Pharmacokinetics of Chiral Dendrimer-Triamine-Coordinated Gd-MRI Contrast Agents Evaluated by in Vivo MRI and Estimated by in Vitro QCM
Abstract
:1. Introduction
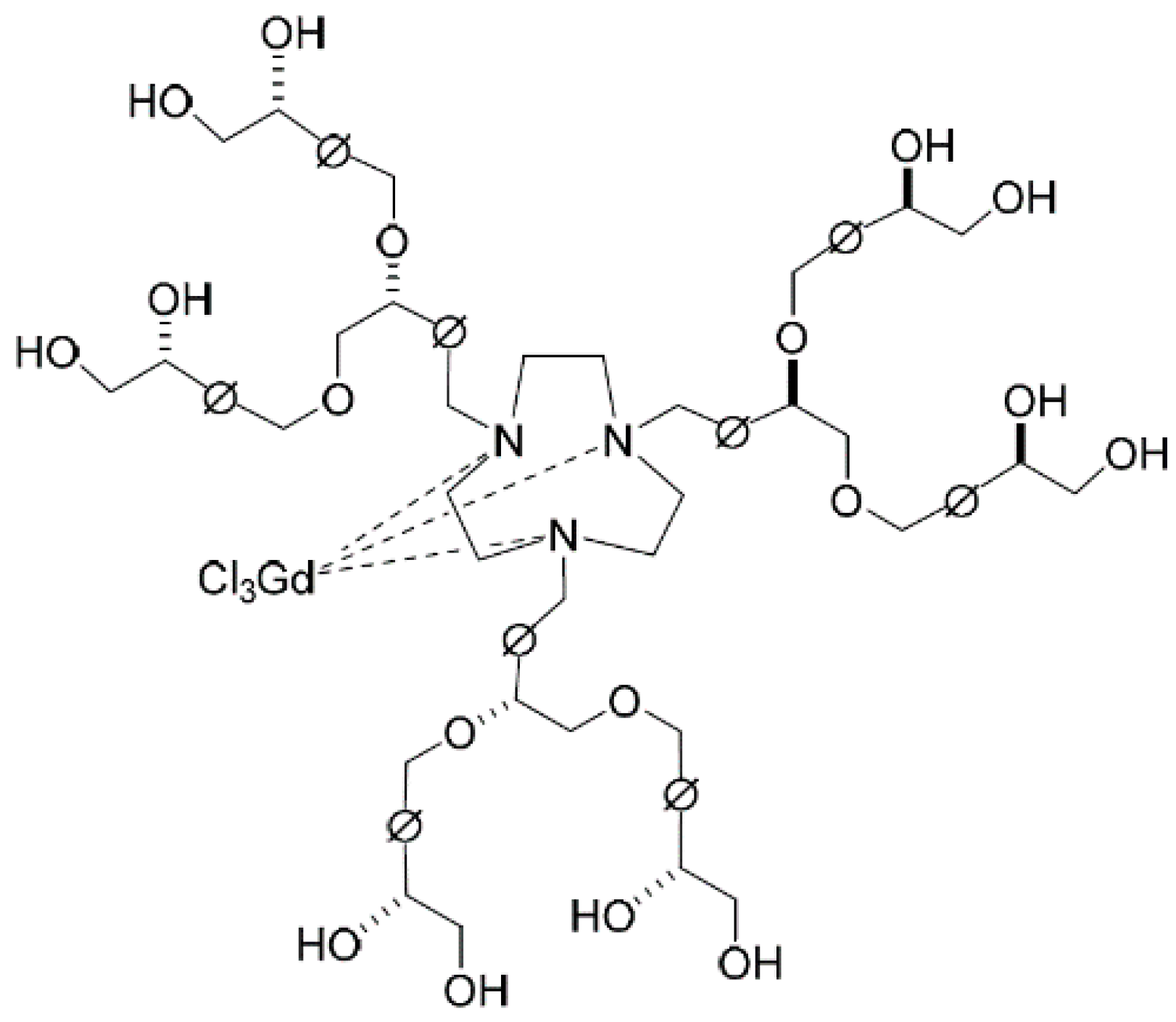
2. Experimental Section
2.1. General Methods
2.2. Synthesis of 3rd-Generation Chiral Dendrimer-Triamine-Coordinated Gd-MRI CAs (Scheme 1)
2.2.1. 2nd-Generation Chiral Dendron with Polyol End-Groups 6
2.2.2. 3rd-Generation Chiral Dendron with Polyacetonide End-Group 7

2.2.3. 3rd-Generation Chiral Dendron 8 from 7 by Deprotection
2.2.4. 3rd-Generation Chiral Dendron 9 from 8 by Bromination
2.2.5. 3rd-Generation Chiral Dendrimer with Polyacetonide End-Group 10
2.2.6. 3rd-Generation Chiral Dendrimer with Polyol End-Group 11
2.2.7. 3rd-Generation Chiral Dendrimer-Triamine-Coordinated Gadolinium Complex 3
2.3. Relaxation Time Measurements
2.4. Cytotoxicity Assay
2.5. Quartz Crystal Microbalance (Affinix-Q®) Analysis
2.6. Evaluation of the Body Distribution of Chiral Dendrimer Gd-MRI CAs by in Vivo MRI
3. Results and Discussion
3.1. Synthesis of 2nd- and 3rd-Generation Chiral Dendrimer-Triamine-Coordinated Gd-MRI CAs
3.2. Functional Evaluation of Chiral Dendrimer-Triamine-Coordinated Gd Complexes, 2-(R), 2-(S), 3-(R), and 3-(S), as Highly Sensitive MRI Contrast Agents
3.2.1. Functional Evaluation of Chiral Dendrimer Gd Complexes by in Vitro MRI

| Sample | r1 ((mmol/L)−1·s−1) |
