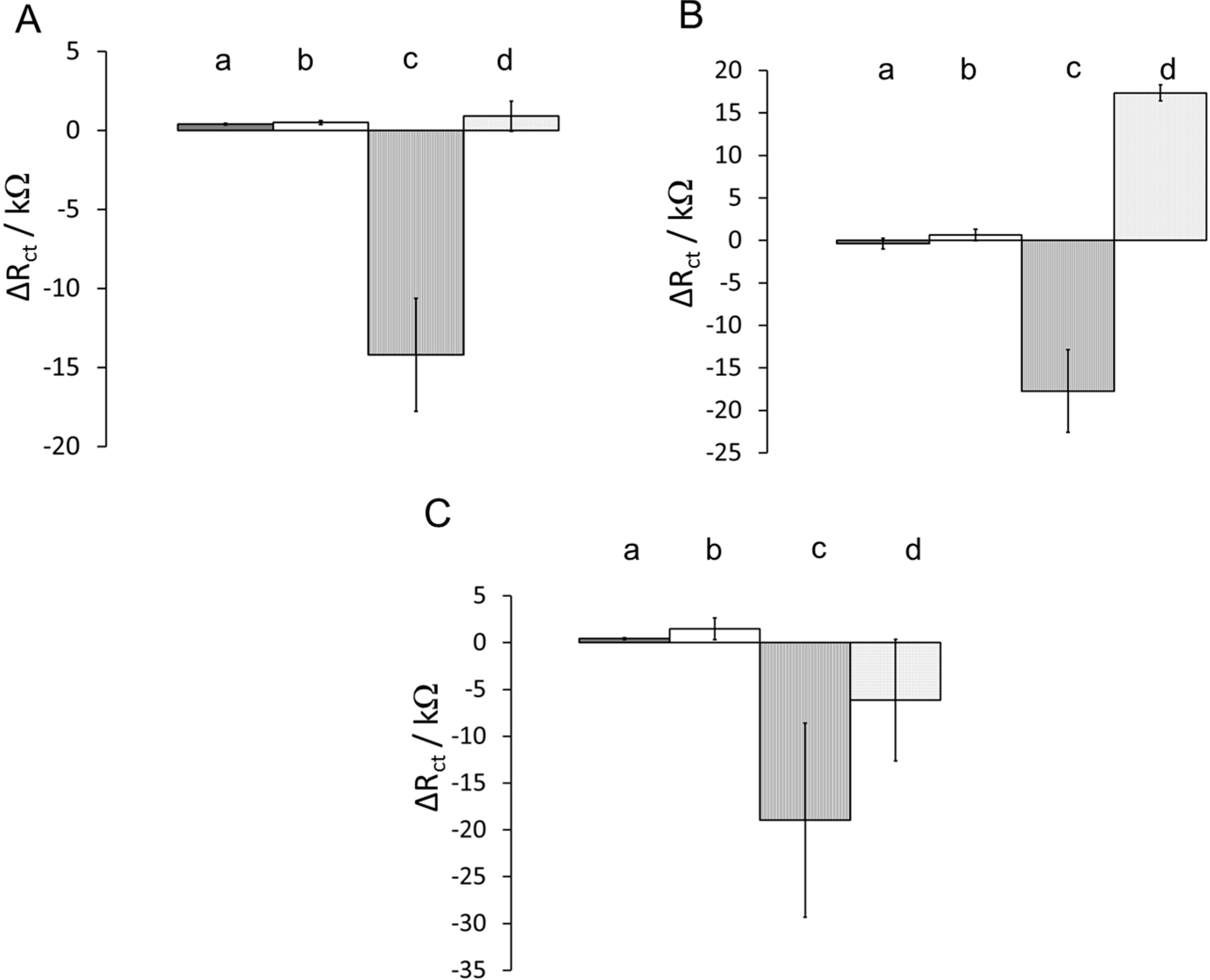
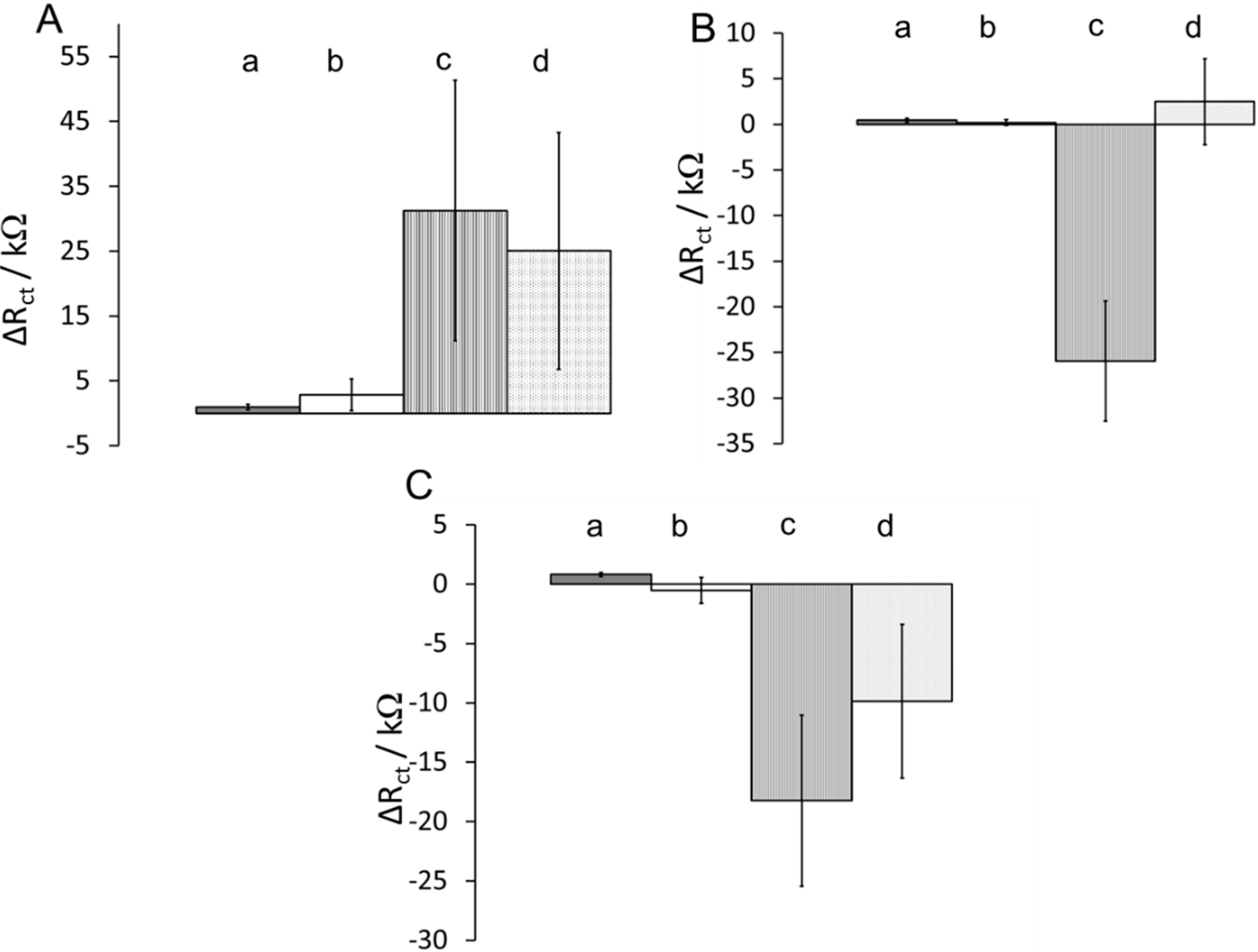
3.3. Effects of Surface Composition on Protein Adsorption
| Combination | Blocking Agent | Diluent | Hydrophobicity |
|---|---|---|---|
| a | Ethanolamine | 2-mercaptoethanol | Highly hydrophilic |
| b | Ethanolamine | hexanethiol | Partially hydrophilic |
| c | n-butylamine | hexanethiol | Highly hydrophobic |
| d | n-butylamine | 2-mercaptoethanol | Partially hydrophobic |
4. Conclusions/Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rivas, D.; Luo, D.; Cai, X.; Valera, F.S.; Dontha, N. DNA-Modified Electrode for the Detection of Aromatic Amines. Anal. Chem. 1996, 68, 4365–4369. [Google Scholar] [CrossRef]
- Lin, S.; Drake, L.R.; Rayson, G.D. Applications of Frontal Affinity Chromatography to the Study of Interactions between Metal Ions and a Complex Biomaterial. Anal. Chem. 1996, 68, 4087–4093. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.P.; Kasemo, B. Monte Carlo simulation of the kinetics of protein adsorption. Proteins 1998, 30, 177–182. [Google Scholar] [CrossRef]
- Zhdanov, V.P.; Kasemo, B. Van der Waals Interaction during Protein Adsorption on a Solid Covered Thin Film. Langmuir 2001, 17, 5407–5409. [Google Scholar] [CrossRef]
- Sigal, G.B.; Mrksich, M.; Whitesides, G.M. Effect of surface wettability on the adsorption of proteins and detergents. J. Am. Chem. Soc. 1998, 120, 3464–3473. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Ratner, B.D.; Barbosa, M.A. Protein adsorption on mixtures of hydroxyl- and methylterminated alkanethiols self-assembled monolayers. J. Biomed. Mater. Res. A 2003, 67, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Miyahara, Y. Interpretation of Protein Adsorption through Its Intrinsic Electric Charges: A Comparative Study Using a Field-Effect Transistor, Surface Plasmon Resonance, and Quartz Crystal Microbalance. Langmuir 2012, 28, 14730–14738. [Google Scholar] [CrossRef] [PubMed]
- Tirado, J.D.; Acevedo, D.; Bretz, R.L.; Abruna, H.D. Adsorption Dynamics of Electroactive Self-Assembling Molecules. Langmuir 1994, 10, 1971–1979. [Google Scholar] [CrossRef]
