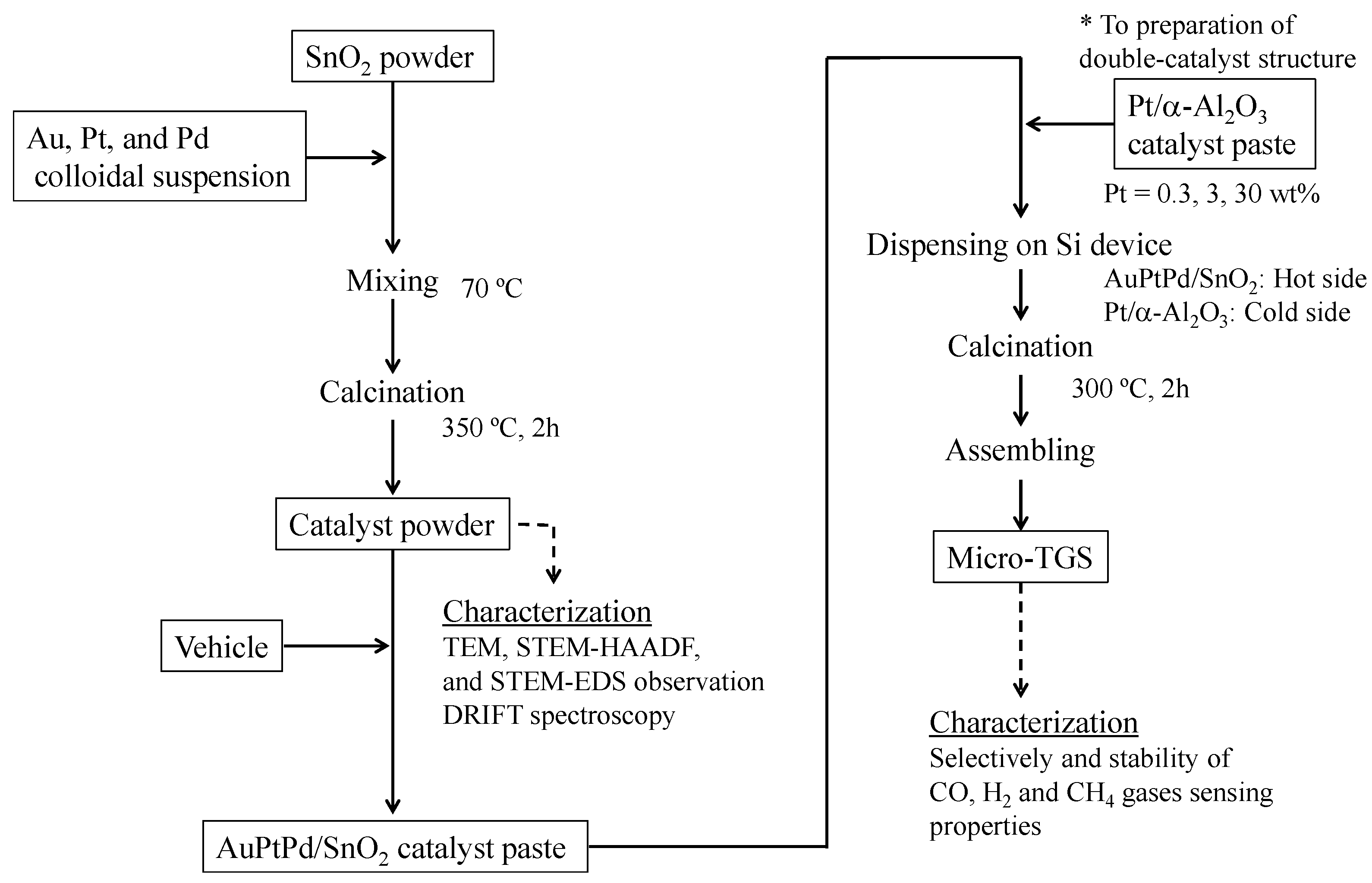
3.1. Catalyst Microstructure
Figure 5 shows the TEM images of the AuPtPd/SnO
2 catalyst powder. Both well-dispersed SnO
2 particles (20–50 nm) (
Figure 5a), and small metal particles (3 nm) supported on SnO
2 particles can clearly be seen (
Figure 5b). It should be noted here that the high combustion performance of the catalyst is affected by its porous structure and particle size. The grain size of the aggregated metal particles was several nanometers, similar to the starting colloidal particle, resulting in relatively good dispersion. However, to investigate whether each metal element (
i.e., Pt, Pd, and Au) is alloyed or not, the catalyst was analyzed by STEM and elemental mapping.
Figure 5.
Transmission electron microscopy (TEM) images of the AuPtPd/SnO2 catalyst powder: (a) low magnification (17,500) and (b) high magnification (88,000).
Figure 5.
Transmission electron microscopy (TEM) images of the AuPtPd/SnO2 catalyst powder: (a) low magnification (17,500) and (b) high magnification (88,000).