|---|---|
| Gd-DTPA | 4.6 |
| 2-(R) | 11.4 |
| 2-(S) | 11.1 |
| 3-(R) | 16.3 |
| 3-(S) | 16.4 |
3.2.2. Cytotoxicity of Chiral Dendrimer Gd-MRI CAs
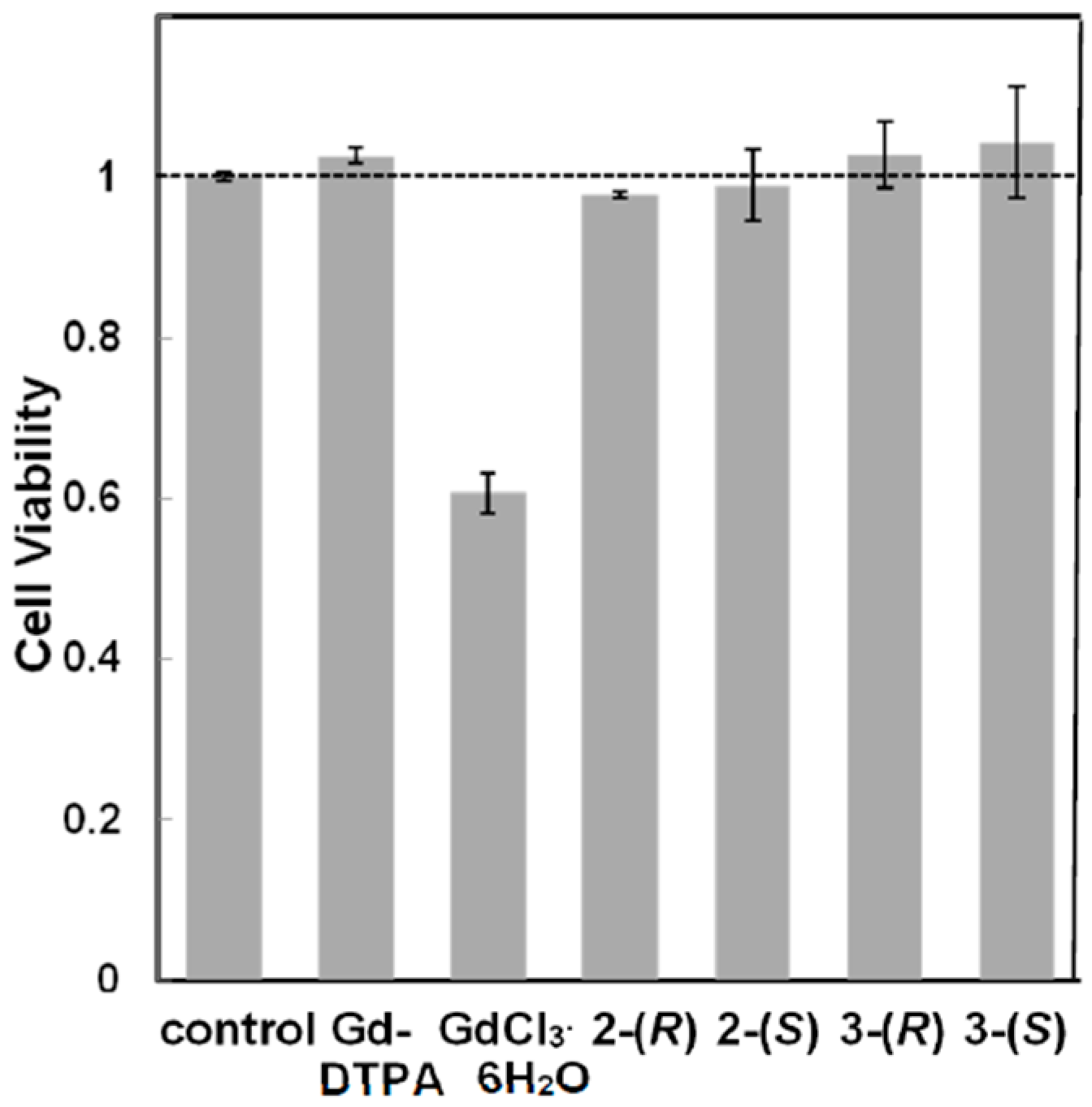
3.2.3. Functional Evaluation and Pharmacokinetics of Chiral Dendrimer Gd-MRI CAs by in Vivo MRI

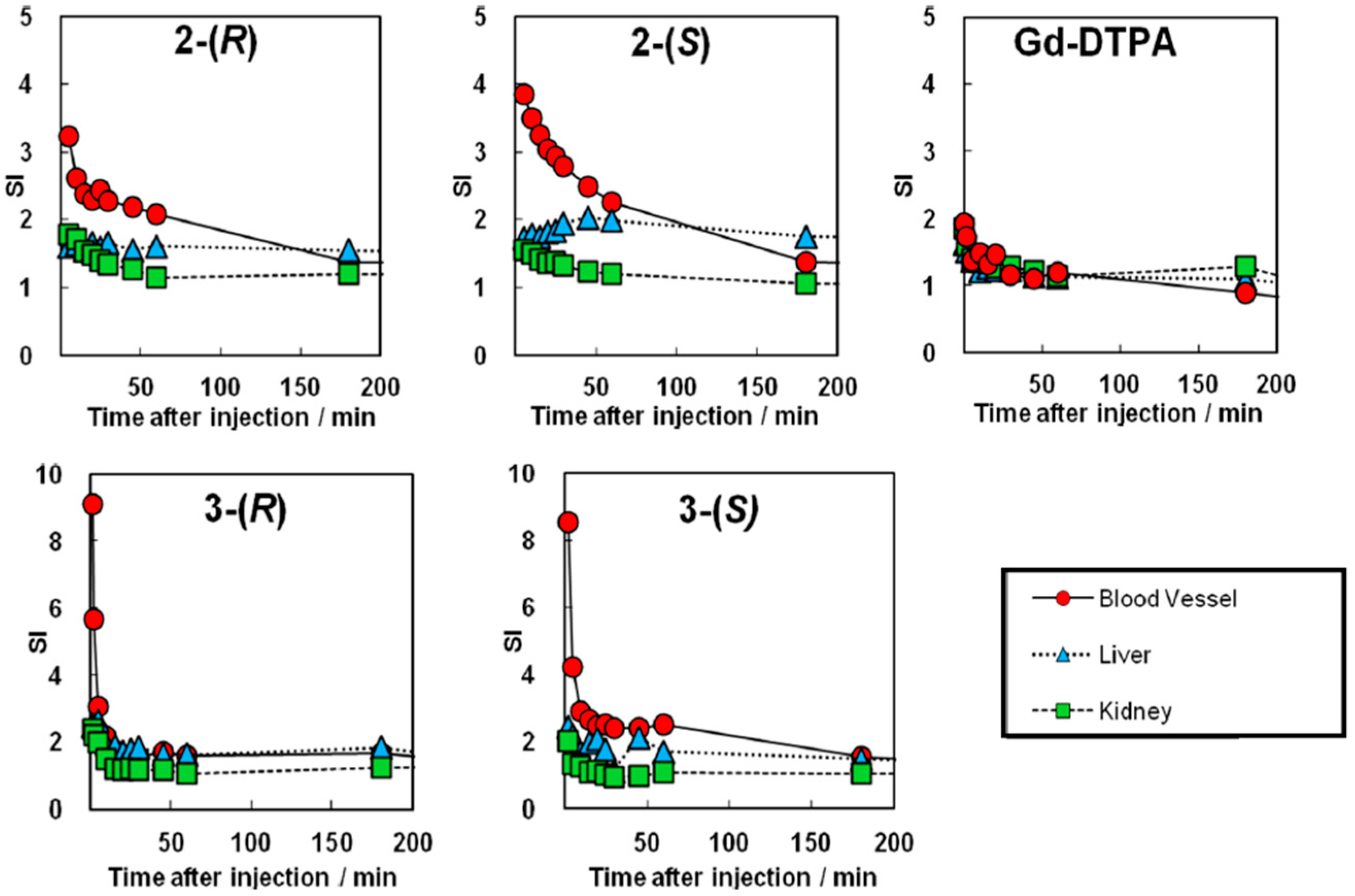
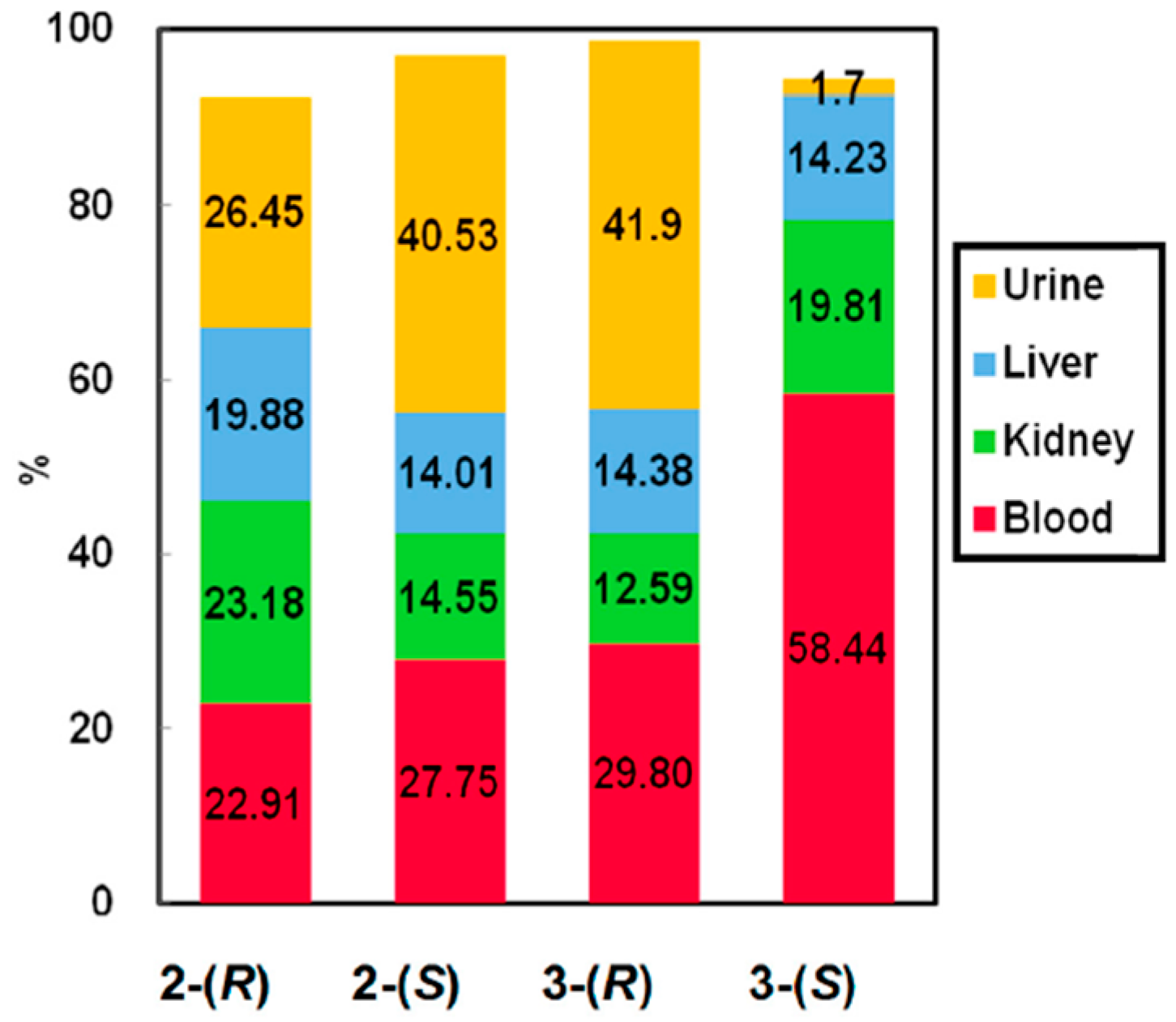
3.2.4. Affinity of Chiral Dendrimer Gd-MRI CAs with Plasma Protein Estimated by in Vitro Quartz Crystal Microbalance Measurement

| Sample | Kd (mol/L) | Ka (mol/L−1) | ΔFmax |
|---|---|---|---|
| 2-(R) | 1.42 × 10−9 | 7.04 × 108 | 13.7 |
| 2-(S) | 2.49 × 10−11 | 4.02 × 1010 | 58.3 |
| 3-(R) | 1.02 × 10−9 | 9.80 × 108 | 969 |
| 3-(S) | 5.57 × 10−11 | 1.80 × 1010 | 83.8 |
4. Conclusions/Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bammer, R.; Skare, S.; Newbould, R.; Liu, C.; Thijs, V.; Ropele, S.; Clayton, D.B.; Krueger, G.; Moseley, M.E.; Glover, G.H. Foundations of advanced magnetic resonance imaging. NeuroRx 2005, 2, 167–196. [Google Scholar] [CrossRef] [PubMed]
- Edelman, R.R.; Warach, S. Magnetic resonance imaging (Second of Two Parts). N. Engl. J. Med. 1993, 328, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Edelman, R.R.; Warach, S. Magnetic resonance imaging (First of Two Parts). N. Engl. J. Med. 1993, 328, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Bottrill, M.; Kwok, L.; Long, N.J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 