Integration of a Miniature Quartz Crystal Microbalance with a Microfluidic Chip for Amyloid Beta-Aβ42 Quantitation
Abstract
:1. Introduction
2. Experimental
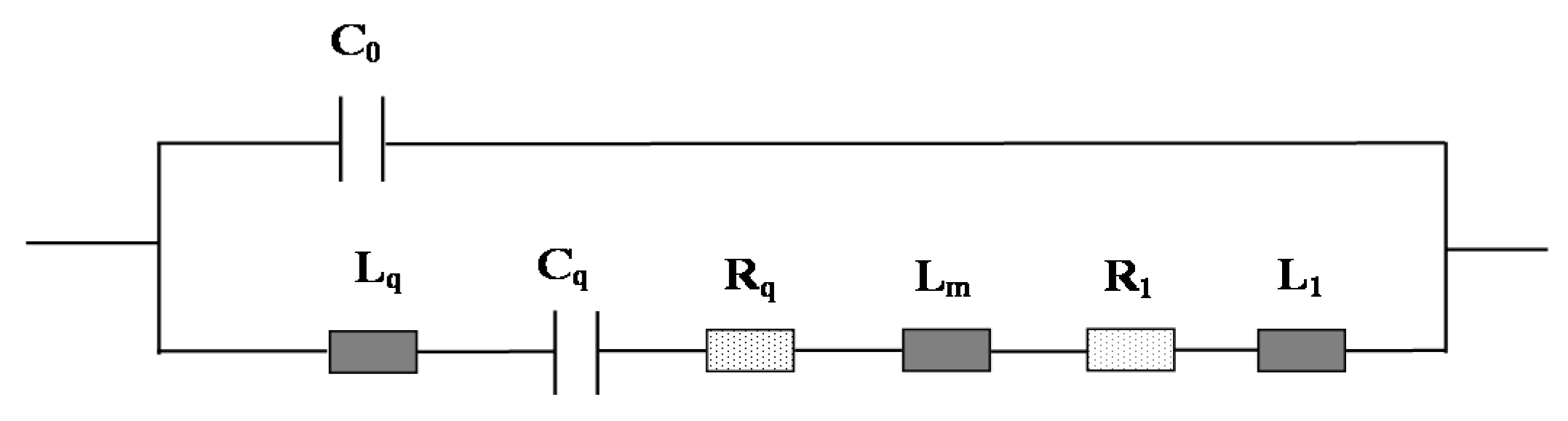
2.1. Principle of MQCM in a Liquid Microfluidic System
2.2. Chemicals and Reagents
2.3. Fabrication of PDMS-mQCM Microfluidic Device
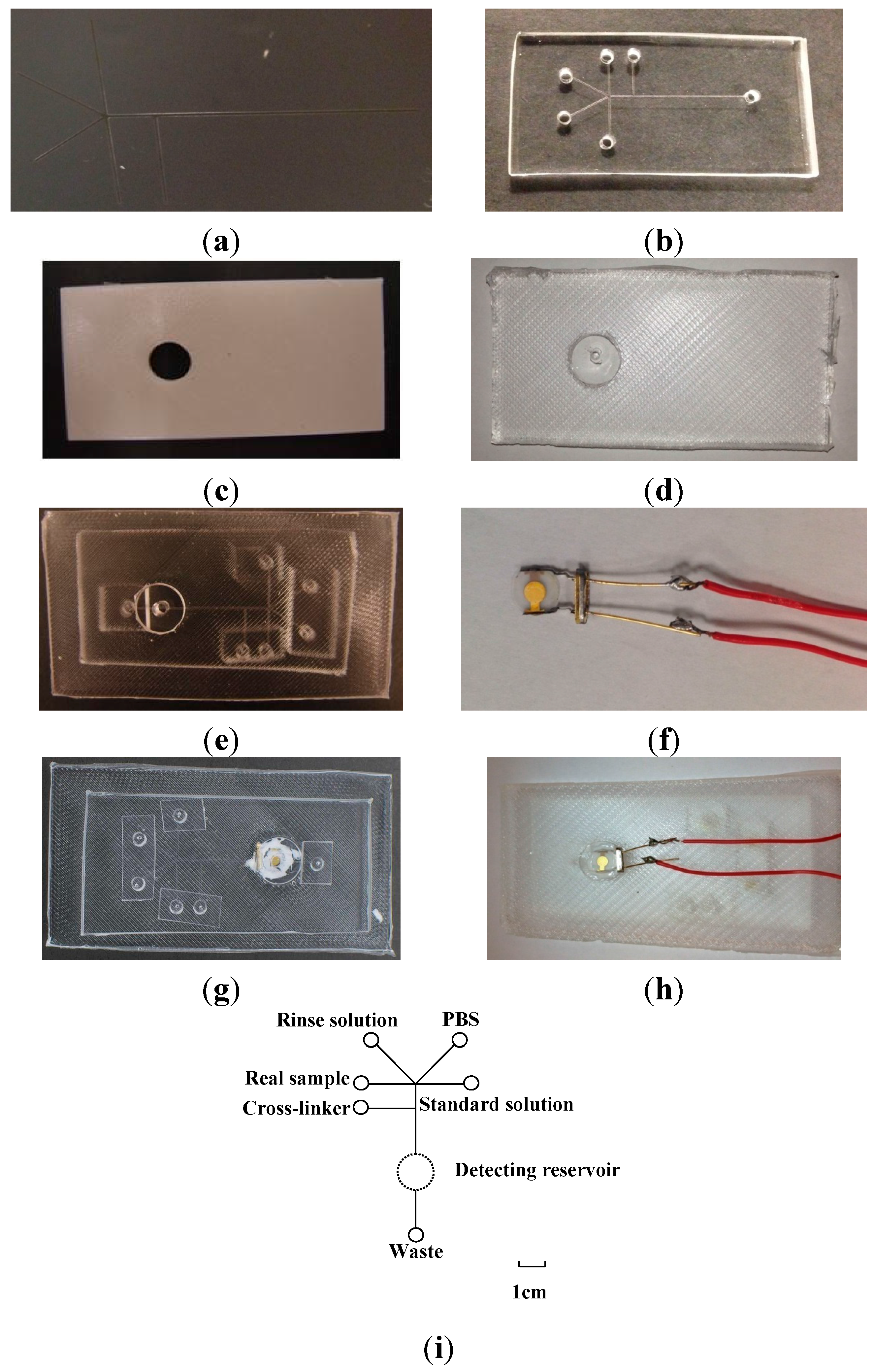
2.3.1. Si Mold
2.3.2. ABS Mold
2.3.3. PDMS Microfluidic Chip and Mounting PDMS Slab Molding
2.3.4. PDMS-mQCM Microfluidic Device
2.3.5. Layout of PDMS-mQCM Microfluidic Device
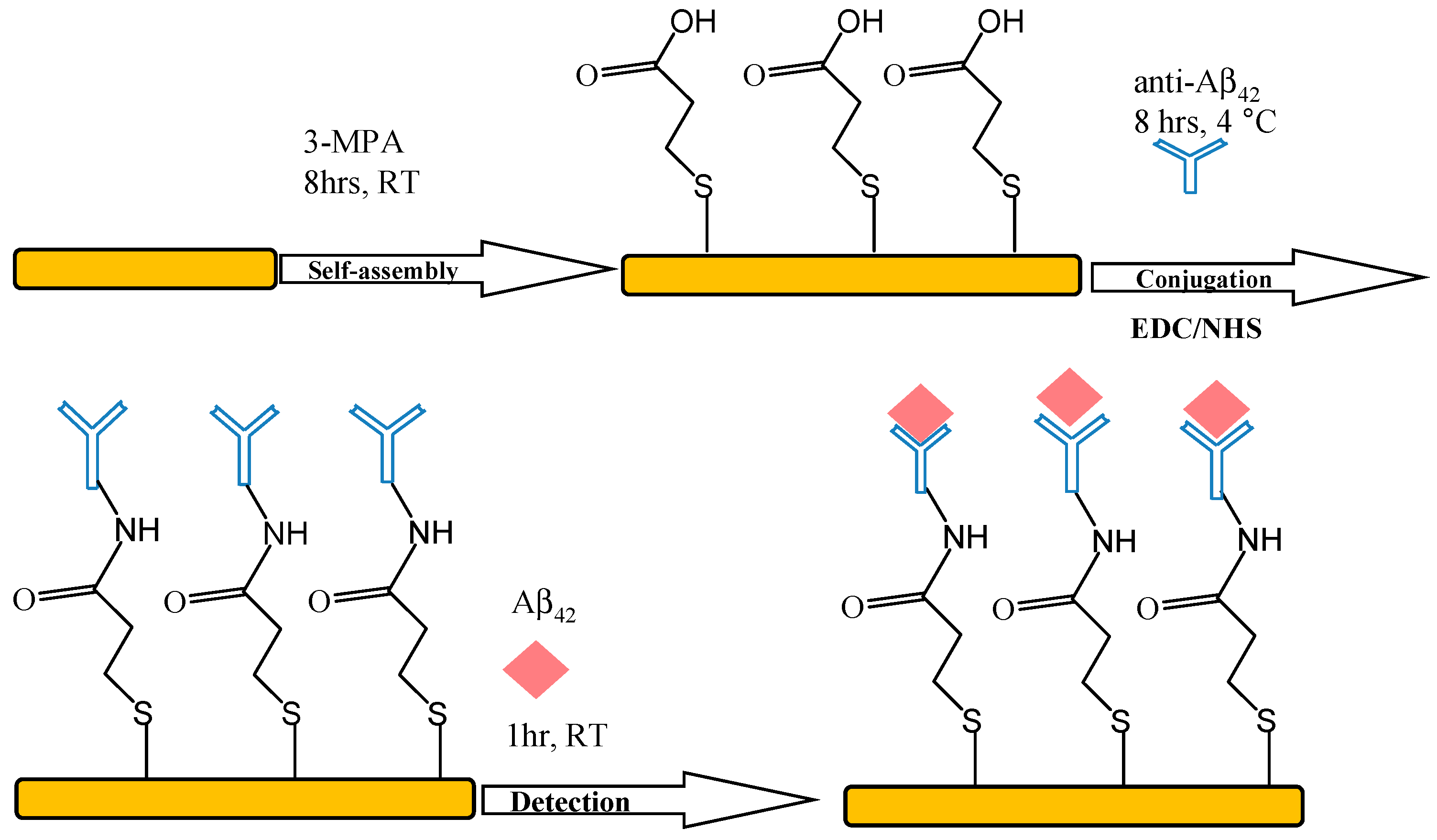
2.4. Immobilization of Aβ42 Antibody
2.5. Measurements
3. Results and Discussion