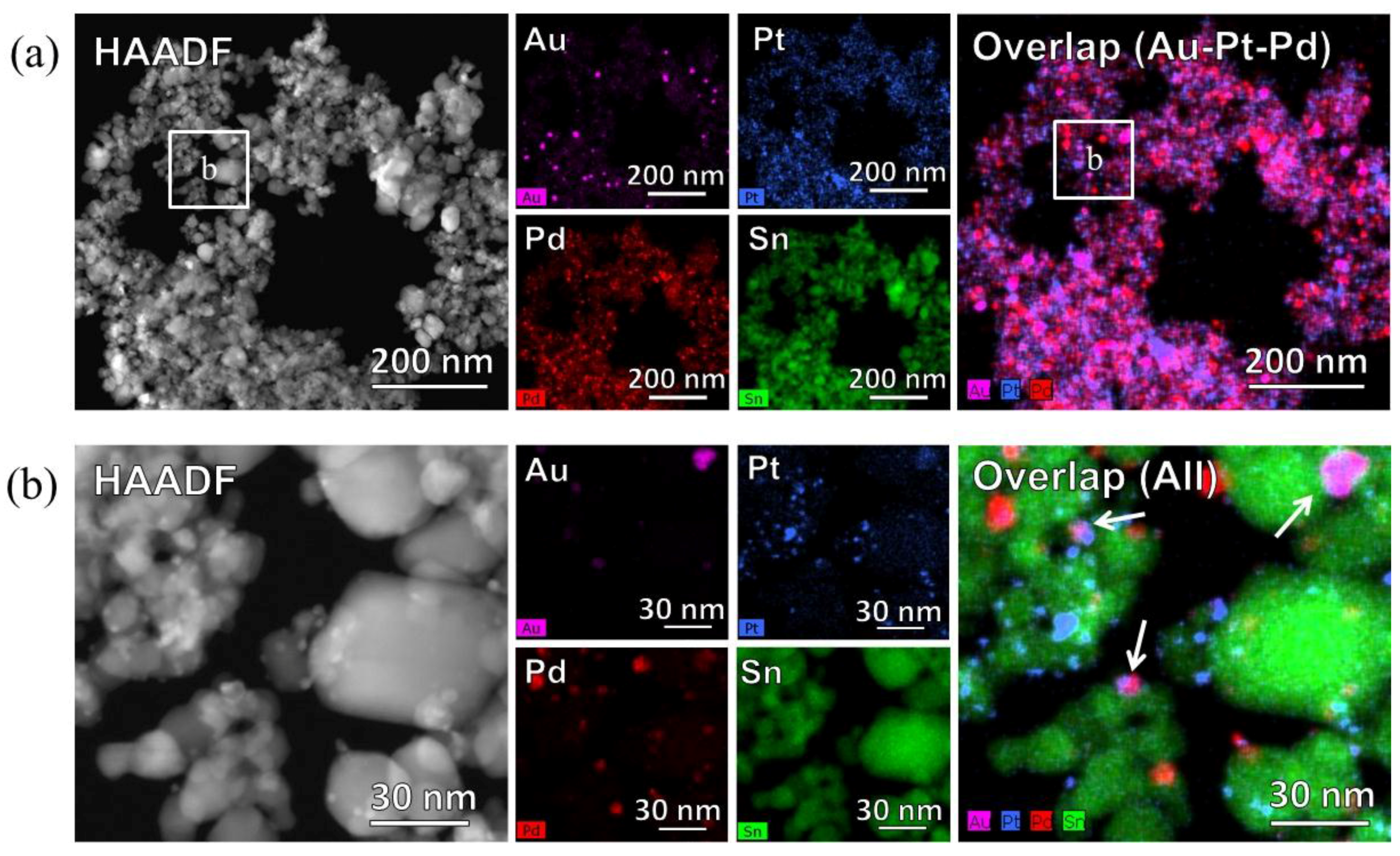
Figure 6 shows the High-angle annular dark field scanning transmission electron microscopy (STEM-HAADF) images and STEM-energy dispersive X-ray spectroscopy (STEM-EDS) mapping of the Au, Pt, Pd, and Sn distributions in the catalyst powder. Low magnification images (
Figure 6a) indicate that the noble metals were dispersed over the SnO
2 particles. However, in the high magnification images (
Figure 6b), only the Pt and Pd nanoparticles were deposited and dispersed on SnO
2 particles, while the Au nanoparticles aggregated as 10 nm particles, overlapping with Pt and Pd, as indicated by arrows in the overlap image of
Figure 6b.
Figure 6.
High-angle annular dark field scanning transmission electron microscopy (STEM-HAADF) and STEM-energy dispersive X-ray spectroscopy (STEM-EDS) images of the AuPtPd/SnO2 catalyst powder. (a) STEM-HAADF image and EDS maps of Au, Pt, Pd, Sn, and Overlap (Au-Pt-Pd) at low magnification (110,000), and (b) STEM-HAADF image and EDS maps of Au, Pt, Pd, Sn, and Overlap at high magnification (630,000).
Figure 6.
High-angle annular dark field scanning transmission electron microscopy (STEM-HAADF) and STEM-energy dispersive X-ray spectroscopy (STEM-EDS) images of the AuPtPd/SnO2 catalyst powder. (a) STEM-HAADF image and EDS maps of Au, Pt, Pd, Sn, and Overlap (Au-Pt-Pd) at low magnification (110,000), and (b) STEM-HAADF image and EDS maps of Au, Pt, Pd, Sn, and Overlap at high magnification (630,000).
