Light-Addressable Measurement of in Vivo Tissue Oxygenation in an Unanesthetized Zebrafish Embryo via Phase-Based Phosphorescence Lifetime Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Principle of Operation
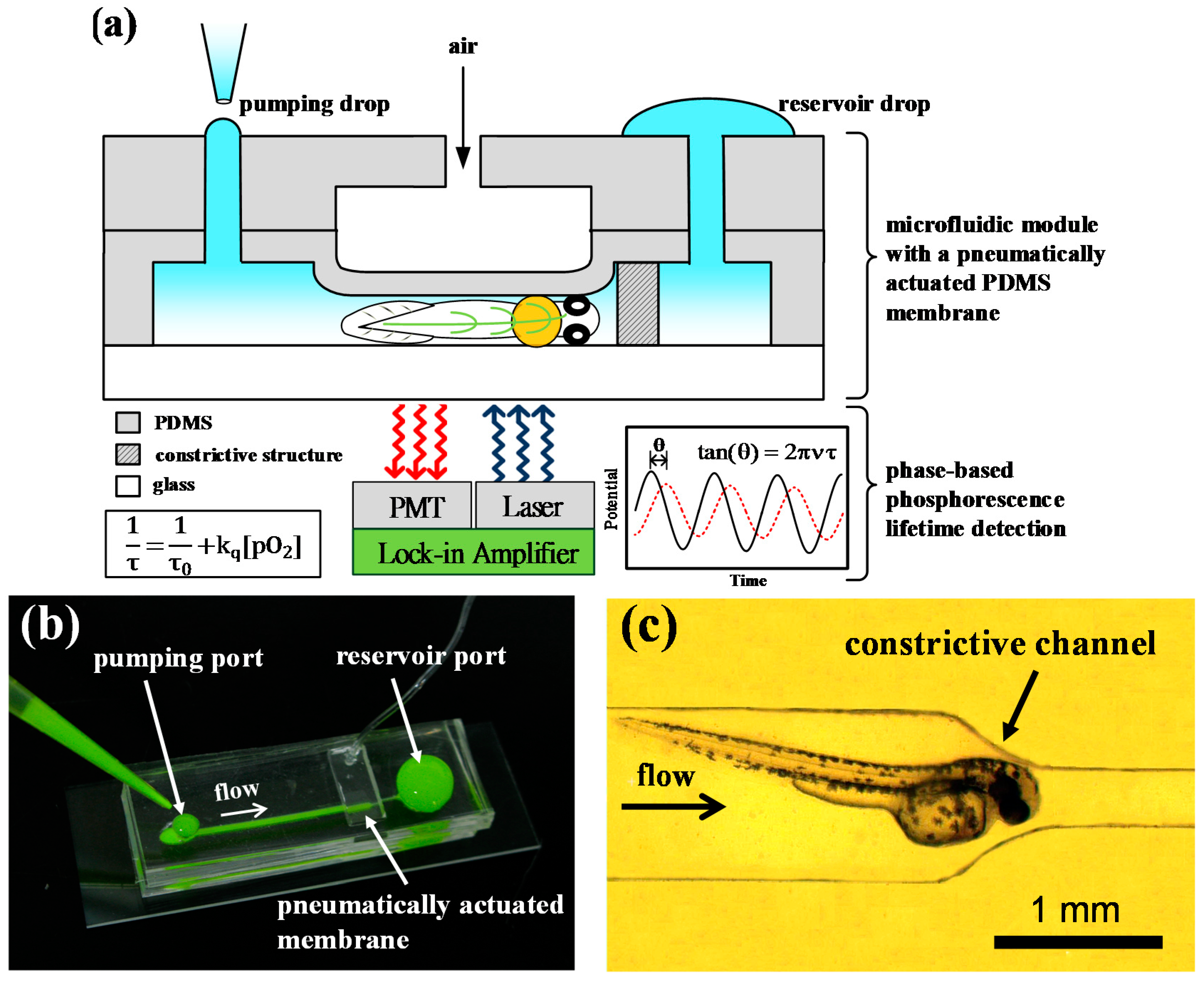
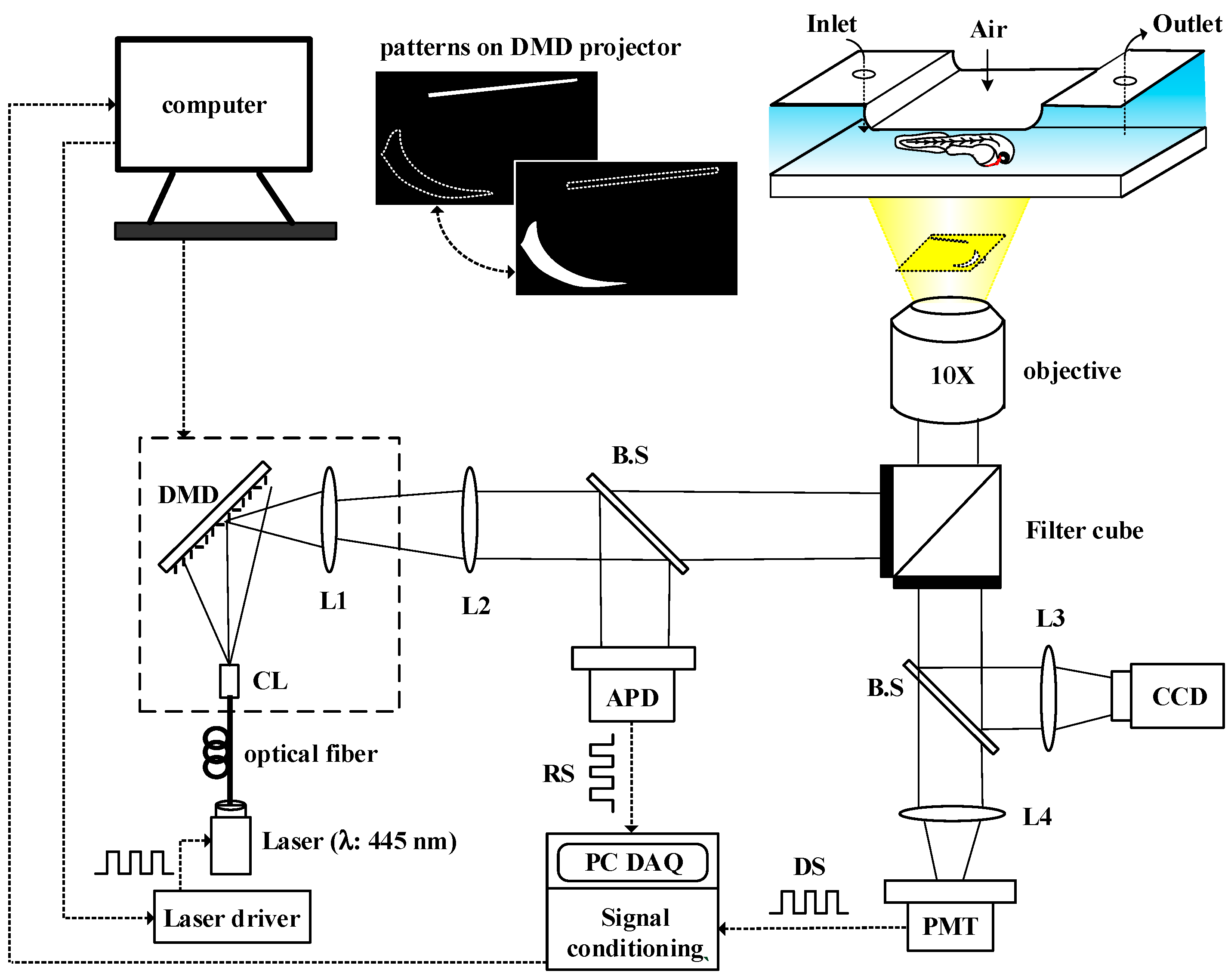
2.2. DMD-Based Light Modulation System for Phase-Based Phosphorescence Lifetime Detection
2.3. Microinjection of Oxyphor G4 into a 48 hpf Zebrafish Embryo
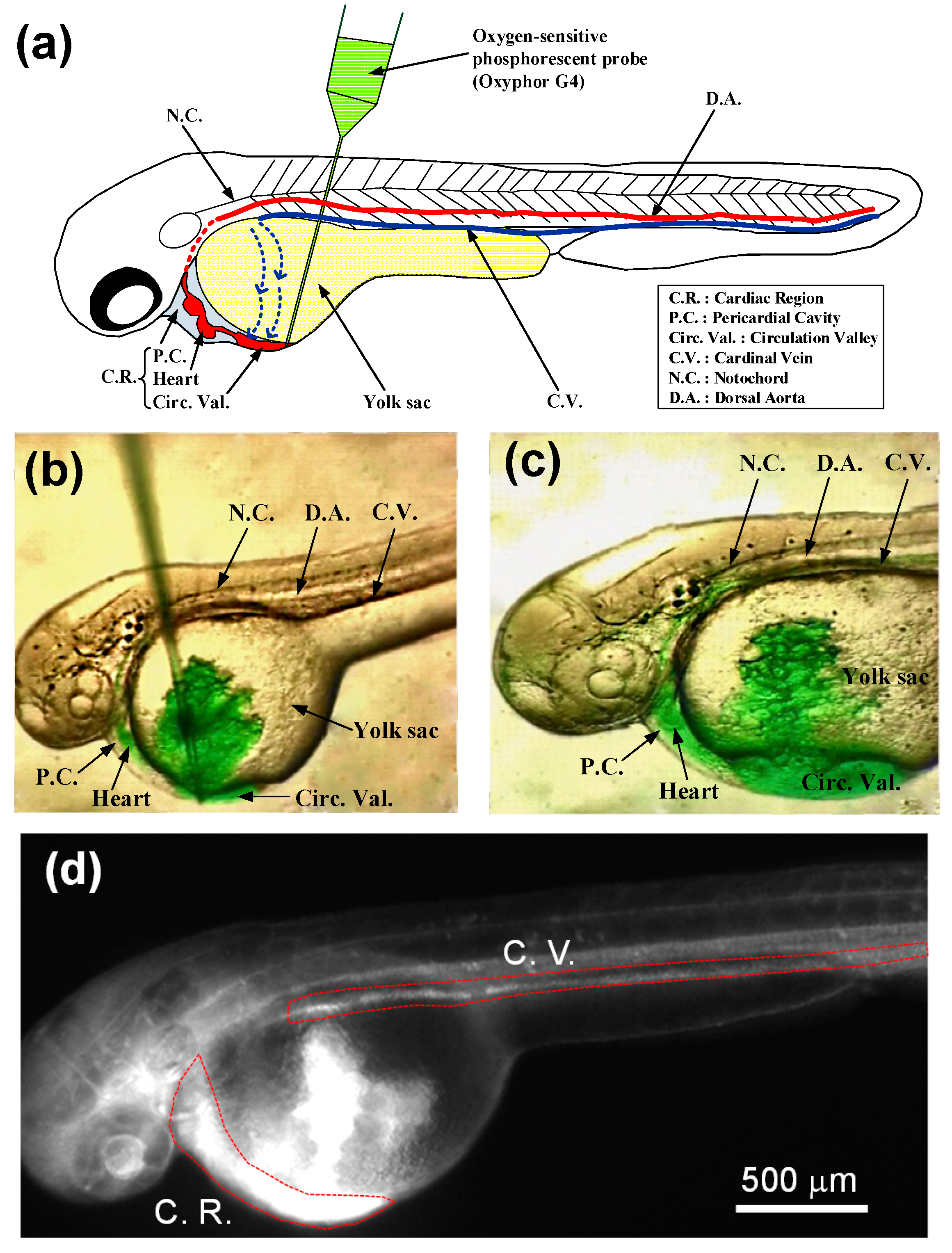
2.4. Fabrication of Microfluidic Devices
3. Results and Discussion
3.1. Survival Rates after Microinjection with Oxyphor G4

3.2. Physiological States of Zebrafish Embryos after Immobilization
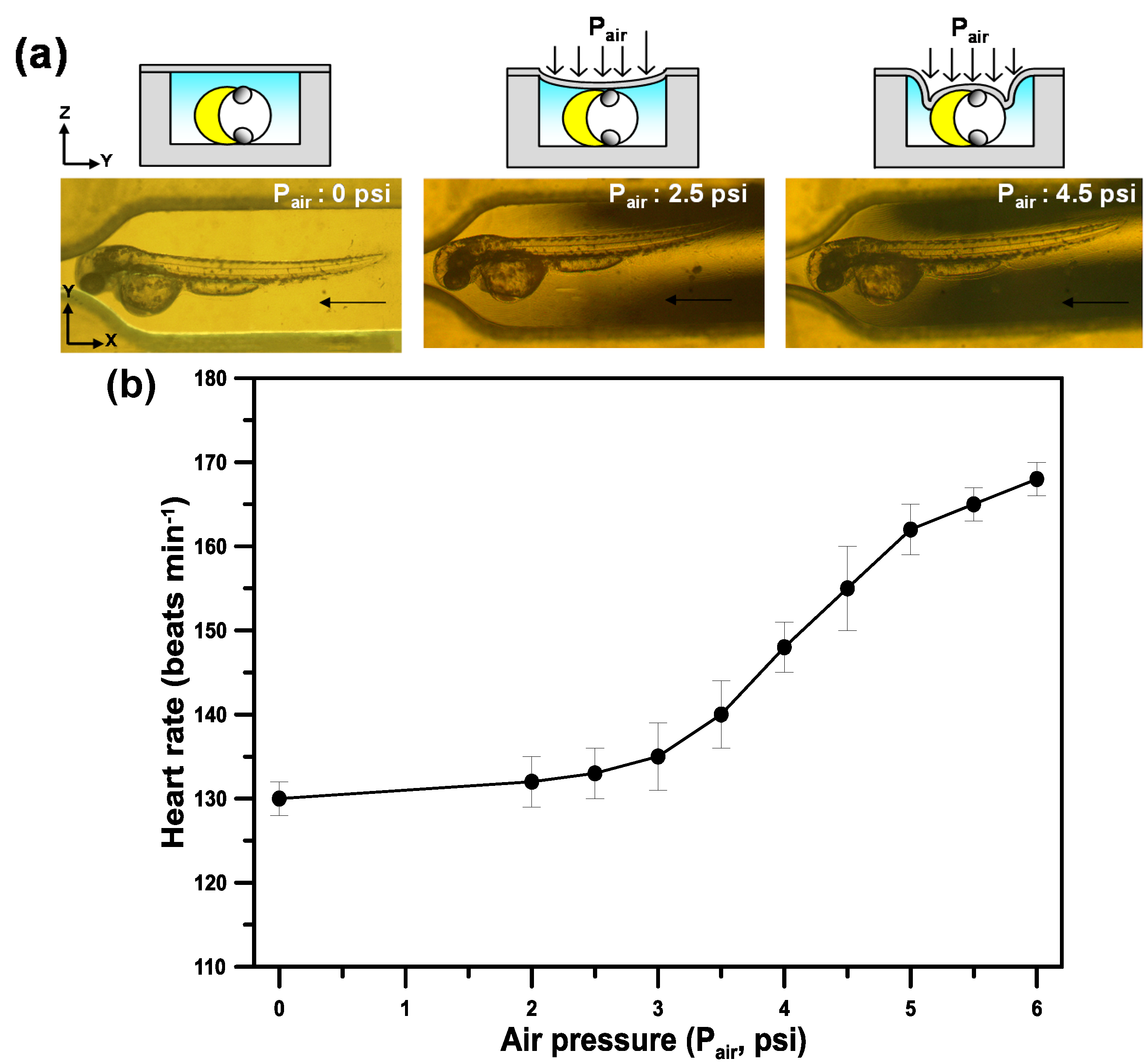
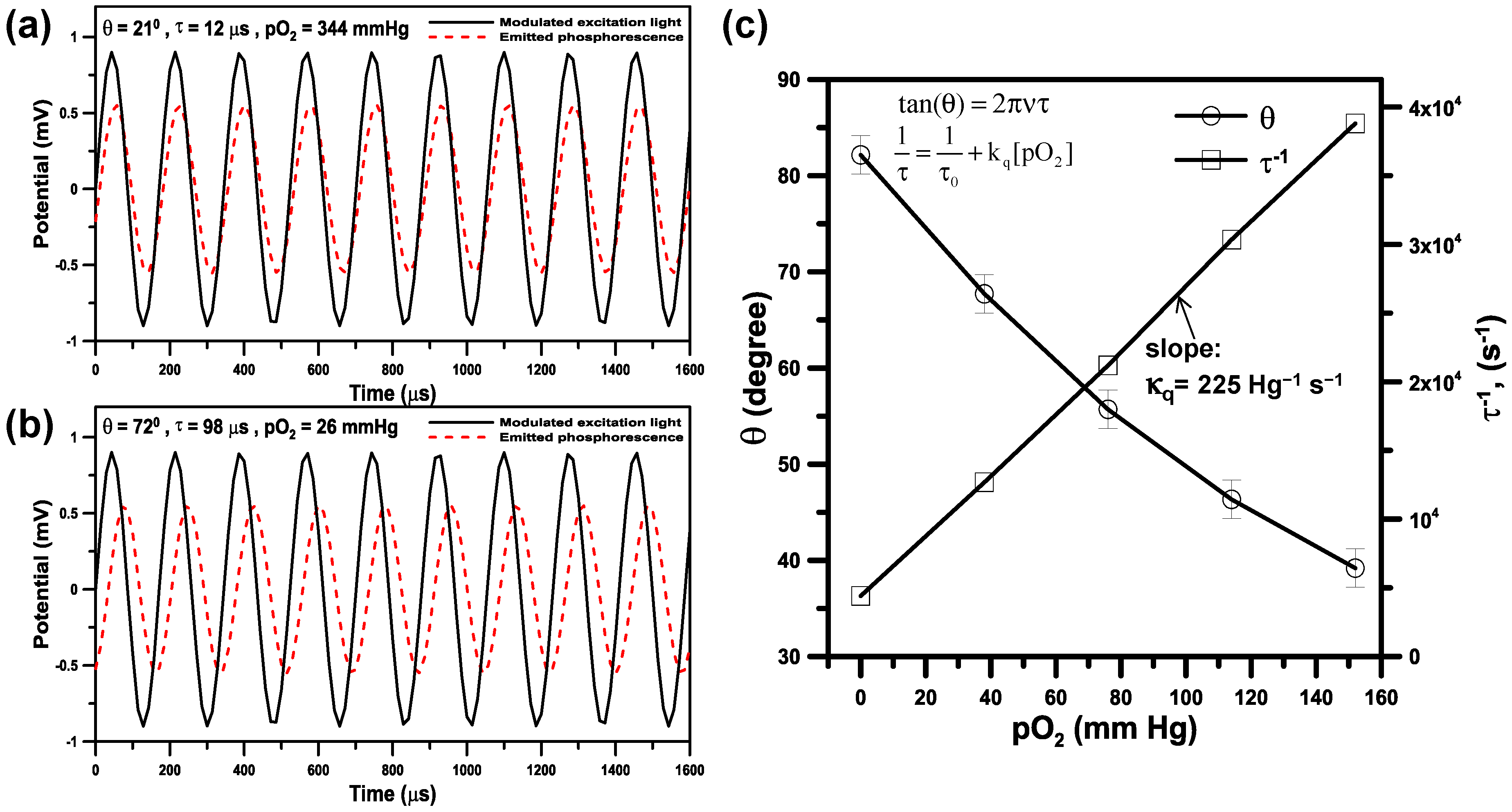
3.3. Phase-Based Phosphorescence Lifetime Detection for Oxygen Measurements
3.4. Light-Addressable Measurement of In Vivo Tissue Oxygenation

3.5. In vivo Tissue Oxygenation under Hypoxia/Reperfusion (HP)

4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Collard, C.D.; Gelman, S. Pathophysiology clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001, 94, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, B.A.; Kassebaum, B.L.; Gitlin, J.D. The zebrafish embryo as a dynamic model of anoxia tolerance. Dev. Dynam. 2008, 237, 1780–1788. [Google Scholar] [CrossRef]