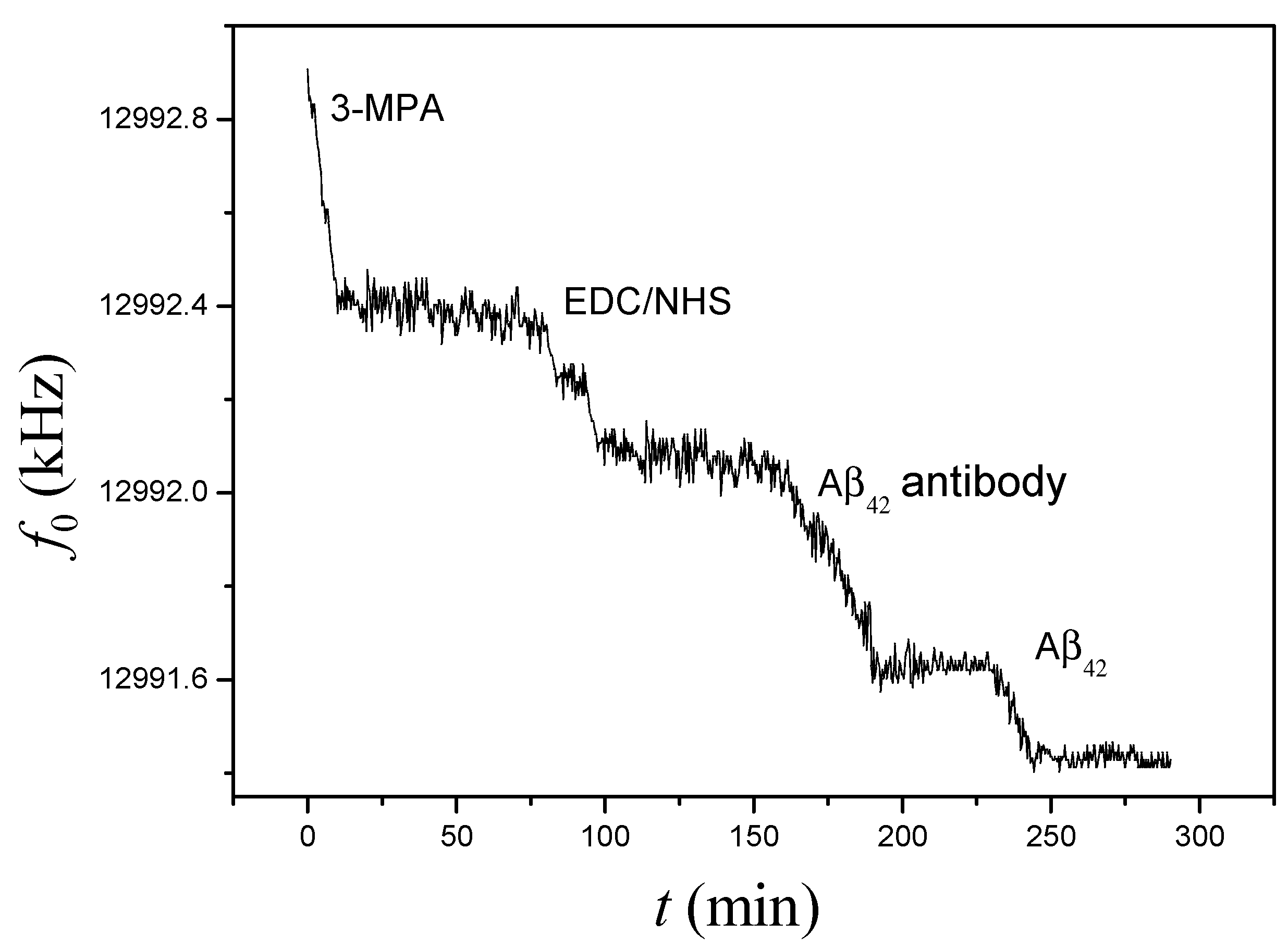
3.1. On-Chip Determination of Aβ42
| Device | Gmax (mS) | Rt (Ω) | ΔRt (Ω) | Δf0 (Hz) | K |
|---|---|---|---|---|---|
| PDMS-mQCM | 3.79 ± 0.02 | 504.5 ± 3.5 | NA | NA | NA |
| Aβ42 antibody-PDMS-mQCM | 1.23 ± 0.05 | 860.6 ± 5.3 | 356.1 ± 0.6 | −1528 ± 3 | 2.94 ± 0.03 |
| Aβ42-Aβ42 antibody-PDMS-mQCM | 1.20 ± 0.03 | 863.8 ± 6.8 | 3.2 ± 0.3 | −218 ± 1 | 46.74 ± 0.50 |
3.2. Calibration Curve
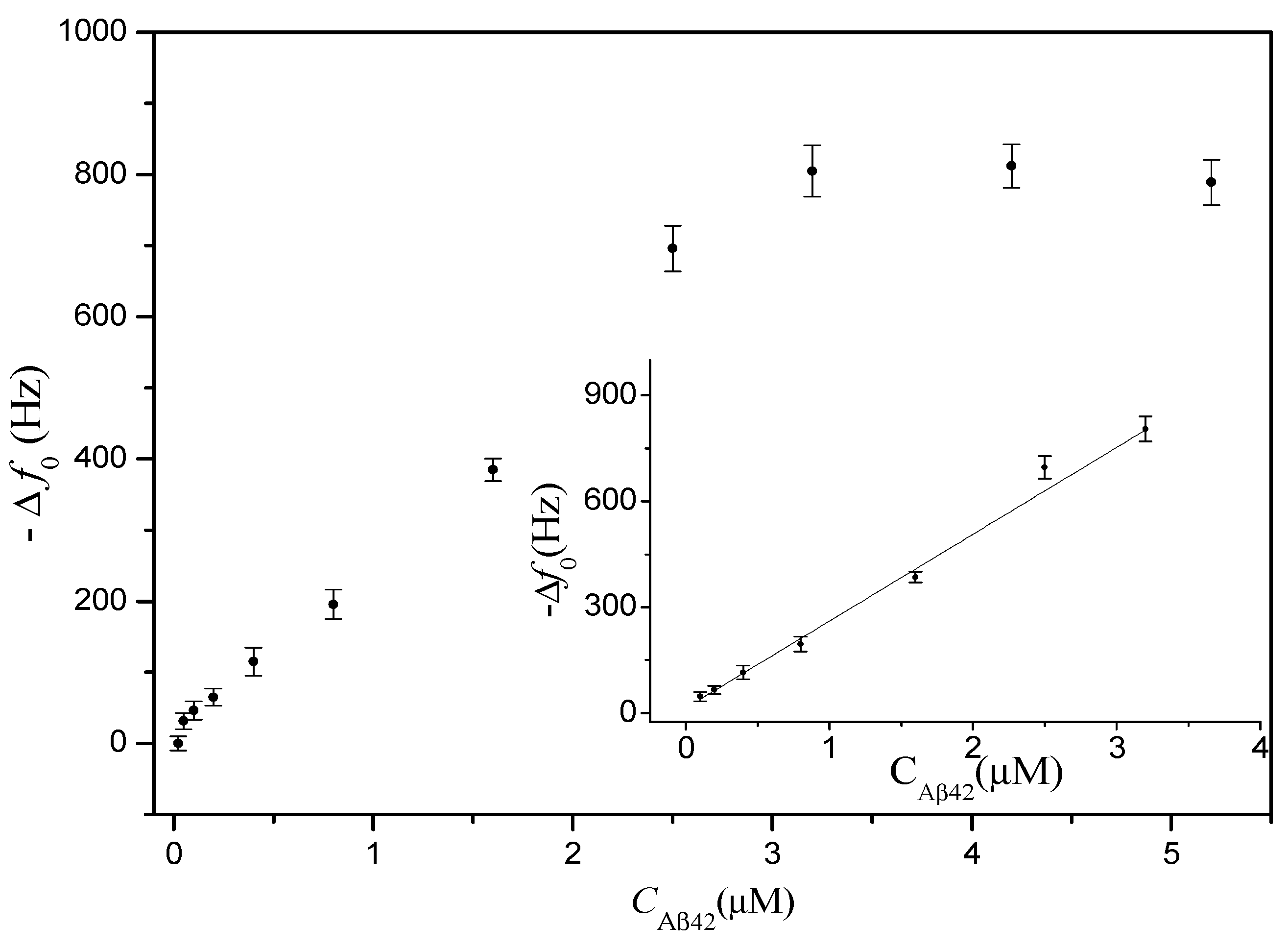
3.3. Real Sample Assay
3.3.1. Non-Specific Binding Assay
| Device | Injected Sample a | Δf0 (Hz) |
|---|---|---|
| PDMS-mQCM | 25% serum | −36.5 ± 3.0 |
| PDMS-mQCM | 75% serum | −89.5 ± 7.8 |