3.2. DRIFT Characterization of the Catalyst
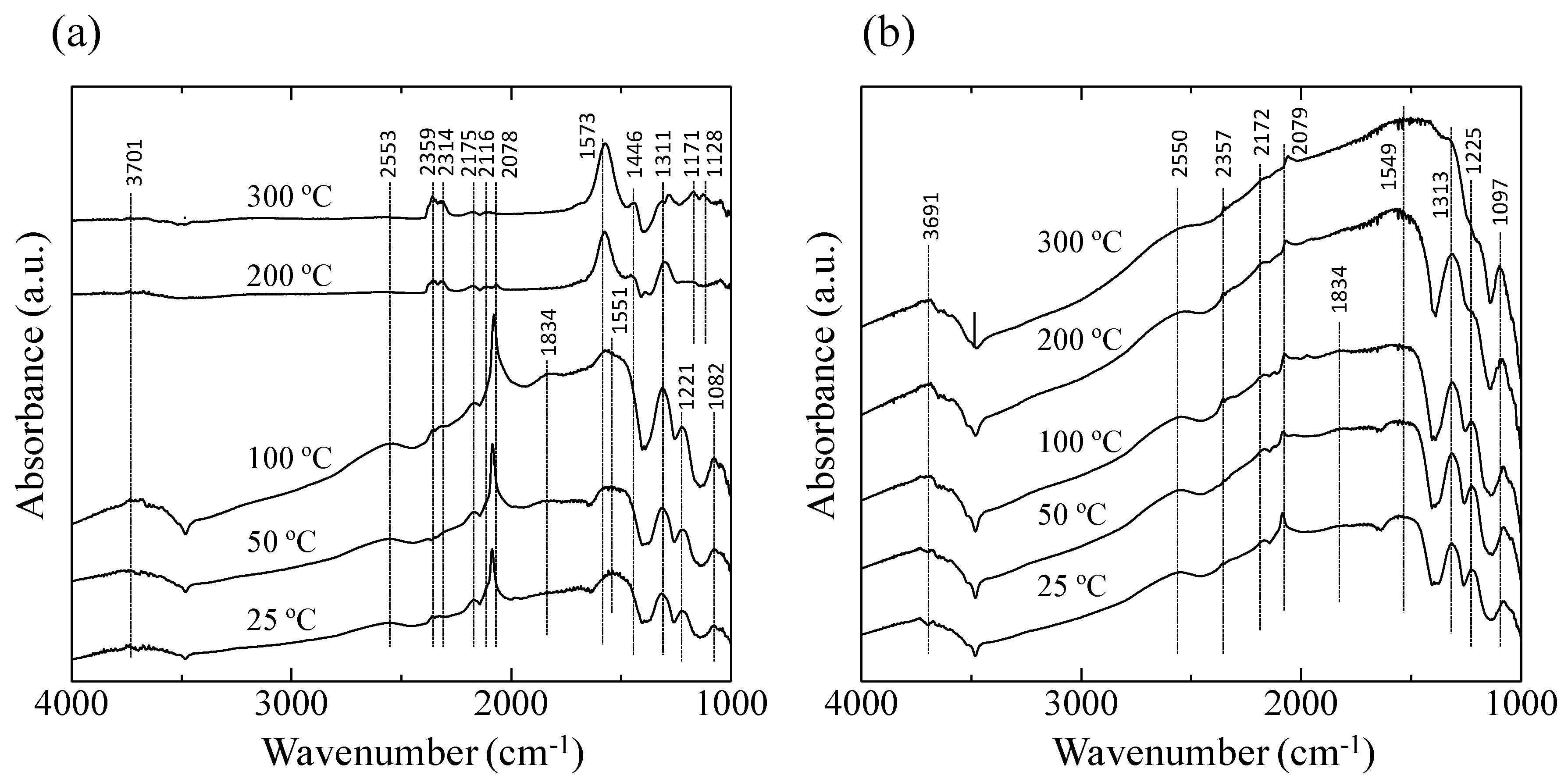
Figure 7 shows the 1000–4000 cm
−1 region of the diffuse reflectance Fourier transform Infrared spectroscopy (DRIFT) spectra of the AuPtPd/SnO
2 catalyst at 25–300 °C in 10,000 ppm CO in both dry air (
Figure 7a) and in Ar (
Figure 7b). As can be seen in
Figure 7a, at lower temperatures (25–100 °C), bands at 1082, 1221, 1311, and 1551 cm
−1 were observed, corresponding to vibrations of the carbonate species on noble metals or SnO
2 [
15,
20]. The bands at 1834, 2078, 2116, and 2175 cm
−1 could be assigned to CO. In particular, the sharp peak at 2078 cm
−1 and the broad peak at 1834 cm
−1 correspond to the linear and bridging bonds of the CO-Pt and CO-Pd bands, respectively [
21]. The peak intensity corresponding to CO adsorption decreased with increasing the catalyst temperature. At catalyst temperatures of 200 and 300 °C, bands at 2314 and 2359 cm
−1, corresponding to CO
2, were observed, confirming the oxidation of CO by the AuPtPd/SnO
2 catalyst. For the experiments employing 10,000 ppm CO in Ar (
Figure 7b), the peaks corresponding to the carbonate species (at 1097, 1225, 1313, 1549, 2079, and 2172 cm
−1) were detected at 25–200 °C. However, in contrast to the experiments employing 10,000 ppm CO in dry air (
Figure 7a), peaks corresponding to CO
2 were not detected.
Figure 7.
Diffuse reflectance Fourier transform Infrared spectroscopy (DRIFT) spectra of the AuPtPd/SnO2 catalyst powder at 25–300 °C, in (a) 10,000 ppm CO in dry air, and (b) 10,000 ppm CO in Ar.
Figure 7.
Diffuse reflectance Fourier transform Infrared spectroscopy (DRIFT) spectra of the AuPtPd/SnO2 catalyst powder at 25–300 °C, in (a) 10,000 ppm CO in dry air, and (b) 10,000 ppm CO in Ar.