- Mendelsohn, B.A.; Malone, J.P.; Townsend, R.R.; Gitlin, J.D. Proteomic analysis of anoxia tolerance in the developing zebrafish embryo. Comp. Biochem. Phys. D 2009, 4, 21–31. [Google Scholar]
- Rouhi, P.; Jensen, L.D.; Cao, Z.Q.; Hosaka, K.; Lanne, T.; Wahlberg, E.; Steffensen, J.F.; Cao, Y.H. Hypoxia-induced metastasis model in embryonic zebrafish. Nat. Protoc. 2010, 5, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.M.; Rico, E.P.; Cordova, S.D.; Pinto, C.B.; Blaser, R.E.; Dias, R.D.; Rosemberg, D.B.; Oliveira, D.L.; Souza, D.O. Evaluation of spontaneous recovery of behavioral and brain injury profiles in zebrafish after hypoxia. Behav. Brain Res. 2013, 253, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.R.; Middleton, R.C.; Le, Q.P.; Shelden, E.A. Hsf1 is essential for the resistance of zebrafish eye and brain tissues to hypoxia/reperfusion injury. PLoS ONE 2011, 6, e22268. [Google Scholar] [CrossRef]
- Dunphy, I.; Vinogradov, S.A.; Wilson, D.F. Oxyphor r2 and g2: Phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal. Biochem. 2002, 310, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Esipova, T.V.; Karagodov, A.; Miller, J.; Wilson, D.F.; Busch, T.M.; Vinogradov, S.A. Two new “protected” oxyphors for biological oximetry: Properties and application in tumor imaging. Anal. Chem. 2011, 83, 8756–8765. [Google Scholar] [CrossRef] [PubMed]
- Pirow, R.; Baumer, C.; Paul, R.J. Crater landscape: Two-dimensional oxygen gradients in the circulatory system of the microcrustacean daphnia magna. J. Exp. Biol. 2004, 207, 4393–4405. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, A.; Mammen, A.; Kubin, J.; Reade, E.; Liu, H.; Mendoza, A.; Greeley, W.J.; Wilson, D.F.; Pastuszko, A. Early regional response of apoptotic activity in newborn piglet brain following hypoxia and ischemia. Neurochem. Res. 2011, 36, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ponticorvo, A.; Dunn, A.K. Simultaneous imaging of oxygen tension and blood flow in animals using a digital micromirror device. Opt. Express 2010, 18, 8160–8170. [Google Scholar] [CrossRef] [PubMed]
- Ragoonanan, T.E.; Beattie, W.S.; Mazer, C.D.; Tsui, A.K.Y.; Leong-Poi, H.; Wilson, D.F.; Tait, G.; Yu, J.; Liu, E.; Noronha, M.; et al. Metoprolol reduces cerebral tissue oxygen tension after acute