| Aβ42 antibody-PDMS-mQCM | 25% serum | 0 |
| Aβ42 antibody-PDMS-mQCM | 75% serum | 0 |
| Aβ42 antibody-PDMS-mQCM | 0.8 µM Aβ42 + 75% serum | −189.7 ± 8.6 |
| Aβ42 antibody-PDMS-mQCM | 1.6 µM Aβ42 + 75% serum | −356.0 ± 9.7 |
3.3.2. Detection of Aβ42 in 75% Human Serum
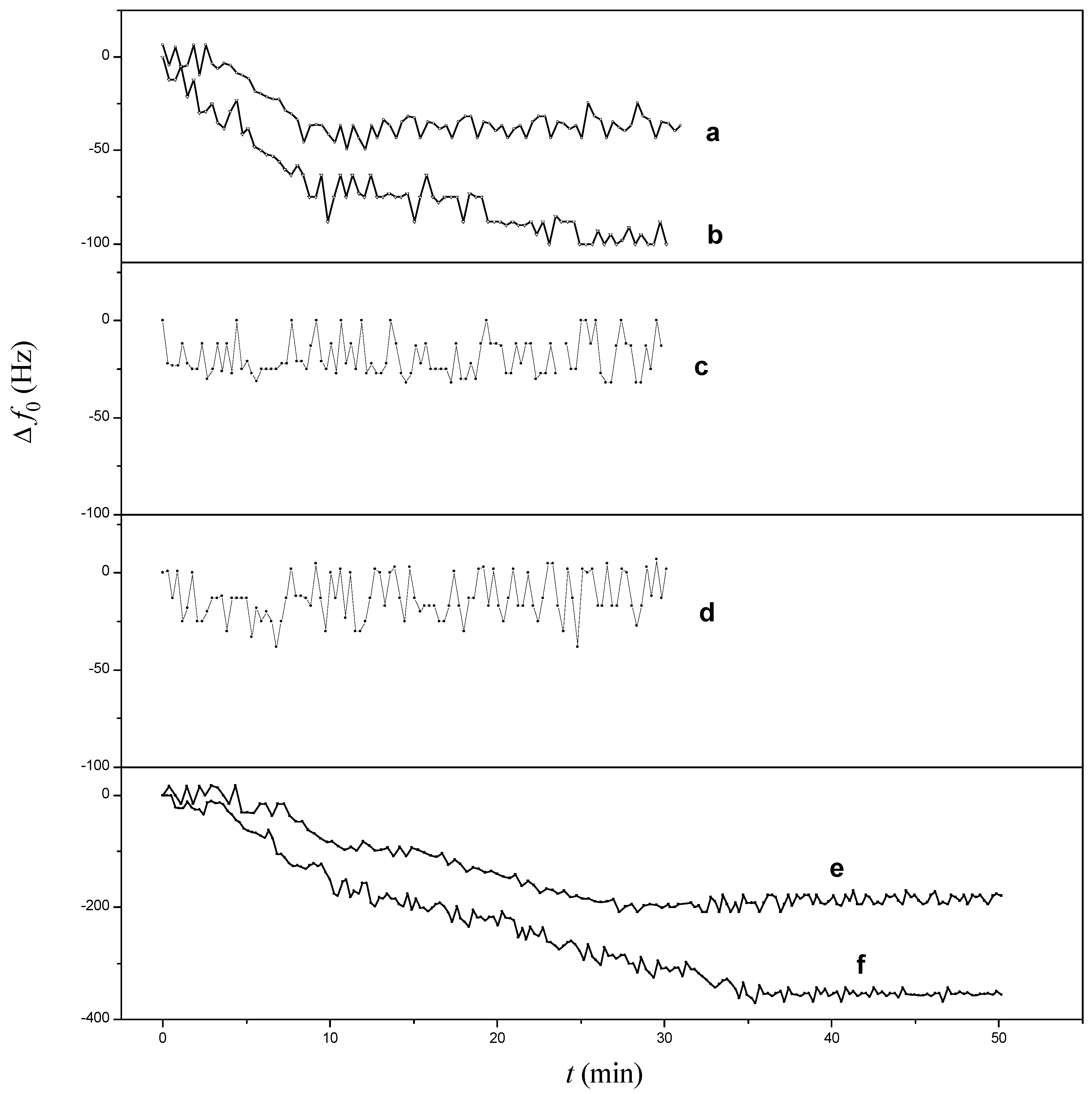
3.4. Reusability
| Device | f0 (Hz) | SD (Hz) | Gmax (mS) | Δf0 (Hz) | ΔGmax (mS) |
|---|---|---|---|---|---|
| PDMS-mQCM | 12.99275 × 106 | 3 | 3.90 ± 0.07 | NA | NA |
| Aβ42 antibody-PDMS-mQCM a | 12.992675 × 106 | 5 | 3.00 ± 0.03 | 75 ± 3 | 0.90 ± 0.01 |