The DRIFT spectra (
Figure 7) clearly showed a peak corresponding to CO adsorption on the Pt and Pd particles at 2078 cm
−1, but no sharp peak corresponding to CO adsorption on the metallic Au site was detected at 2112 cm
−1 as shown in previous researches [
15,
22]. This indicated that less CO oxidation took place on the Au particles compared to the Pt and Pd particles. As shown in the STEM-EDS images (
Figure 6), Au particles formed aggregates, thus increasing their particle size (≥10 nm). This can be explained by previous studies showing that the oxidation reaction of the Au catalyst slowed with increasing particle diameter, with a particle size of <10 nm being required [
12]. In addition, Haruta
et al., reported that the combination of Au with transition metal oxides resulted in high catalytic activities [
23]. Therefore, CO oxidation by Au would be expected to be lower than that of Pt or Pd when considering the large particle size and the effect of non-transition metal (SnO
2) catalysis carriers.
3.3. Sensor Gas Response
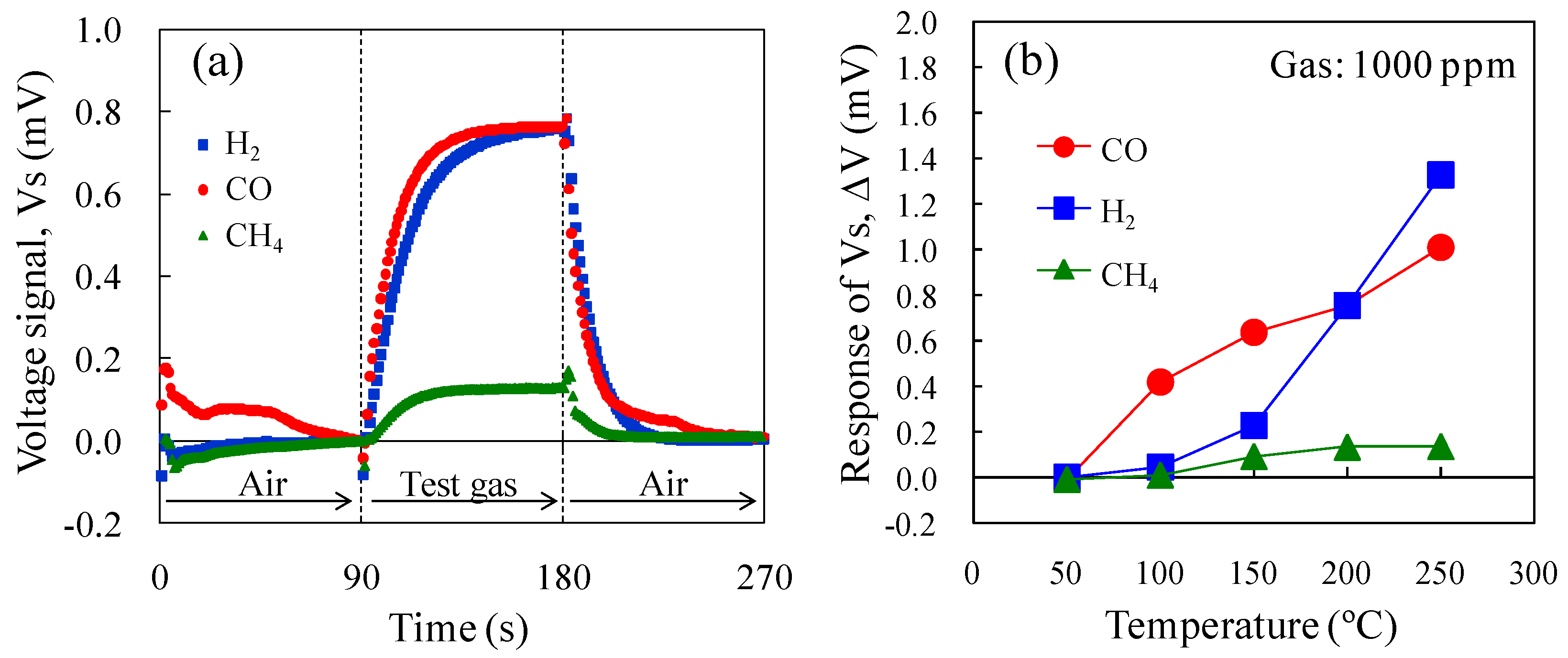
Figure 8a shows the Vs of the micro-TGS with AuPtPd/SnO
2 catalyst at 200 °C for 1000 ppm CO, H
2, and CH
4. The combustion performance of the catalyst is directly related to the Vs of the sensor. At a catalyst temperature of 200 °C, the CO and H
2 sensing properties of the micro-TGS were comparable, while CH
4 combustion was not effective and the Vs was low. The temperature dependence of the ΔV of the AuPtPd/SnO
2 micro-TGS for 1000 ppm CO, H
2, and CH
4 is shown in
Figure 8b. The H
2 and CO sensing signals increased to 1.4 and 1.0 mV at 250 °C, respectively. Up to temperatures of 200 °C, the Vs for CO of this micro-TGS was higher than that of ΔV for H
2. In addition, the CH
4 sensing signal increased slightly to 0.14 mV at 250 °C. Considering our previously reported results [
9] on a micro-TGS with Pd/θ-Al
2O
3 catalyst, to achieve ppm detection of CH
4, a catalyst temperature of 400 °C was required. Thus, the catalyst temperature of 250 °C used in the present study was too low to achieve CH
4 combustion.
Figure 8.
Vs of the AuPtPd/SnO2 micro-TGS for 1000 ppm H2, CO, and CH4 in dry air. (a) Response curves of the micro-TGS at a catalyst temperature of 200 °C, and (b) the ΔV of the micro-TGS at 25–300 °C.