2006, 35, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Pierre, V.C.; Allen, M.J.; Caravan, P. Contrast agents for MRI: 30+ years and where are we going? J. Biol. Inorg. Chem. 2014, 19, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Kotek, J.; Kubícek, V.; Lukes, I. Gadolinium(III) complexes as MRI contrast agents: Ligand design and properties of the complexes. Dalton Trans. 2008, 9226, 3027–3047. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.J.; Datta, A.; Jocher, C.J.; Raymond, K.N. High-relaxivity MRI contrast agents: Where coordination chemistry meets medical imaging. Angew. Chem. Int. Ed. 2008, 47, 8568–8580. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Gale, E.M.; Caravan, P. MR imaging probes: Design and applications. Dalton Trans. 2015, 44, 4804–4818. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. How should nephrologists approach gadolinium-based contrast imaging in patients with kidney disease? Clin. J. Am. Soc. Nephrol. 2008, 3, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Current status of gadolinium toxicity in patients with kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Daftari Besheli, L.; Aran, S.; Shaqdan, K.; Kay, J.; Abujudeh, H. Current status of nephrogenic systemic fibrosis. Clin. Radiol. 2014, 69, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Gadolinium-contrast toxicity in patients with kidney disease: Nephrotoxicity and nephrogenic systemic fibrosis. Curr. Drug Saf. 2008, 3, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Weller, A.; Barber, J.L.; Olsen, O.E. Gadolinium and nephrogenic systemic fibrosis: An update. Pediatr. Nephrol. 2014, 29, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Bumb, A.; Brechbiel, M.W.; Choyke, P. Macromolecular and dendrimer-based magnetic resonance contrast agents. Acta Radiol. 2010, 51, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Brechbiel, M.W. Nano-sized MRI contrast agents with dendrimer cores. Adv. Drug Deliv. Rev. 2005, 57, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Langereis, S.; Dirksen, A.; Hackeng, T.M.; van Genderen, M.H.P.; Meijer, E.W. Dendrimers and magnetic resonance imaging. New J. Chem. 2007, 31, 1152–1155. [Google Scholar] [CrossRef]
- Villaraza, A.J.L.; Bumb, A.; Brechbiel, M.W. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: The interplay between size, function, and pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Campagna, S.; Ceroni, P.; Puntoriero, F. Designing Dendrimers; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Bryant, L.H.; Brechbiel, M.W.; Wu, C.; Bulte, J.W.; Herynek, V.; Frank, J.A. Synthesis and relaxometry of high-generation (g = 5, 7, 9, and 10) PAMAM dendrimer-dota-gadolinium chelates. J. Magn. Reson. Imaging 1999, 9, 348–352. [Google Scholar] [CrossRef]