hemodilution in rats. Anesthesiology 2009, 111, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Tsytsarev, V.; Arakawa, H.; Borisov, S.; Pumbo, E.; Erzurumlu, R.S.; Papkovsky, D.B. In vivo imaging of brain metabolism activity using a phosphorescent oxygen-sensitive probe. J. Neurosci. Meth. 2013, 216, 146–151. [Google Scholar] [CrossRef]
- Wilson, D.F. Measuring in vivo metabolite levels in brain. Sleep 2011, 34, 837–837. [Google Scholar] [PubMed]
- Yu, J.; Ramadeen, A.; Tsui, A.K.Y.; Hu, X.; Zou, L.; Wilson, D.F.; Esipova, T.V.; Vinogradov, S.A.; Leong-Poi, H.; Zamiri, N.; et al. Quantitative assessment of brain microvascular and tissue oxygenation during cardiac arrest and resuscitation in pigs. Anaesthesia 2013, 68, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Huntosova, V.; Gay, S.; Nowak-Sliwinska, P.; Rajendran, S.K.; Zellweger, M.; van den Bergh, H.; Wagnieres, G. In vivo measurement of tissue oxygenation by time-resolved luminescence spectroscopy: Advantageous properties of dichlorotris(1, 10-phenanthroline)-ruthenium(II) hydrate. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Pastuszko, P.; Liu, H.P.; Mendoza-Paredes, A.; Schultz, E.; Markowitz, S.D.; Greeley, W.J.; Wilson, D.F.; Pastuszko, A. Brain oxygen and metabolism is dependent on the rate of low-flow cardiopulmonary bypass following circulatory arrest in newborn piglets. Eur. J. Cardio-Thorac. 2007, 31, 899–905. [Google Scholar] [CrossRef]
- Vinogradov, S.A.; Fernandez-Searra, M.A.; Dugan, B.W.; Wilson, D.F. Frequency domain instrument for measuring phosphorescence lifetime distributions in heterogeneous samples. Rev. Sci. Instrum. 2001, 72, 3396–3406. [Google Scholar] [CrossRef]
- Wilson, D.F.; Vinogradov, S.A.; Grosul, P.; Vaccarezza, M.N.; Kuroki, A.; Bennett, J. Oxygen distribution and vascular injury in the mouse eye measured by phosphorescence-lifetime imaging. Appl. Opt. 2005, 44, 5239–5248. [Google Scholar] [CrossRef] [PubMed]
- Mammen, A.; Kubin, J.; Greeley, W.J.; Schears, G.J.; Pastuszko, P.; Wilson, D.F.; Pastuszko, A. Effect of hypoxia on expression of selected proteins involved in regulation of apoptotic activity in striatum of newborn piglets. Neurochem. Res. 2011, 36, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.D.; Ponticorvo, A.; Ford, T.N.; Dunn, A.K. Microvascular oxygen quantification using two-photon microscopy. Opt. Lett. 2008, 33, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.M.; Bedell, V.M.; Boczek, N.J.; Essner, J.J.; Balciunas, D.; Clark, K.J.; Ekker, S.C. Score imaging: Specimen in a corrected optical rotational enclosure. Zebrafish 2010, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bischel, L.L.; Mader, B.R.; Green, J.M.; Huttenlocher, A.; Beebe, D.J. Zebrafish entrapment by restriction array (zebra) device: A low-cost, agarose-free zebrafish mounting technique for automated imaging. Lab Chip 2013, 13, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Beebe, D.J. A passive pumping method for microfluidic devices. Lab Chip 2002, 2, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Hsu, Y.H.; Wu, C.W.; Wu, C.J. Light-addressable measurements of cellular oxygen consumption rates in microwell arrays based on phase-based phosphorescence lifetime detection. Biomicrofluidics 2012, 6, 044118. [Google Scholar] [CrossRef]
- Huang, S.H.; Huang, K.S.; Yu, C.H.; Gong, H.Y. Metabolic profile analysis of a single developing zebrafish embryo via monitoring of oxygen consumption rates within a microfluidic device. Biomicrofluidics 2013, 7, 064107. [Google Scholar] [CrossRef]
- Huang, S.H.; Tsai, C.H.; Wu, C.W.; Wu, C.J. Light-directed, spatially addressable oxygen detection in a hydrogel microarray based on phase-based lifetime detection using a digital micromirror device. Sens. Actuat. A-Phys. 2011, 165, 139–146. [Google Scholar] [CrossRef]
- Grillitsch, S.; Medgyesy, N.; Schwerte, T.; Pelster, B. The influence of environmental pO2 on hemoglobin oxygen saturation in developing zebrafish danio rerio. J. Exp. Biol. 2005, 208, 309–316. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-H.; Yu, C.-H.; Chien, Y.-L. Light-Addressable Measurement of in Vivo Tissue Oxygenation in an Unanesthetized Zebrafish Embryo via Phase-Based Phosphorescence Lifetime Detection. Sensors 2015, 15, 8146-8162. https://doi.org/10.3390/s150408146
Huang S-H, Yu C-H, Chien Y-L. Light-Addressable Measurement of in Vivo Tissue Oxygenation in an Unanesthetized Zebrafish Embryo via Phase-Based Phosphorescence Lifetime Detection. Sensors. 2015; 15(4):8146-8162. https://doi.org/10.3390/s150408146
Chicago/Turabian StyleHuang, Shih-Hao, Chu-Hung Yu, and Yi-Lung Chien. 2015. "Light-Addressable Measurement of in Vivo Tissue Oxygenation in an Unanesthetized Zebrafish Embryo via Phase-Based Phosphorescence Lifetime Detection" Sensors 15, no. 4: 8146-8162. https://doi.org/10.3390/s150408146