Figure 8.
Vs of the AuPtPd/SnO2 micro-TGS for 1000 ppm H2, CO, and CH4 in dry air. (a) Response curves of the micro-TGS at a catalyst temperature of 200 °C, and (b) the ΔV of the micro-TGS at 25–300 °C.
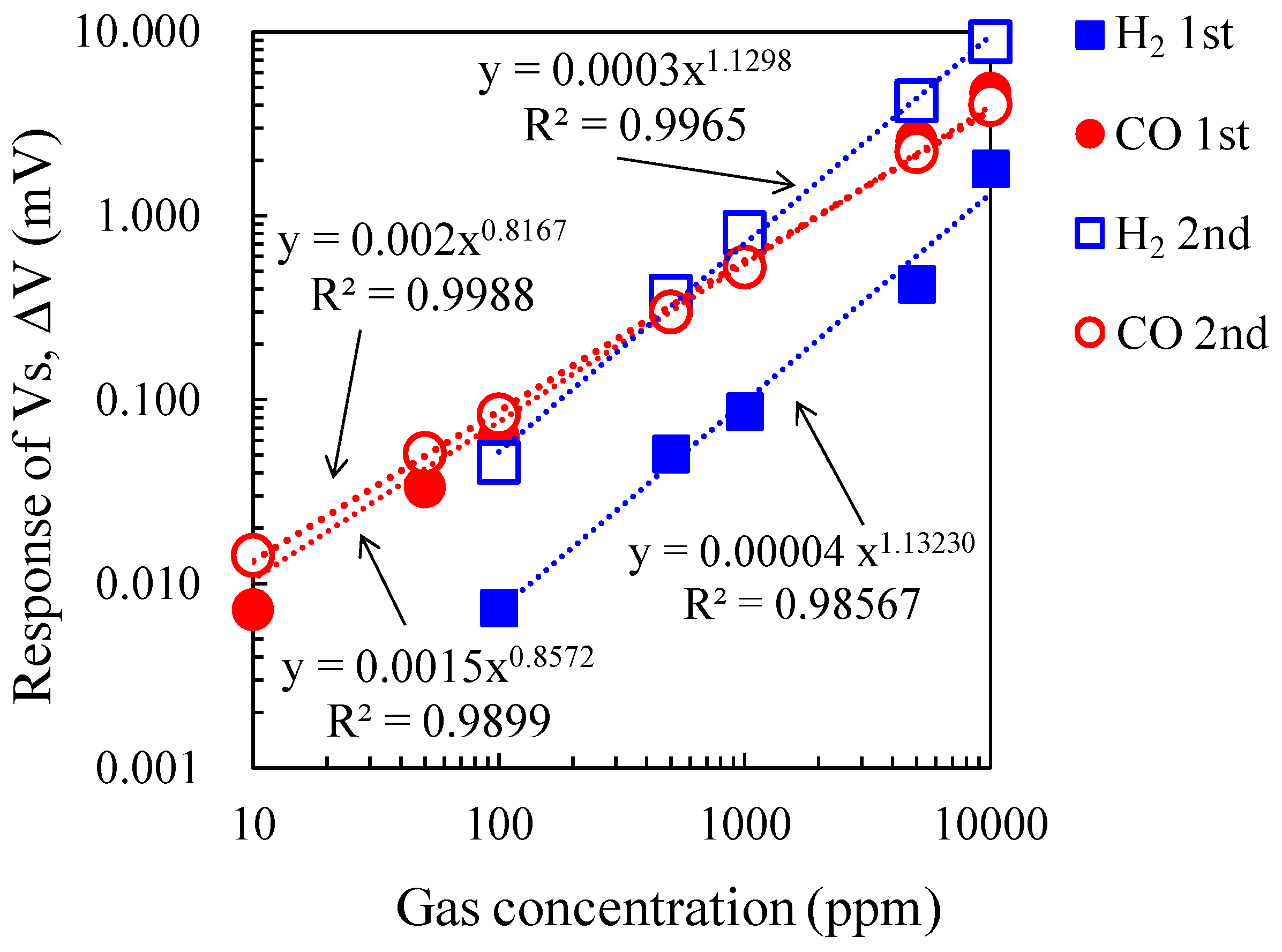
Figure 9 shows the double logarithmic plot of gas concentration and ΔV of the micro-TGS with AuPtPd/SnO
2 catalyst at 200 °C for 10–10,000 ppm of CO and H
2. The plots were fitted to the power approximation curve “
y = axb”, and the sensor ΔV for these gases showed good linear dependence. While the gas concentration dependence of ΔV for CO was unchanged, the ΔV of the second measurement for H
2 increased linearly, and the values became comparable to those of the ΔV for CO.