- Kojima, C.; Turkbey, B.; Ogawa, M.; Bernardo, M.; Regino, C.A.; Bryant, L.H., Jr.; Choyke, P.L.; Kono, K.; Kobayashi, H. Dendrimer-based MRI contrast agents: The effects of pegylation on relaxivity and pharmacokinetics. Nanomedicine 2011, 7, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Labieniec, M.; Watala, C. PAMAM dendrimers—Diverse biomedical applications. Facts and unresolved questions. Cent. Eur. J. Biol. 2009, 4, 434–451. [Google Scholar] [CrossRef]
- Wiener, E.C.; Brechbiel, M.W.; Brothers, H.; Magin, R.L.; Gansow, O.A.; Tomalia, D.A.; Lauterbur, P.C. Dendrimer-based metal chelates: A new class of magnetic resonance imaging contrast agents. Magn. Reson. Med. 1994, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawamoto, S.; Star, R.A.; Waldmann, T.A.; Brechbiel, M.W.; Choyke, P.L. Activated clearance of a biotinylated macromolecular MRI contrast agent from the blood pool using an avidin chase. Bioconjug. Chem. 2003, 14, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R., Jr. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Langereis, S.; de Lussanet, Q.G.; van Genderen, M.H.P.; Backes, W.H.; Meijer, E.W. Multivalent contrast agents based on gadolinium-diethylenetriaminepentaacetic acid-terminated poly(propylene imine) dendrimers for magnetic resonance imaging. Macromolecules 2004, 37, 3084–3091. [Google Scholar] [CrossRef]