- Leopold, M.C.; Bowden, E.F. The Measurement of the Rate of Adsorption of Electroactive Cytochrome c to Modified Gold Electrodes by Electrochemical Impedance Spectroscopy. Langmuir 2002, 18, 5283–5286. [Google Scholar]
- Leopold, M.C.; Bowden, E.F. Influence of Gold Substrate Topography on the Voltammetry of Cytochrome c Adsorbed on Carboxylic Acid Terminated Self-Assembled Monolayers. Langmuir 2002, 18, 2239–2245. [Google Scholar] [CrossRef]
- Heli, H.; Sattarahmady, N.; Jabbari, A.; Moosavi-Movahedi, A.A.; Hakimelahi, G.H.; Tsai, F.J. Adsorption of human serum albumin onto glassy carbon surface-applied to albumin-modified electrode: Mode of protein-ligand interactions. J. Electroanal. Chem. 2007, 610, 67–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Fung, Y.; Sun, H.; Zhu, D.; Yao, S. Studies of protein adsorption on polymer coatings surface by combining quartz crystal microbalance with electrochemical impedance methods. Sens. Actuators B Chem. 2005, 108, 933–942. [Google Scholar] [CrossRef]
- Jackson, D.; Omanovic, S.; Roscoe, S.G. Electrochemical studies of the adsorption behavior of serum proteins on titanium. Langmuir 2000, 16, 5449–5457. [Google Scholar] [CrossRef]
- Oliva, F.Y.; Avalle, L.B.; Macagno, V.A.; De Pauli, C.P. Study of human serum albumin-TiO2 nanocrystalline electrodes interaction by impedance electrochemical spectroscopy. Biophys. Chem. 2001, 91, 141–155. [Google Scholar] [CrossRef]
- Oliva, F.Y.; Avalle, L.B.; Cám, O.R. Electrochemical behavior of human serum albumin-TiO2 nanocrystalline electrodes studied as a function of pH: Part 1. Voltammetric response. J. Electroanal. Chem. 2002, 534, 19–29. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Roscoe, S.G. Electrochemical studies of the interfacial behavior of insulin. J. Colloid Interface Sci. 1996, 184, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Philips, R.K.R.; Omanovic, S.; Roscoe, S.G. Electrochemical studies of the effect of temperature on the adsorption of yeast alcohol dehydrogenase at Pt. Langmuir 2001, 17, 2471–2477. [Google Scholar] [CrossRef]
- Roscoe, S.G.; Fuller, K.L.; Robitalle, G. An electrochemical study of the effect of temperature on the adsorption behavior of β-lactoglobulin. J. Colloid Interface Sci. 1992, 152, 429–441. [Google Scholar] [CrossRef]