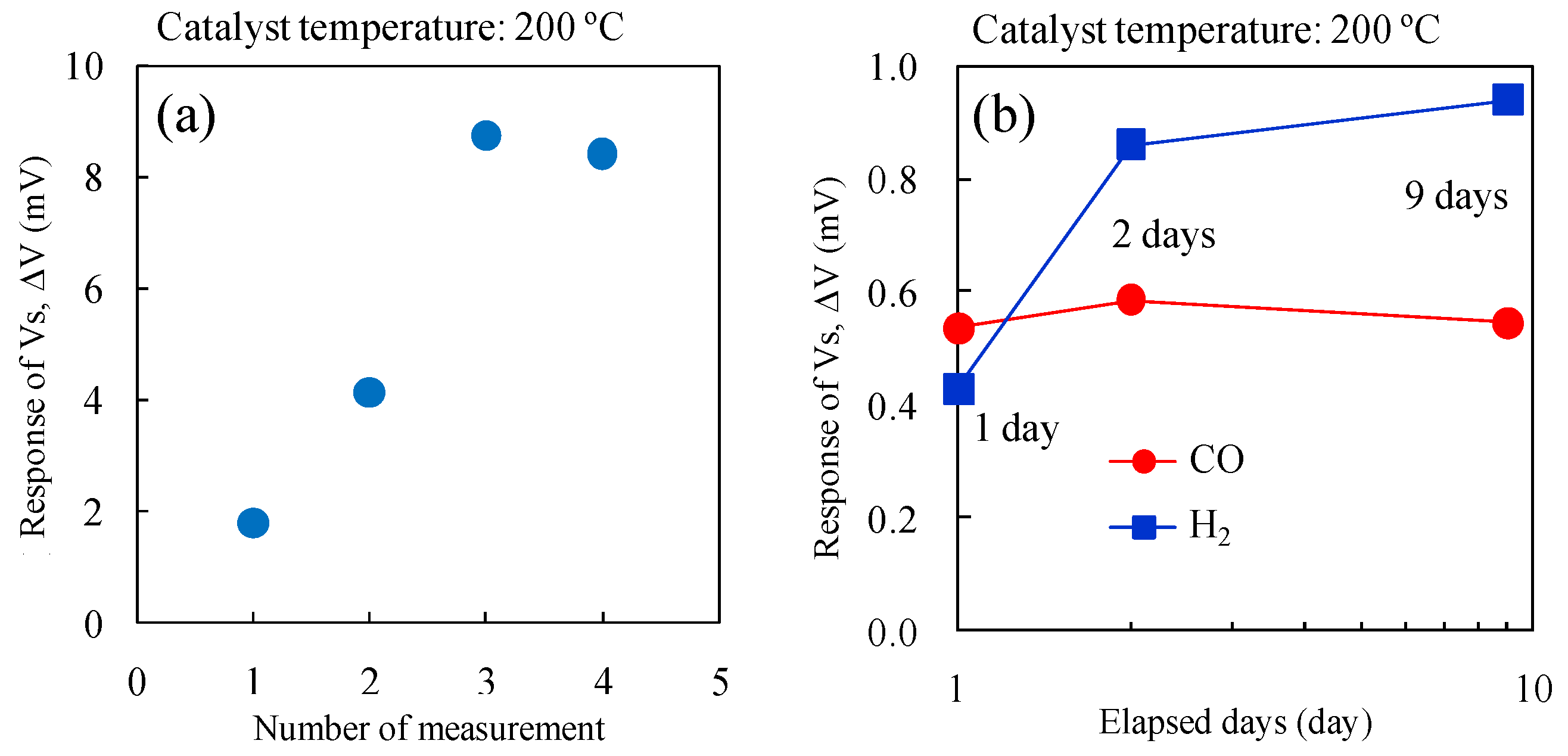
Figure 10a shows the change in ΔV for the micro-TGS with AuPtPd/SnO
2 during repeated measurements with 10,000 ppm H
2 at a catalyst temperature of 200 °C. The long-term stability of the ΔV of the micro-TGS at 1000 ppm CO or H
2 in dry air at 200 °C is shown in
Figure 10b. The CO response of the micro-TGS remained fairly constant, while the H
2 response increased with time. It therefore appears that the chemical state, composition, or dispersed state of Pt and Pd nanoparticles on SnO
2 was altered by pretreatment with 10,000 ppm H
2 in dry air.
Figure 9.
ΔV against CO and H2 concentration for the micro-TGS, from 10–10,000 ppm in dry air.
Figure 9.
ΔV against CO and H2 concentration for the micro-TGS, from 10–10,000 ppm in dry air.
Figure 10.
Gas response of the micro-TGS with AuPtPd/SnO2 catalyst: (a) ΔV against time for the micro-TGS with 10,000 ppm H2 in dry air; and (b) long-term stability of ΔV from the micro-TGS with AuPtPd/SnO2 catalyst for 1000 ppm H2 in dry air or 1000 ppm CO in dry air at 200 °C for 1–9 days.
Figure 10.
Gas response of the micro-TGS with AuPtPd/SnO2 catalyst: (a) ΔV against time for the micro-TGS with 10,000 ppm H2 in dry air; and (b) long-term stability of ΔV from the micro-TGS with AuPtPd/SnO2 catalyst for 1000 ppm H2 in dry air or 1000 ppm CO in dry air at 200 °C for 1–9 days.
We previously reported the CO response of the micro-TGS with noble metal-loaded cobalt oxide catalysts [
5,
6,
7]. The ΔV of the AuPtPd/SnO
2 micro-TGS for CO was comparable to that of the micro-TGS with either AuPtPd/Co
3O
4 or AuPtPd/CoO catalysts [
7]. In addition, the stability of ΔV of the micro-TGS with AuPtPd/SnO
2 catalyst observed in this study was higher than that of the previously reported micro-TGS with Au/Co
3O
4 catalyst [
6]. These results indicate that the AuPtPd/SnO
2 micro-TGS is useful as a CO gas sensor. Furthermore, this study revealed that this micro-TGS is required to control the varying ΔV of H
2 in order to improve its CO selectivity against H
2.