- Wiener, E.C.; Auteri, F.P.; Chen, J.W.; Brechbiel, M.W.; Gansow, O.A.; Schneider, D.S.; Belford, R.L.; Clarkson, R.B.; Lauterbur, P.C. Molecular dynamics of ion-chelate complexes attached to dendrimers. J. Am. Chem. Soc. 1996, 118, 7774–7782. [Google Scholar] [CrossRef]
- Miyake, Y.; Kimura, Y.; Ishikawa, S.; Tsujita, H.; Miura, H.; Narazaki, M.; Matsuda, T.; Tabata, Y.; Yano, T.; Toshimitsu, A.; et al. Synthesis and functional evaluation of chiral dendrimer-triamine-coordinated Gd complexes as highly sensitive MRI contrast agents. Tetrahedron Lett. 2012, 53, 4580–4583. [Google Scholar] [CrossRef]
- Miyake, Y.; Kimura, Y.; Orito, N.; Imai, H.; Matsuda, T.; Toshimitsu, A.; Kondo, T. Synthesis and functional evaluation of chiral dendrimer-triamine-coordinated Gd complexes with polyaminoalcohol end-groups as highly sensitive MRI contrast agents. Tetrahedron 2015, 71, 4438–4444. [Google Scholar] [CrossRef]
- Fee, C.J. Label-free, real-time interaction and adsorption analysis 2: Quartz crystal microbalance. In Protein Nanotechnology, 2nd ed.; Gerrard, J.A., Ed.; Springer: New York, NY, USA, 2013; pp. 313–322. [Google Scholar]
- Sauerbrey, G. Use of quartz crystal vibrator for weighting thin films on a microbalance. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. The kidneys and body fluids (Unit V), The body fluid compartments: Extracellular and intracellular fluids; Interstitial fluid and edema (Chapter 25). In Textbook of Medical Physiology, 10th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2000; pp. 264–278. [Google Scholar]