- Omanovic, S.; Roscoe, S.G. Interfacial behavior of beta-lactoglobulin at a stainless steel surface: An electrochemical impedance spectroscopy study. J. Colloid Interface Sci. 2000, 227, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Stryker, G.A.; Mernaugh, R.L.; Yu, L.; Yan, H.P.; Zeng, X.Q. Single-chain fragment variable antibody piezoimmunosensors. Anal. Chem. 2005, 77, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.C.; Luther, G.W., III; Waite, J.H. The adsorption of the adhesive protein of the blue mussel mytilus edulis L onto type 304L stainless steel. J. Colloid Interface Sci. 1994, 168, 206–216. [Google Scholar] [CrossRef]
- Merritt, K.; Brown, S.A.; Sharkey, N.A. The binding of metal salts and corrosion products to cells and proteins in vitro. J. Biomed. Mater. Res. 1984, 18, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.; Fujii, H.; Saitoh, Y.; Koizumi, K.; Aozuka, Y.; Sekine, K.; Yamada, M.; Saiki, I.; Nishikawa, K. Aminopeptidase N (APN/CD13) is selectively expressed in vascular endothelial cells and plays multiple roles in angiogenesis. Cancer Lett. 2006, 243, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wickstrom, M.; Larsson, R.; Nygrem, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Chaturvedi, P.; Batra, S.K. Emerging roles of MUC4 in cancer: A novel target for diagnosis and therapy. Cancer Res. 2007, 67, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Bresalier, R.S. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.P.; Chakraborty, S.; Souchek, J.; Batra, S.K. Mucin-based targeted pancreatic cancer therapy. Curr. Pharm. Des. 2012, 18, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Soudy, R.; Ahmed, S.; Kaur, K. NGR peptide ligands for targeting CD13/APN identified through peptide array screening resemble fibronectin sequences. ACS Comb. Sci. 2012, 14, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mathews, A.S.; Byeon, N.; Lavasanifar, A.; Kaur, K. Peptide arrays for screening cancer specific peptides. Anal. Chem. 2010, 82, 7533–7541. [Google Scholar] [CrossRef] [PubMed]