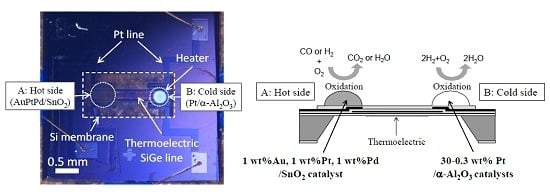
3.4. Double Catalyst Structure
We previously attempted implementing the double catalyst micro-TGS structure for use as a hydrogen sensor [
19], along with calorimetric-TGS devices [
8] to control sensor selectivity. In the present study, to enhance CO selectivity, changes in the CO and H
2 sensing properties of the micro-TGS with AuPtPd/SnO
2 catalyst containing a double catalyst structure were investigated. The AuPtPd/SnO
2 catalyst, which was deposited on the hot side (point A) of the micro-TGS device, burned both H
2 and CO gases. In contrast, the Pt/α-Al
2O
3 catalyst, which was deposited on the cold side (point B) of the micro-TGS device, oxidized only H
2. We therefore aimed for the combustion heat of H
2 (Q
H2) to deduct from the combustion heat of H
2 and CO (Q
H2 + Q
CO) for the AuPtPd/SnO
2 catalyst, by employing various Pt contents for the Pt/α-Al
2O
3 catalyst.
Figure 11 shows the temperature dependence of the ΔV of the double catalyst micro-TGS with 1000 ppm H
2 in dry air and 1000 ppm CO in dry air. The “0Pt” indicated the different batch of the same composition of AuPtPd/SnO
2 micro-TGS. For the H
2 gas response (
Figure 11a), the values of ΔV for 3Pt and 30Pt were negative at all catalyst temperature, indicating a higher Q
H2 for Pt/α-Al
2O
3 than for AuPtPd/SnO
2. The ΔV of 0.3Pt was negative at all catalytic temperatures except 250 °C. Thus, the amount of Pt in the Pt/α-Al
2O
3 catalyst greatly influenced the H
2 sensitivity of the AuPtPd/SnO
2 micro-TGS, which is in agreement with previously reported calorimetric-TGS devices [
8]. In addition, the ΔV of 0Pt (in the absence of Pt/α-Al
2O
3 catalyst on the cold side) was positive, and increased with increasing catalyst temperature. For the CO response (
Figure 11b), the ΔV values of 3Pt and 30Pt were also negative between 50–250 °C, indicating that the Q
CO from Pt/α-Al
2O
3 containing high quantities of Pt is significant in the double catalyst micro-TGS system. Furthermore, ΔV of 0.3Pt increased upon increasing the catalyst temperature to 150 °C, and then decreased gradually at higher temperatures.
Figure 11a,b indicates that the Pt/α-Al
2O
3 catalyst oxidized both H
2 and CO, and that the combustion heats of both 30Pt and 3Pt were higher than that of the AuPtPd/SnO
2 catalyst, due to a negative ΔV. The high Pt content in the Pt/α-Al
2O
3 catalyst therefore appeared to accelerate the oxidation reaction of H
2 and CO. Indeed, this Pt/α-Al
2O
3 catalyst is a combustion catalyst for various gases, including both H
2 and CO. With an increase in Pt content on the cold (point B) side of the Pt/α-Al
2O
3 catalyst, a decrease in the ΔV of H
2 and CO was observed for the micro-TGS. Thus, ΔV varied in the order: 0Pt > 0.3Pt ~ 3Pt > 30Pt. Considering these results, an optimal Pt content of <3 wt% should be used in the Pt/α-Al
2O
3 catalyst to give the best CO selectivity.
Figure 11.
The ΔV of the single and double catalyst structure micro-TGS with AuPtPd/SnO2 and 0.3–30 wt% Pt/α-Al2O3 catalyst at 200 °C for (a) 1000 ppm H2, and (b) 1000 ppm CO in dry air.
Figure 11.
The ΔV of the single and double catalyst structure micro-TGS with AuPtPd/SnO2 and 0.3–30 wt% Pt/α-Al2O3 catalyst at 200 °C for (a) 1000 ppm H2, and (b) 1000 ppm CO in dry air.
