Sensors for Highly Toxic Gases: Methylamine and Hydrogen Chloride Detection at Low Concentrations in an Ionic Liquid on Pt Screen Printed Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical Reagents
2.2. Electrochemical Experiments
2.3. Gas-Mixing Setup
3. Results and Discussion
3.1. Methylamine Gas
3.1.1. Electrochemical Oxidation of Methylamine Gas on Pt SPEs
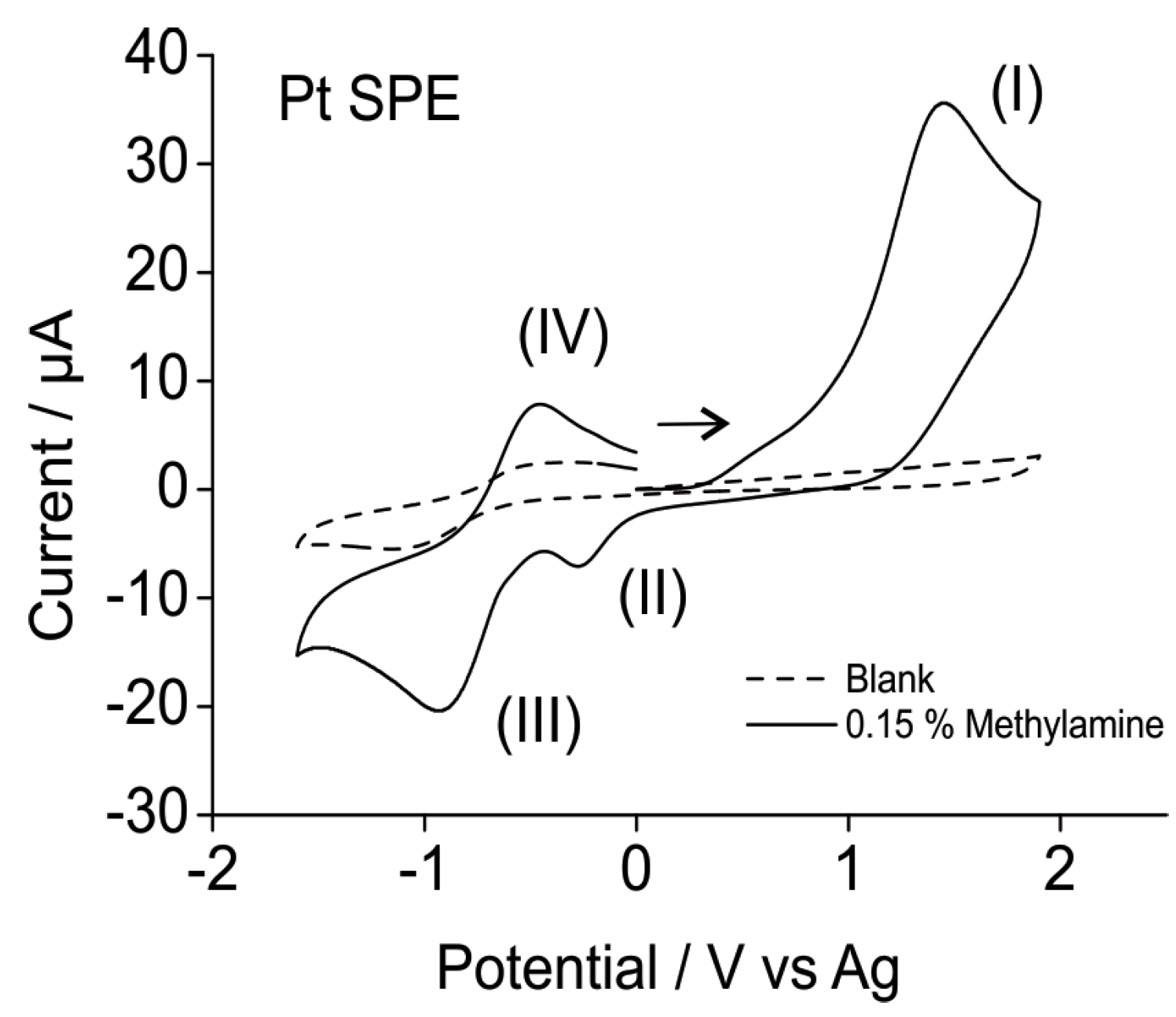
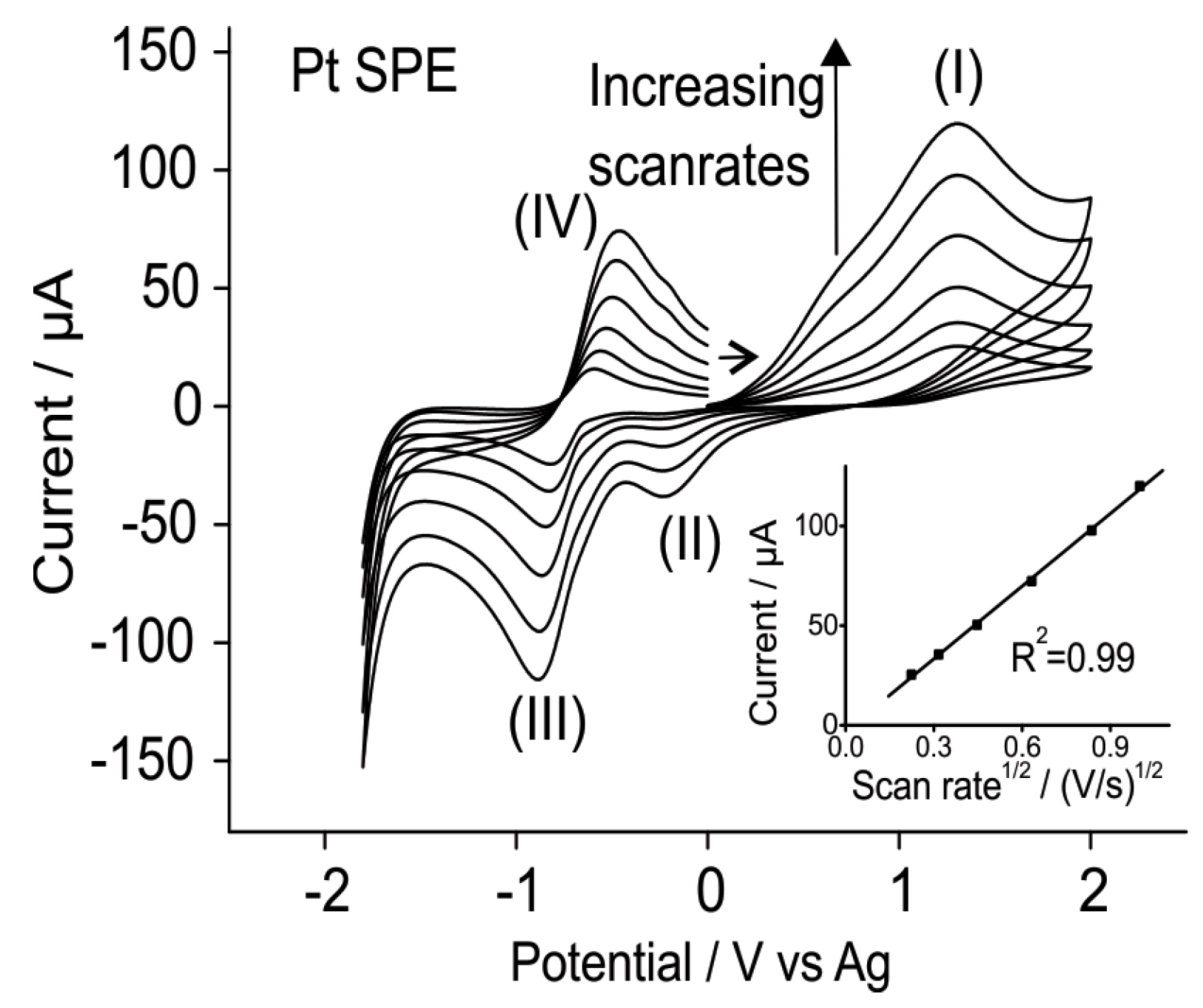
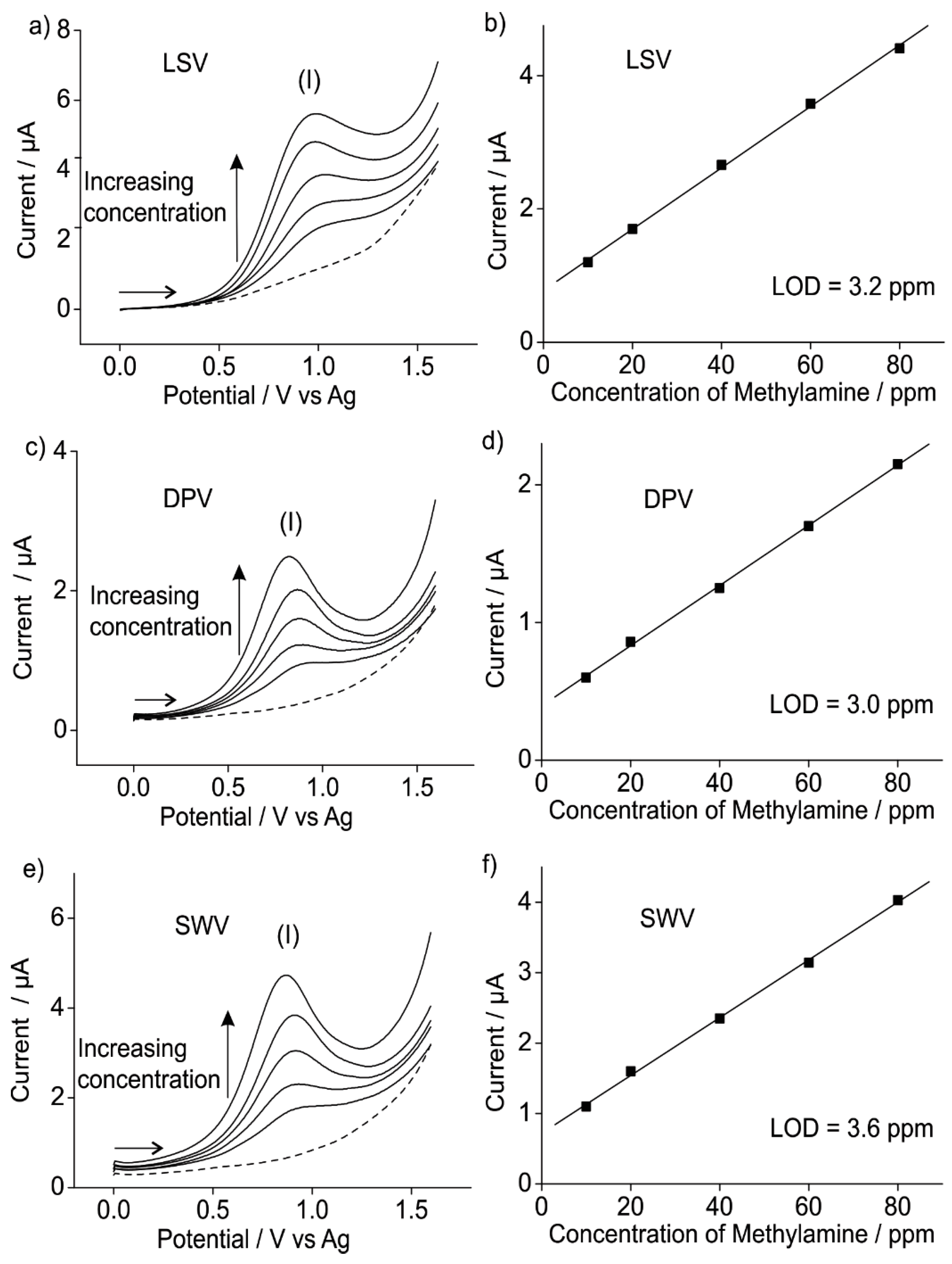
3.1.2. Analytical Utility of Methylamine Gas on Pt SPEs
3.2. Hydrogen Chloride
3.2.1. Electrochemical Oxidation of Hydrogen Chloride Gas on Pt SPEs
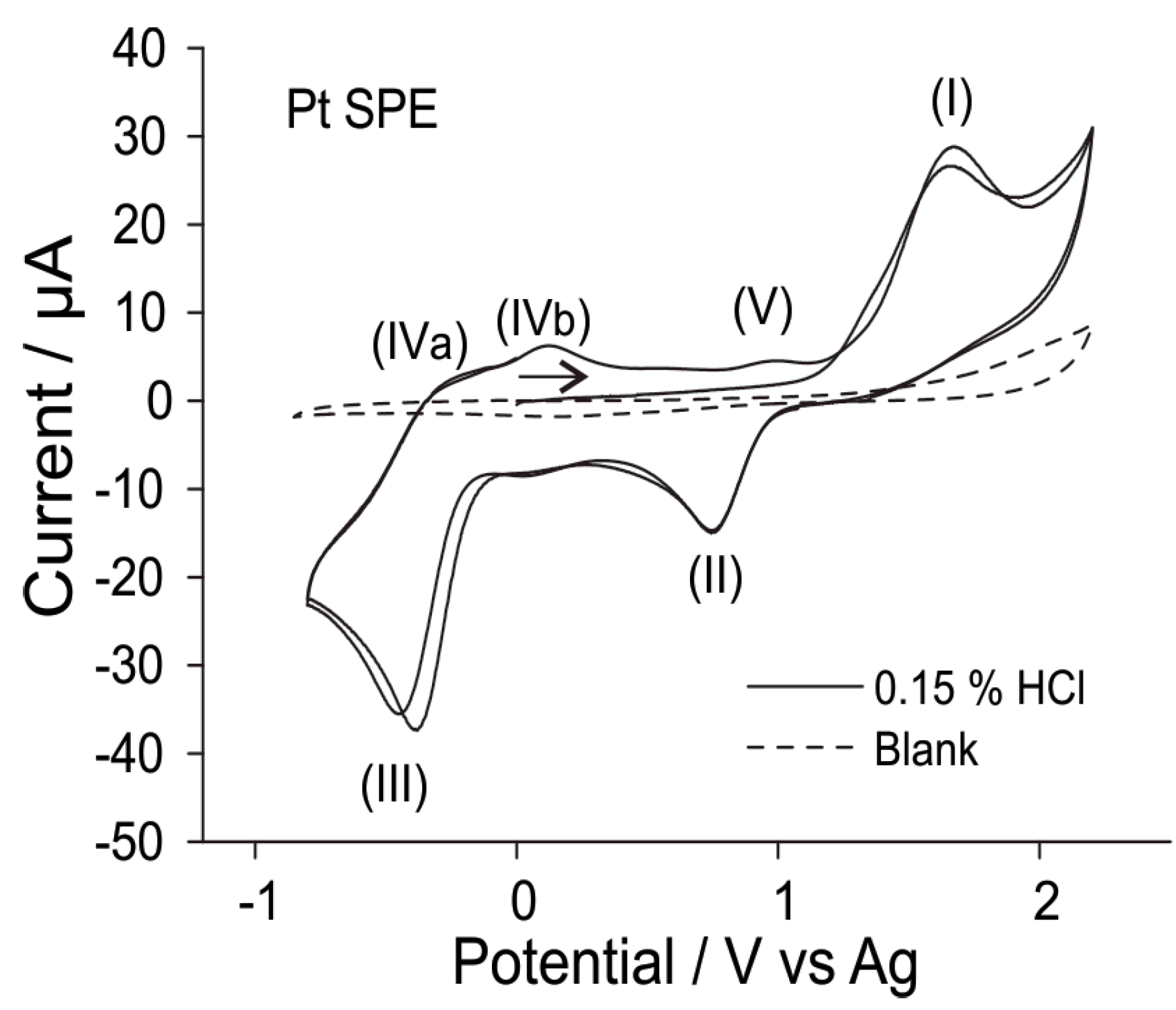
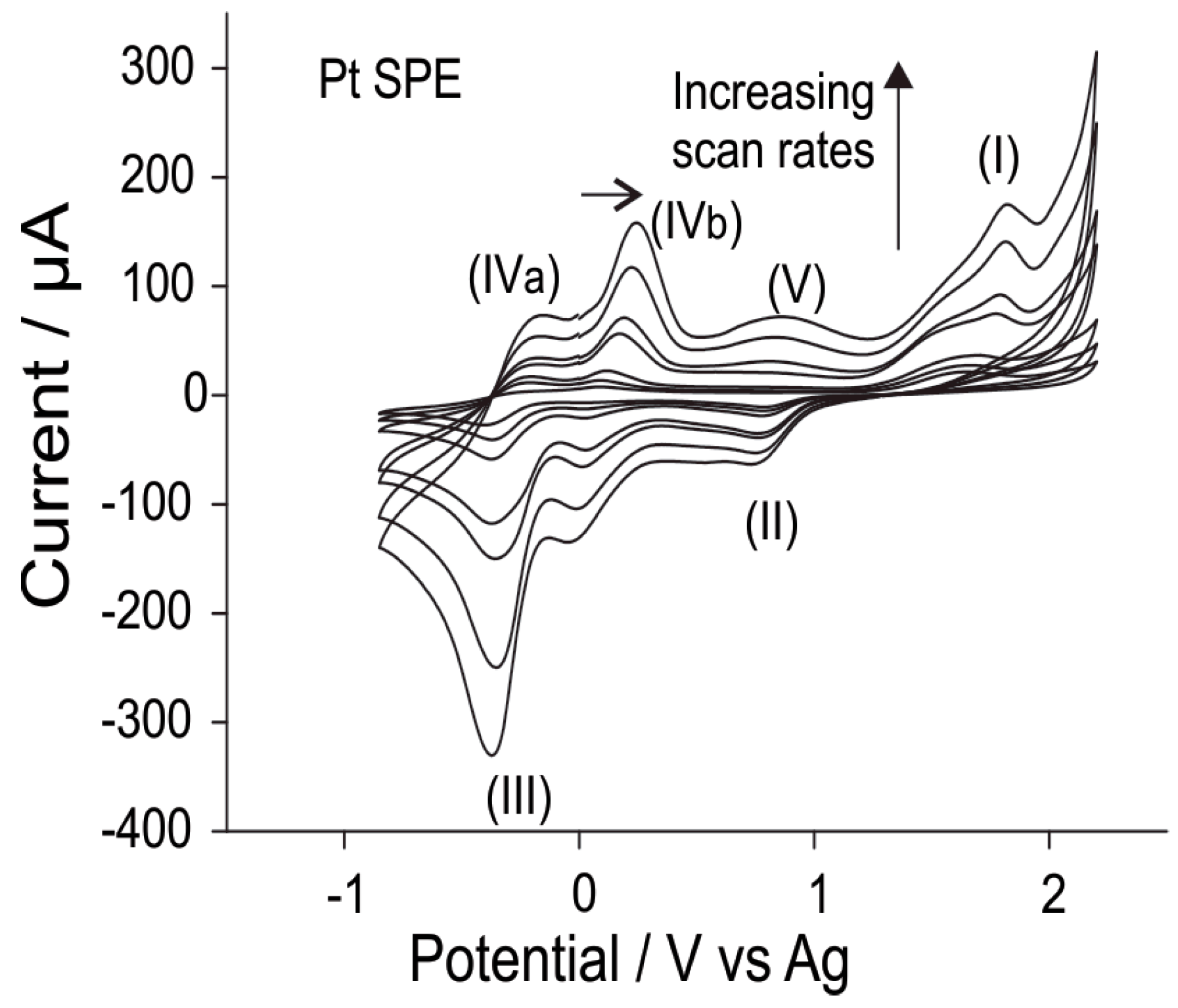
3.2.2. Analytical Utility of Hydrogen Chloride Gas on Pt SPEs
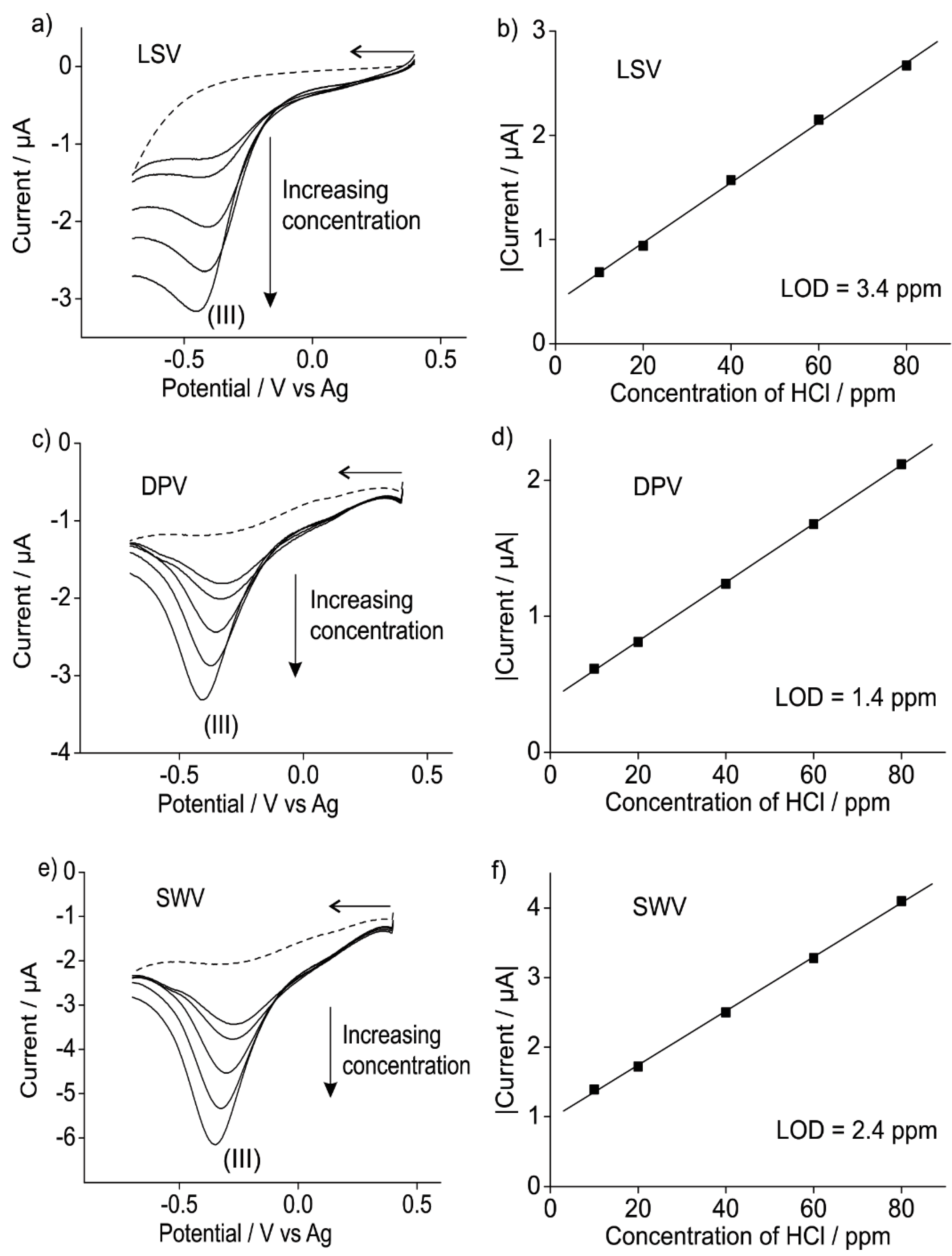