- Chaudhuri, P.; Wieghardt, K. The chemistry of 1,4,7-triazacyclononane and related tridentate macrocyclic compounds. Prog. Inorg. Chem. 1987, 35, 329–436. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyake, Y.; Ishikawa, S.; Kimura, Y.; Son, A.; Imai, H.; Matsuda, T.; Yamada, H.; Toshimitsu, A.; Kondo, T. Pharmacokinetics of Chiral Dendrimer-Triamine-Coordinated Gd-MRI Contrast Agents Evaluated by in Vivo MRI and Estimated by in Vitro QCM. Sensors 2015, 15, 31973-31986. https://doi.org/10.3390/s151229900
Miyake Y, Ishikawa S, Kimura Y, Son A, Imai H, Matsuda T, Yamada H, Toshimitsu A, Kondo T. Pharmacokinetics of Chiral Dendrimer-Triamine-Coordinated Gd-MRI Contrast Agents Evaluated by in Vivo MRI and Estimated by in Vitro QCM. Sensors. 2015; 15(12):31973-31986. https://doi.org/10.3390/s151229900
Chicago/Turabian StyleMiyake, Yuka, Syungo Ishikawa, Yu Kimura, Aoi Son, Hirohiko Imai, Tetsuya Matsuda, Hisatsugu Yamada, Akio Toshimitsu, and Teruyuki Kondo. 2015. "Pharmacokinetics of Chiral Dendrimer-Triamine-Coordinated Gd-MRI Contrast Agents Evaluated by in Vivo MRI and Estimated by in Vitro QCM" Sensors 15, no. 12: 31973-31986. https://doi.org/10.3390/s151229900





