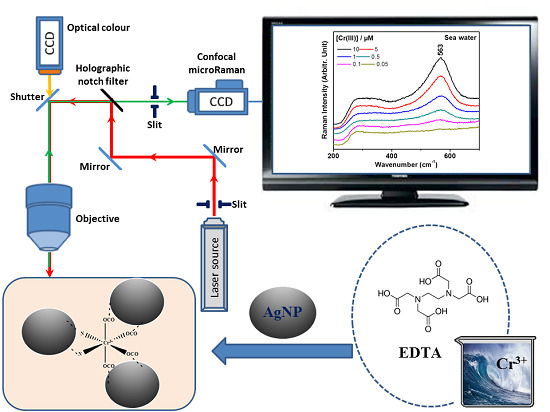
Silver Nanoparticle-Enhanced Resonance Raman Sensor of Chromium(III) in Seawater Samples
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Equipment and Characterization Methods
2.3. Sample Preparation
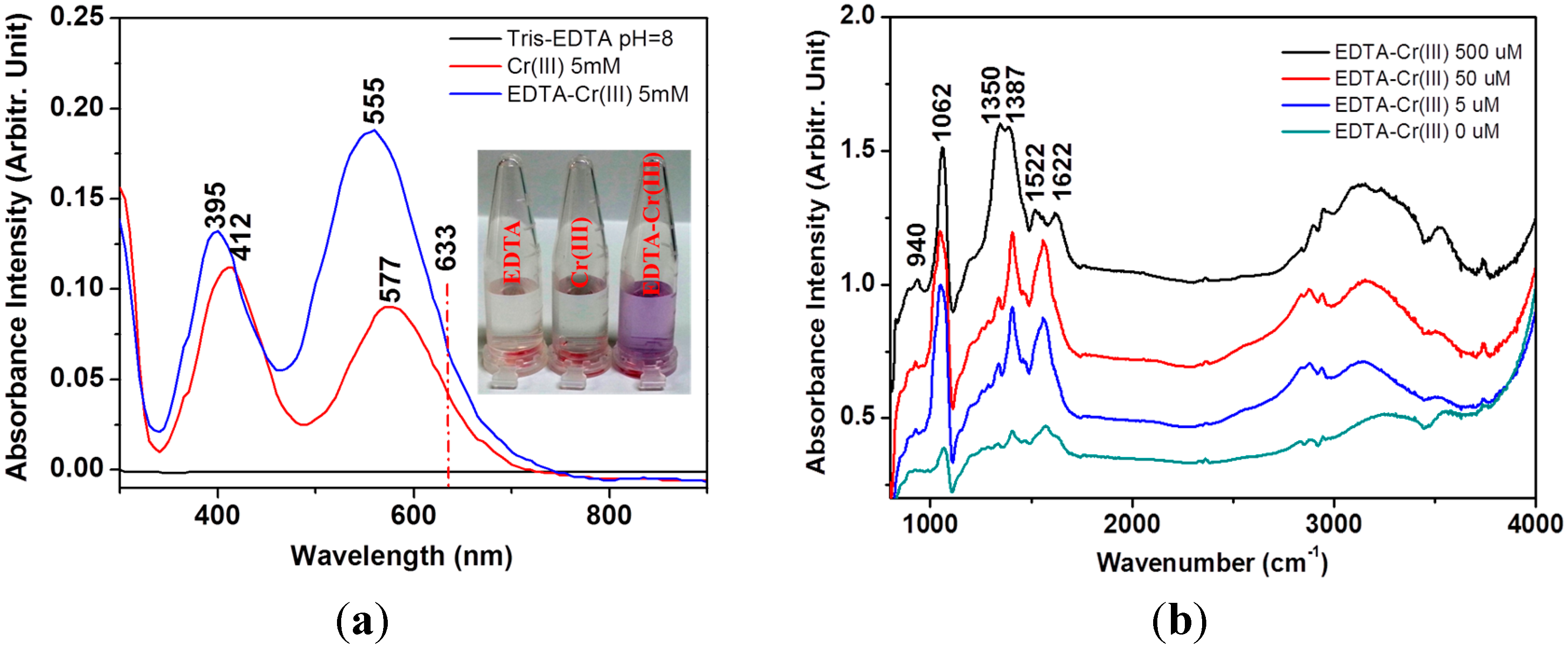
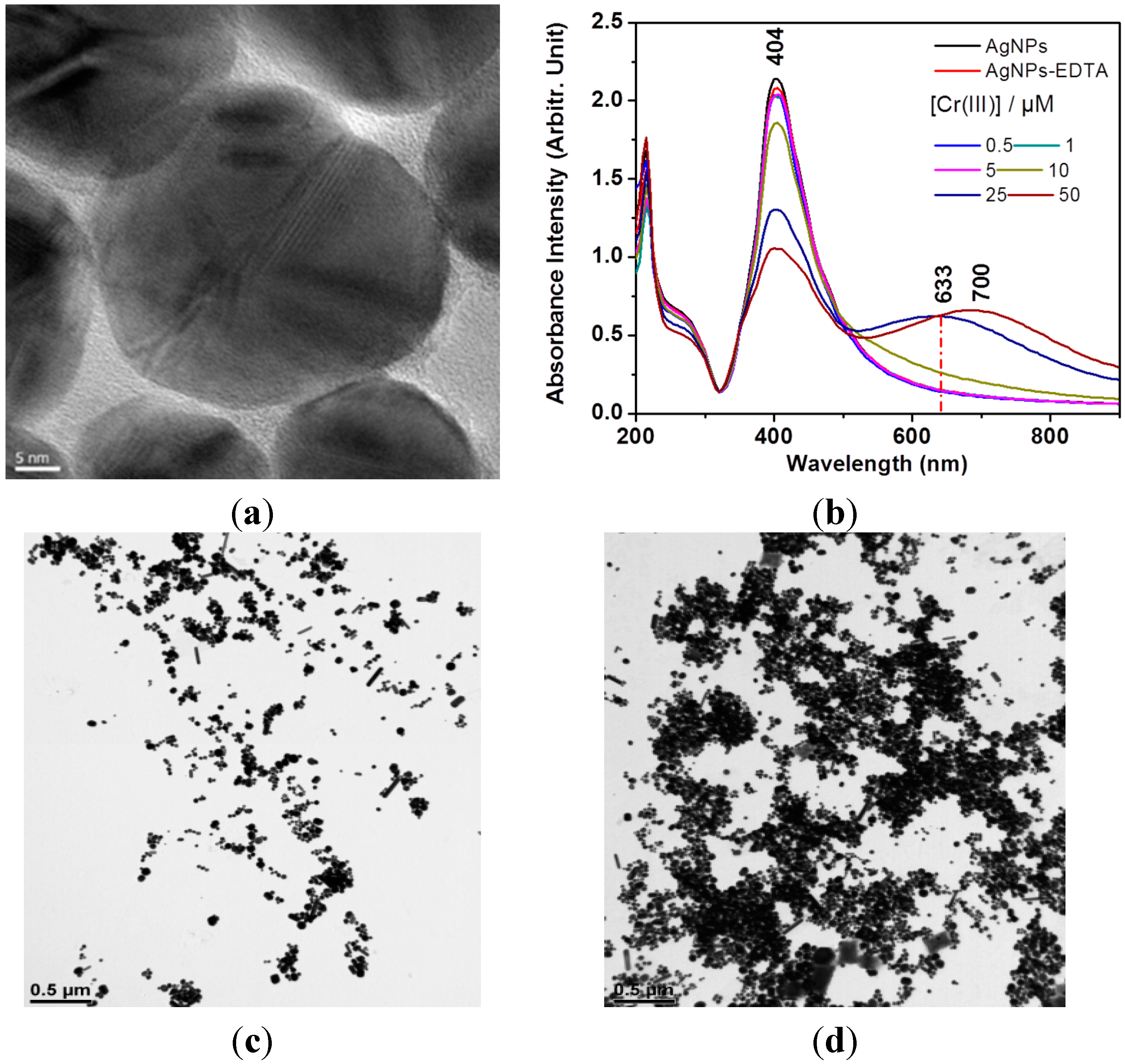
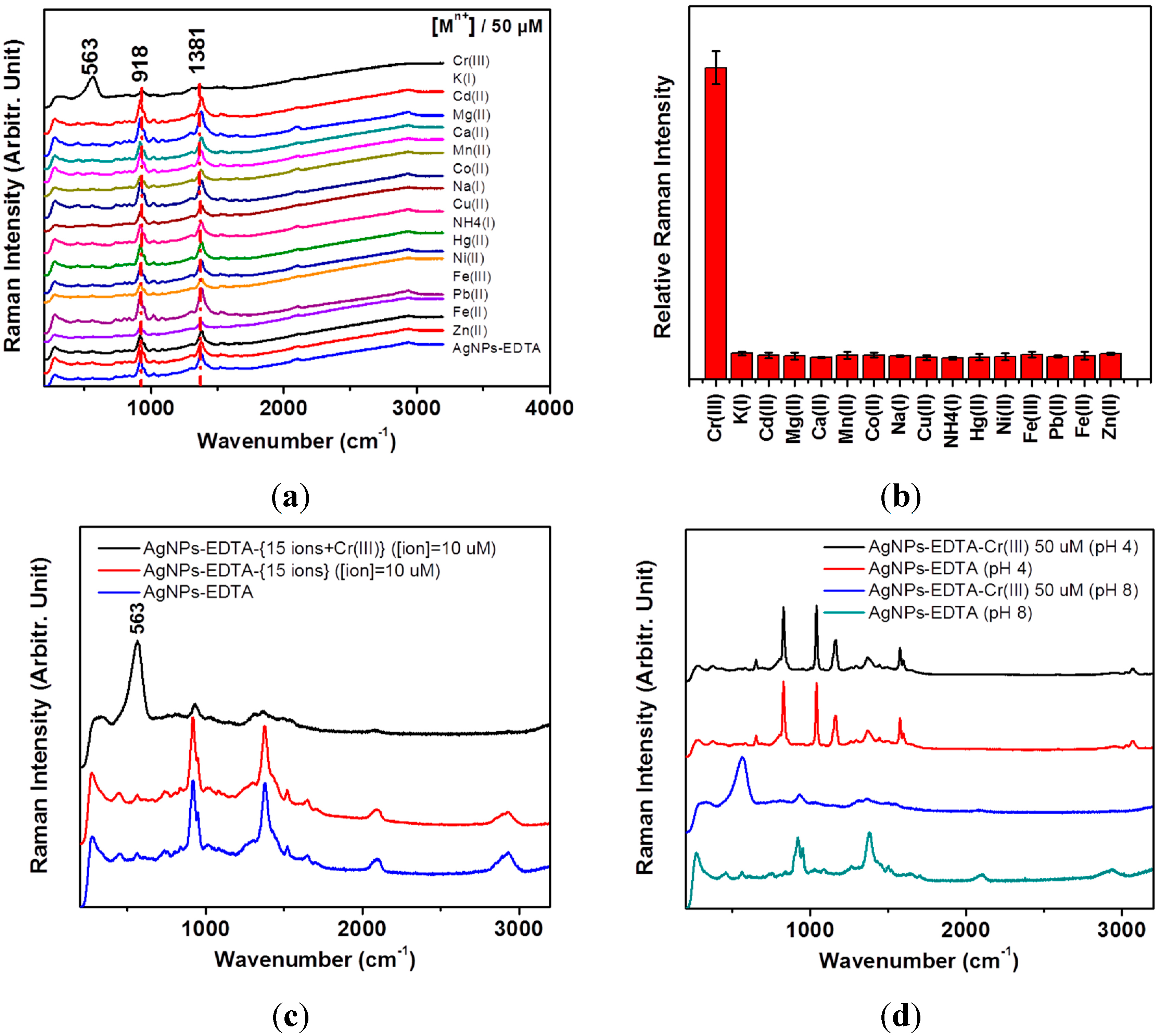
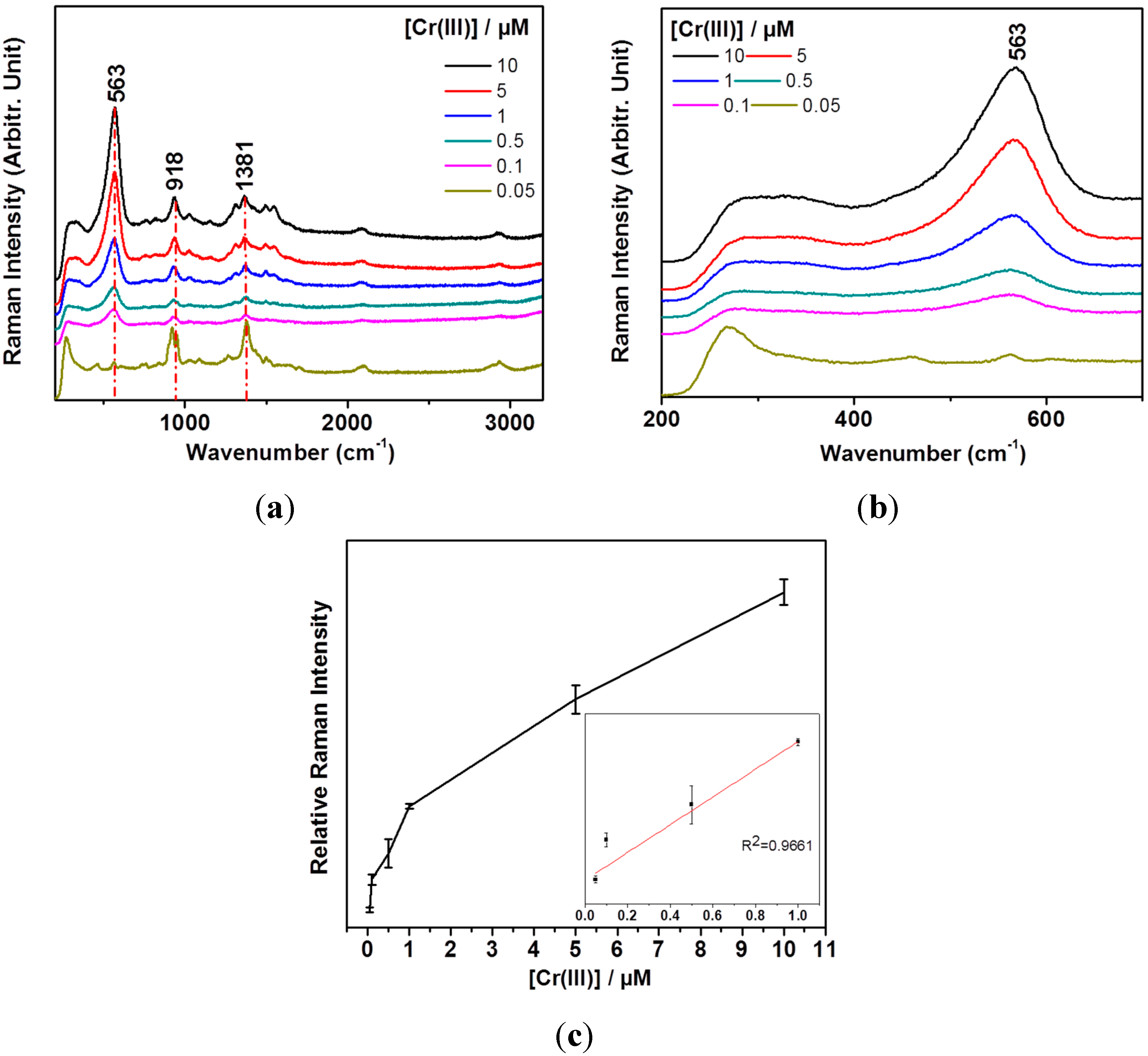
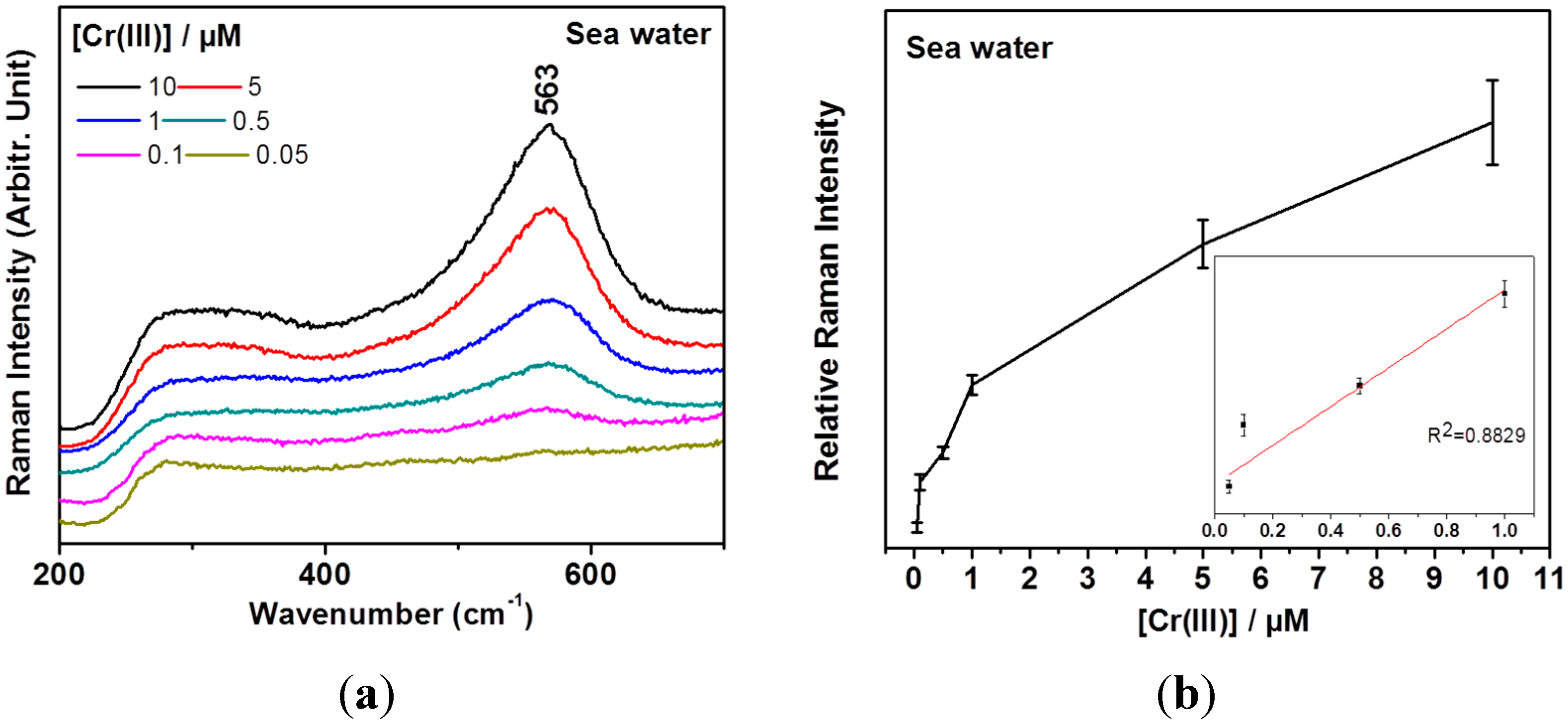
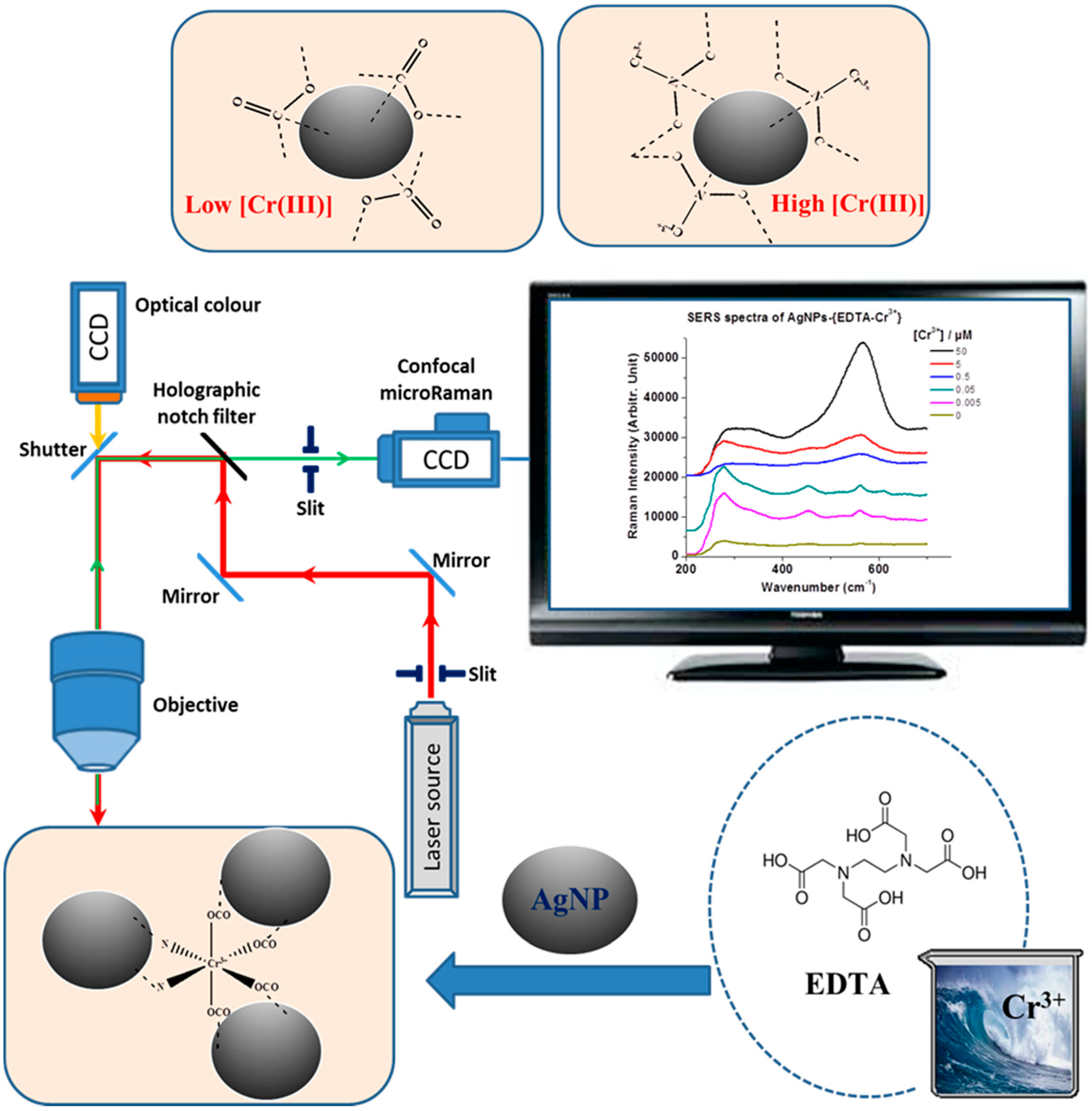
3. Results and Discussion
| Sample | Compositions | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Cd | Mg | Ca | Mn | Co | Na | Cu | Hg | Ni | Fe | Pb | Zn | Cr | |
| Distilled | ND * | ND | ND | 0.03 | ND | ND | 1.05 | ND | ND | ND | ND | ND | ND | ND |
| Seawater | 521.78 | ND | 1,059.81 | 344.90 | 0.03 | ND | 10,001.92 | 0.02 | ND | ND | ND | ND | 0.02 | ND |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aragay, G.; Pons, J.; Merkoci, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Johnson, R.C.; Hupp, J.T. Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Lee, H.; Xu, L.; Koh, D.; Nyayapathi, N.; Oh, K.W. Various on-chip sensors with microfluidics for biological applications. Sensors 2014, 14, 17008–17036. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jamal Deen, M.; Kumar, S.; Ravi Selvaganapathy, P. Raman spectroscopy for in-line water quality monitoring—Instrumentation and potential. Sensors 2014, 14, 17275–17303. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.L.; Stratis-Cullum, D.N.; Hankus, M.E. A nanosensor for TNT detection based on molecularly imprinted polymers and surface enhanced Raman scattering. Sensors 2011, 11, 2700–2714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, X.; Huang, Y.; Li, Z.; Zhang, Z. Rapid detection of polychlorinated biphenyls at trace levels in real environmental samples by surface-enhanced Raman scattering. Sensors 2011, 11, 10851–10858. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Rodriguez-Loureiro, I.; Correa-Duarte, M.A.; Lee, Y.H.; Ling, X.Y.; de Abajo, F.J.G.; Alvarez-Puebla, R.A. Chemical speciation of heavy metals by surface-enhanced Raman scattering spectroscopy: Identification and quantification of inorganic- and methyl-mercury in water. Nanoscale 2014, 6, 8368–8375. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Perricone, N.V.; Bagchi, D.; Yasmin, T.; Stohs, S.J. Comparing metabolic effects of six different commercial trivalent chromium compounds. J. Inorg. Biochem. 2008, 102, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B.; Love, S.T. The need for combined inorganic, biochemical, and nutritional studies of chromium(III). Chem. Biodivers. 2012, 9, 1923–1241. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.R.; Dadfarnia, S.; Shabani, A.M.H. Speciation and determination of ultra trace amounts of chromium by solidified floating organic drop microextraction (SFODME) and graphite furnace atomic absorption spectrometry. J. Hazard. Mater. 2011, 186, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Karak, D.; Banerjee, A.; Sahana, A.; Guha, S.; Lohar, S.; Adhikari, S.S.; Das, D. 9-Acridone-4-carboxylic acid as an efficient Cr(III) fluorescent sensor: trace level detection, estimation and speciation studies. J. Hazard. Mater. 