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murugappan, K.; Lee, J.; Silvester, D.S. Comparative study of screen printed electrodes for ammonia gas sensing in ionic liquids. Electrochem. Commun. 2011, 13, 1435–1438. [Google Scholar] [CrossRef]
- Lee, J.; Murugappan, K.; Arrigan, D.W.M.; Silvester, D.S. Oxygen reduction voltammetry on platinum macrodisk and screen-printed electrodes in ionic liquids: Reaction of the electrogenerated superoxide species with compounds used in the paste of Pt screen-printed electrodes? Electrochim. Acta 2013, 101, 158–168. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Compton, R.G. Amperometric gas detection: A review. Int. J. Electrochem. Sci. 2014, 9, 7152–7181. [Google Scholar]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.; O’Malley, E.; Morrin, A.; Smyth, M.R.; Killard, A.J. An aqueous ammonia sensor based on an inkjet-printed polyaniline nanoparticle-modified electrode. Analyst 2008, 133, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.A.; Kampouris, D.K.; Kadara, R.O.; Jenkinson, N.; Banks, C.E. Next generation screen printed electrochemical platforms: Non-enzymatic sensing of carbohydrates using copper (II) oxide screen printed electrodes. Anal. Methods 2009, 1, 183–187. [Google Scholar] [CrossRef]
- Murugappan, K.; Arrigan, D.W.M.; Silvester, D.S. Electrochemical behaviour of chlorine on platinum microdisk and screen printed electrodes in a room temperature ionic liquid. J. Phys. Chem. C 2015, 119, 23572–23579. [Google Scholar] [CrossRef]