- Gruzman, A.; Hidmi, A.; Katzhendler, J.; Haj-Yehie, A.; Sasson, S. Synthesis and characterization of new and potent alpha-lipoic acid derivatives. Bioorganic Med. Chem. 2004, 12, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Hoefling, M.; Monti, S.; Corni, S.; Gottschalk, K.E. Interaction of β-sheet folds with a gold surface. PLoS ONE 2011, 6, e20925. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Lee, S.; Muller, M.; Rezwan, K.; Spencer, N. Porcine gastric mucin (PGM) at the water/poly(dimethylsiloxane) (PDMS) interface: Influence of pH and ionic strength on its conformation, adsorption, and aqueous lubrication properties. Langmuir 2005, 21, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Hara, Y.; Kubota, T.; Shiba, K.; Hosaki, S. A family with high serum leucine aminopeptidase activity derived from a novel variant CD13. Clin. Chem. 1998, 44, 215–220. [Google Scholar] [PubMed]
- Moulton, S.E.; Barisci, J.N.; Bath, A.; Stella, R.; Wallace, G.G. Investigation of protein adsorption and electrochemical behavior at a gold electrode. J. Colloid Interface Sci. 2002, 261, 312–319. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Y.; Xu, M.; Li, Z.; Yuan, Y.; Yao, S. Crystal quartz impedance and electrochemical impedance measurements during adsorption of bovine serum albumin onto bare and cystaine- or thiophenol-modified gold electrodes. J. Electroanal. Chem. 1999, 478, 1–8. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzeciakiewicz, H.; Esteves-Villanueva, J.; Soudy, R.; Kaur, K.; Martic-Milne, S. Electrochemical Characterization of Protein Adsorption onto YNGRT-Au and VLGXE-Au Surfaces. Sensors 2015, 15, 19429-19442. https://doi.org/10.3390/s150819429
Trzeciakiewicz H, Esteves-Villanueva J, Soudy R, Kaur K, Martic-Milne S. Electrochemical Characterization of Protein Adsorption onto YNGRT-Au and VLGXE-Au Surfaces. Sensors. 2015; 15(8):19429-19442. https://doi.org/10.3390/s150819429
Chicago/Turabian StyleTrzeciakiewicz, Hanna, Jose Esteves-Villanueva, Rania Soudy, Kamaljit Kaur, and Sanela Martic-Milne. 2015. "Electrochemical Characterization of Protein Adsorption onto YNGRT-Au and VLGXE-Au Surfaces" Sensors 15, no. 8: 19429-19442. https://doi.org/10.3390/s150819429