| Aβ42 antibody-PDMS-mQCM b | 12.991205 × 106 | 9 | 1.38 ± 0.02 | 1470 ± 8 | 1.62 ± 0.02 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tickler, A.K.; Wade, J.D.; Separovic, F. The role of Abeta peptides in Alzhermer’s disease. Protein Pept. Lett. 2005, 12, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Iwatsubo, T. Secretase as a therapeutic target for treatment of Alzhermer’s disease. Curr. Pharm. Des. 2006, 12, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Kakano, K.; Endo, S.; Mukaiyama, A.; Chon, H.; Matumura, H.; Koga, Y.; Kanaya, S. Structure of amyloid beta fragments in aqueous environments. FEBS J. 2006, 273, 150–158. [Google Scholar]
- Indeykina, M.I.; Popov, I.A.; Kozin, S.A.; Kononikhin, A.S.; Kharybin, O.N.; Tsvetkov, P.O.; Makarov, A.A.; Nikolaev, E.N. Capabilities of MS for analytical quantitative determination of the ratio of γ- and βAsp7 isoforms of the amyloid-β peptide in binary mixtures. Anal. Chem. 2011, 83, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Portelius, E.; Brinkmalm, A.W.; Zetterberg, H.; Blennow, K. Determination of α-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. J. Proteom. Res. 2006, 5, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, J.V.; Ahmed, A.; Griffiths, H.H.; Pollock, N.M.; Hooper, N.M.; Millner, P.A. A label-free electrical impedimetric biosensor for the specific detection of Alzheimer’s amyliod-beta oligomers. Biosens. Bioelectron. 