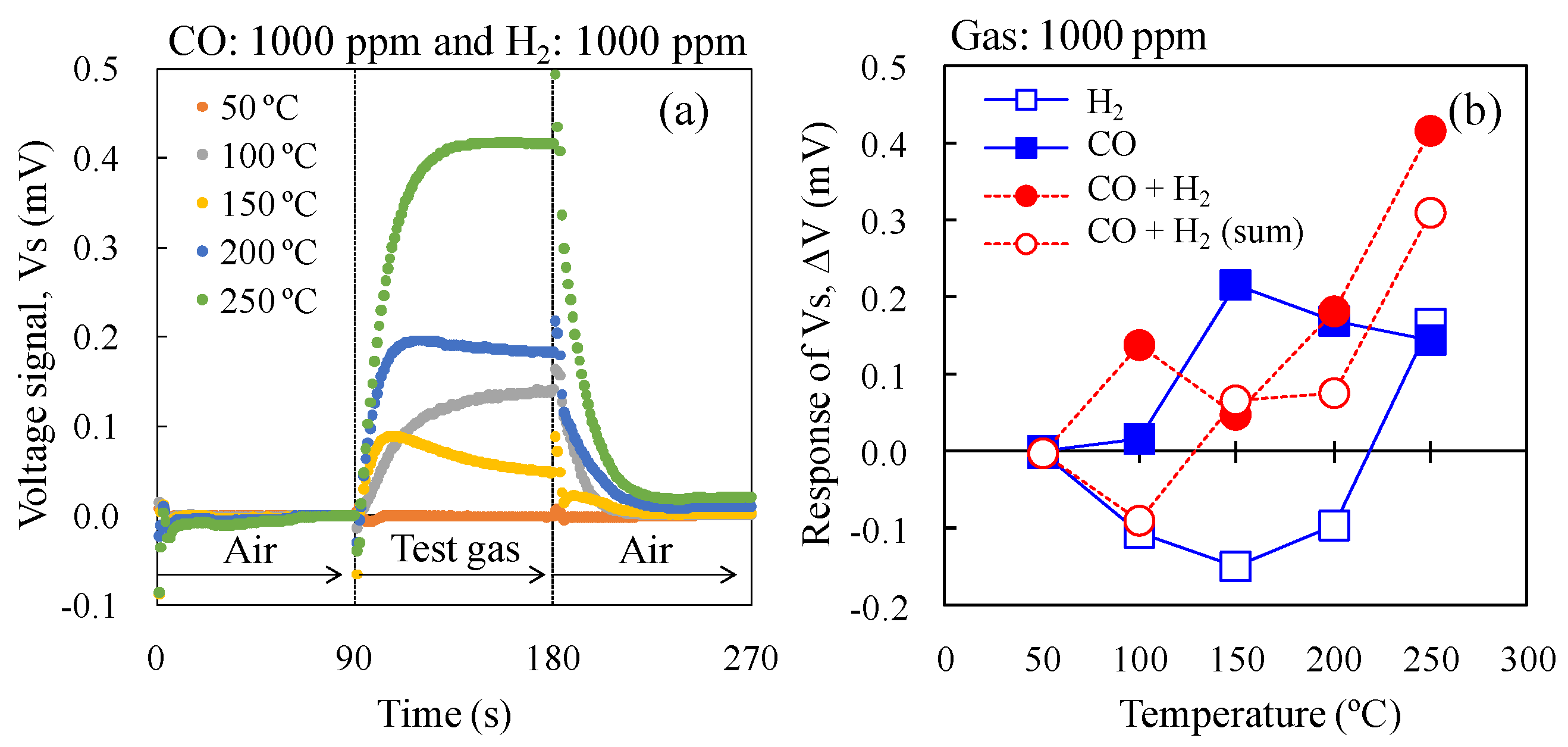
To investigate the CO selectively against H
2, the ΔV of 0.3Pt to CO and H
2 mixed gas in dry air was investigated (
Figure 12), with the ΔV of 0.3Pt to CO and H
2 shown in
Figure 11 re-plotted in
Figure 12b. At a catalyst temperature of 100 °C, the ΔV of 0.3Pt to the mixed gas was large, whereas those to CO and H
2 were small and negative, respectively. It appears that the presence of H
2 accelerated the oxidation of CO in the mixed gas on the AuPtPd/SnO
2 catalyst. The combustion heat of the 0.3 wt% Pt/α-Al
2O
3 catalyst increased at 150 °C, due to the gradual decrease in ΔV with increasing temperature. In addition, between 150–250 °C, the ΔV of CO and H
2 mixed gases increased with increasing catalyst temperature. At a catalyst temperature of 200 °C, the ΔV of 0.3Pt for the mixed gases was comparable to that for CO, indicating that 0.3Pt shows good CO selectivity against H
2. Furthermore, at 250 °C, the ΔV of 0.3Pt to the mixed gas reached its highest value. However, the ΔV of 0.3Pt to H
2 was positive while that to the mixed gas was comparable to the sum of the values for CO and H
2. This indicates that the selectivity to CO of 0.3Pt decreased at a catalyst temperature of 250 °C. We therefore concluded that 0.3Pt gave the best CO selectivity against H
2 on the double catalyst micro-TGS system at 200 °C.
Figure 12.
Vs of the double catalyst micro-TGS with AuPtPd/SnO2 and 0.3 wt% Pt/α-Al2O3 catalysts. (a) Response curves of the micro-TGS at catalyst temperatures of 25–300 °C for mixed gas (1000 ppm CO and 1000 ppm H2) in dry air; (b) Catalyst temperature dependence of the ΔV of the micro-TGS for 1000 ppm CO, 1000 ppm H2, mixed gas (1000 ppm CO + 1000 ppm H2), and calculated data (the sum of single gas data).
Figure 12.
Vs of the double catalyst micro-TGS with AuPtPd/SnO2 and 0.3 wt% Pt/α-Al2O3 catalysts. (a) Response curves of the micro-TGS at catalyst temperatures of 25–300 °C for mixed gas (1000 ppm CO and 1000 ppm H2) in dry air; (b) Catalyst temperature dependence of the ΔV of the micro-TGS for 1000 ppm CO, 1000 ppm H2, mixed gas (1000 ppm CO + 1000 ppm H2), and calculated data (the sum of single gas data).
From
Figure 12b, the ΔV of experimental data of mixed gas was higher than that of calculated data (the sum of single gas data), at catalyst temperature of 100 °C. This result indicated that, at 100 °C, the combustion of 0.3 wt% Pt/α-Al
2O
3 for CO and H
2 (cold side) was inhibited by adsorption of CO, compared to the case of AuPtPd/SnO
2 catalyst (Hot side). On the other hand, the CO combustion on the AuPtPd/SnO
2 catalyst (hot side) was relatively not inhibited much. Therefore combustion heat is generated and ΔV of experimental data showed the positive value. However the ΔV of experimental data was similar to that of calculated data from 150 °C and more. Therefore, at catalyst temperature of 200 °C, the tendency of ΔV of mixed gas could be estimated from the sum of ΔV of pure H
2 and pure CO.