2011, 188, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, R.A.; Gismera, M.J.; Sevilla, M.T.; Procopio, J.R. Potentiometric screen-printed bisensor for simultaneous determination of chromium(III) and chromium(VI). Electroanalysis 2011, 23, 287–294. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.S.; Meng, X.Y.; Zhang, Y.Y.; Yang, L.; Li, Z.H.; Zhang, J.H.; Wang, X.R.; Liu, J.Q.; Lu, S.Y.; et al. Production of a monoclonal antibody and development of an immunoassay for detection of Cr(III) in water samples. Chemosphere 2013, 93, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, H.; Yang, L.; Liu, J. Sensitive and selective SERS probe for trivalent chromium detection using citrate attached gold nanoparticles. Nanoscale 2012, 4, 6442–6448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Q.; Wang, T.; Yun, Z.; Li, G.; Liu, J.; Jiang, G. Facile preparation of glutathione-stabilized gold nanoclusters for selective determination of chromium(III) and chromium(VI) in environmental water samples. Anal. Chim. Acta 2013, 770, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, J.; Jin, Y. 11-Mercaptoundecanoic acid directed one-pot synthesis of water-soluble fluorescent gold nanoclusters and their use as probes for sensitive and selective detection of Cr3+ and Cr6+. J. Mater. Chem. C 2013, 1, 138–143. [Google Scholar] [CrossRef]
- Dang, Y-Q.; Li, H-W.; Wang, B.; Li, L.; Wu, Y. Selective detection of trace Cr3+ in aqueous solution by using 5,5'-dithiobis (2-nitrobenzoic acid)-modified gold nanoparticles. ACS Appl. Mater. Interfaces 2009, 1, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.I.; Dasary, S.S.R.; Singh, A.K.; Glenn, Z.; Jamison, H.; Ray, P.C.; Yu, H. Sensitive and selective detection of trivalent chromium using hyper Rayleigh scattering with 5,5'-dithio-bis-(2-nitrobenzoic acid)-modified gold nanoparticles. Sens. Actuators B Chem. 2013, 178, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cai, H-H.; Yang, F.; Lin, D.; Yang, P-H.; Cai, J. Highly sensitive detection of chromium(III) ions by resonance Rayleigh scattering enhanced by gold nanoparticles. Spectrochim. Acta 2014, 118A, 776–781. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, S.; Kaur, A.; Aree, T.; Kaur, N.; Singh, N.; Bakshi, M.S. Aqueous-phase synthesis of copper nanoparticles using organic nanoparticles: Application of assembly in detection of Cr3+. ACS Sustainable Chem. Eng. 2014, 2, 982–990. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.; Lan, C.; Fu, Q.; Huang, C.; Luo, Z.; Jiang, T.; Tang, Y. Silver nanoparticle enhanced Raman scattering-based lateral flow immunoassays for ultra-sensitive detection of the heavy metal chromium. Nanotechnology 2014, 25, 495501. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, G. The monothiocyanate complexes of chromium ion(III) on the silver electrode by the surface enhanced Raman scattering. Spectrochim. Acta 2005, 62A, 415–419. [Google Scholar] [CrossRef]
- Cao, D.; Fan, J.; Qiu, J.; Tu, Y.; Yan, J. Masking method for improving selectivity of gold nanoclusters in fluorescence determination of mercury and copper ions. Biosens. Bioelectron. 2013, 42, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, K.J.; Manesse, M.; McIlwee, H.A.; Schauer, C.L.; Boukherroub, R.; Szunerits, S. Selective detection of hexachromium ions by localized surface Plasmon resonance measurements using gold nanoparticles/chitosan composite interfaces. Analyst 2009, 134, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, K.; Kawai, K. Raman spectral study on the solution structure of the chromium(III)-EDTA complex. Inorg. Chem. 1986, 25, 3711–3713. [Google Scholar] [CrossRef]
- Krishnan, K.; Plane, R.A. Raman spectra of ethylenediaminetetraacetic acid and its metal complexes. J. Am. Chem. Soc. 1968, 90, 3195–3200. [Google Scholar] [CrossRef]
- Wagner, C.C.; Baran, E.J. Vibrational spectra of two Fe(III)/EDTA complexes useful for iron supplementation. Spectrochim Acta 2010, 75A, 807–810. [Google Scholar] [CrossRef]
- Singh, D.K.; Ganbold, E-O.; Cho, E-M.; Cho, K-H.; Kim, D.; Choo, J.; Kim, S.; Lee, C.M.; Yang, S.I.; Joo, S-W. Detection of the mycotoxin citrinin using silver substrates and Raman spectroscopy. J. Hazard. Mater. 2014, 265, 89–95. [Google Scholar] [CrossRef]
- Cong, V.T.; Ganbold, E-O.; Saha, J.K.; Jang, J.; Min, J.; Choo, J.; Kim, S.; Song, N.W.; Son, S.J.; Lee, S.B.; et al. Gold nanoparticle silica nanopeapods. J. Am. Chem. Soc. 2014, 136, 3833–3841. [Google Scholar] [CrossRef]
- Wheeler, W.D.; Legg, J.I. Solution structure of the chromium(III) complex with EDTA by deuteron NMR spectroscopy. Inorg. Chem. 1984, 23, 3798–3802. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies—Tables and Charts, 2nd ed.; John Wiley and Sons Ltd.: Chichester, UK, 1994. [Google Scholar]
- Lombardi, J.R.; Birke, R.L. A unified view of surface-enhanced Raman scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, N.H.; Joo, S.-W. Silver Nanoparticle-Enhanced Resonance Raman Sensor of Chromium(III) in Seawater Samples. Sensors 2015, 15, 10088-10099. https://doi.org/10.3390/s150510088
Ly NH, Joo S-W. Silver Nanoparticle-Enhanced Resonance Raman Sensor of Chromium(III) in Seawater Samples. Sensors. 2015; 15(5):10088-10099. https://doi.org/10.3390/s150510088
Chicago/Turabian StyleLy, Nguyễn Hoàng, and Sang-Woo Joo. 2015. "Silver Nanoparticle-Enhanced Resonance Raman Sensor of Chromium(III) in Seawater Samples" Sensors 15, no. 5: 10088-10099. https://doi.org/10.3390/s150510088