- Carter, M.T.; Stetter, J.R.; Findlay, M.W.; Patel, V. Rational design of amperometric gas sensors with ionic liquid electrolytes. ECS Trans. 2014, 64, 95–103. [Google Scholar] [CrossRef]
- Compressed Gas Association, Inc. Handbook of Compressed Gases; Springer US: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Pohanish, R.P. Sittig’s Handbook of Toxic and Hazardous Chemicals and Carcinogens: A-K; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Murugappan, K.; Kang, C.; Silvester, D.S. Electrochemical oxidation and sensing of methylamine gas in room temperature ionic liquids. J. Phys. Chem. C 2014, 118, 19232–19237. [Google Scholar] [CrossRef]
- Aldous, L.; Silvester, D.S.; Pitner, W.R.; Compton, R.G.; Lagunas, M.C.; Hardacre, C. Voltammetric studies of gold, protons, and [HCl2]- in ionic liquids. J. Phys. Chem. C 2007, 111, 8496–8503. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Silvester, D.S.; Aldous, L.; Hardacre, C.; Compton, R.G. Effect of water on the electrochemical window and potential limits of room-temperature ionic liquids. J. Chem. Eng. Data 2008, 53, 2884–2891. [Google Scholar] [CrossRef]
- Silvester, D.S.; Compton, R.G. Electrochemistry in room temperature ionic liquids: A review and some possible applications. Z. Phys. Chem. 2006, 220, 1247–1274. [Google Scholar] [CrossRef]
- Zhao, C.; Bond, A.M.; Compton, R.G.; O’Mahony, A.M.; Rogers, E.I. Modification and implications of changes in electrochemical responses encountered when undertaking deoxygenation in ionic liquids. Anal. Chem. 2010, 82, 3856–3861. [Google Scholar] [CrossRef] [PubMed]
- Barrosse-Antle, L.E.; Bond, A.M.; Compton, R.G.; O’Mahony, A.M.; Rogers, E.I.; Silvester, D.S. Voltammetry in room temperature ionic liquids: Comparisons and contrasts with conventional electrochemical solvents. Chem. Asian J. 2010, 5, 202–230. [Google Scholar] [CrossRef] [PubMed]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry; World Scientific: Singapore, 2007. [Google Scholar]
- Silvester, D.S.; Aldous, L.; Hardacre, C.; Compton, R.G. An electrochemical study of the oxidation of hydrogen at platinum electrodes in several room temperature ionic liquids. J. Phys. Chem. B 2007, 111, 5000–5007. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugappan, K.; Silvester, D.S. Sensors for Highly Toxic Gases: Methylamine and Hydrogen Chloride Detection at Low Concentrations in an Ionic Liquid on Pt Screen Printed Electrodes. Sensors 2015, 15, 26866-26876. https://doi.org/10.3390/s151026866
Murugappan K, Silvester DS. Sensors for Highly Toxic Gases: Methylamine and Hydrogen Chloride Detection at Low Concentrations in an Ionic Liquid on Pt Screen Printed Electrodes. Sensors. 2015; 15(10):26866-26876. https://doi.org/10.3390/s151026866
Chicago/Turabian StyleMurugappan, Krishnan, and Debbie S. Silvester. 2015. "Sensors for Highly Toxic Gases: Methylamine and Hydrogen Chloride Detection at Low Concentrations in an Ionic Liquid on Pt Screen Printed Electrodes" Sensors 15, no. 10: 26866-26876. https://doi.org/10.3390/s151026866
APA StyleMurugappan, K., & Silvester, D. S. (2015). Sensors for Highly Toxic Gases: Methylamine and Hydrogen Chloride Detection at Low Concentrations in an Ionic Liquid on Pt Screen Printed Electrodes. Sensors, 15(10), 26866-26876. https://doi.org/10.3390/s151026866