2014, 56, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Suprun, E.V.; Shumyantseva, V.V.; Archakov, A.I. Protein electrochemistry: Application in medicine. Electrochim. Acta 2014, 140, 72–82. [Google Scholar] [CrossRef]
- Liu, L.; He, Q.; Zhao, F.; Xia, N.; Liu, H.; Li, S.; Liu, R.; Zhang, H. Competitive electrochemical immunoassay for detection of β-amyloid (1-42) and total β-amyloid peptides using p-aminophenol redox cycling. Biosens. Bioelectron. 2014, 51, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Sun, X.; Tang, D.; Li, C.; Zhang, L.; Nie, D.X.; Yin, X.X.; Shi, G.Y. Gelsolin bound β-amyloid peptides(1-40/1-42): Electrochemical evaluation of levels of soluble peptide associated with Alzheimer’s disease. Biosens. Bioelectron. 2015, 68, 115–121. [Google Scholar] [CrossRef]
- Clarke, N.J.; Tomlinson, A.J.; Ohyagi, Y.; Younkin, S.; Naylor, S. Detection and quantitation of cellularly derived amyloid L-peptide by immune-precipitation HPLC-MS. FEBS Lett. 1998, 430, 419–423. [Google Scholar] [CrossRef]
- Xing, Y.; Xia, N. Biosensors for the determination of Amyloid-beta peptides and their aggregates with application to Alzheimer’s disease. Anal. Lett. 2015, 48, 879–893. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.T.; Chen, R.; Zhang, D.H.; Wei, X. H.; Chen, F.; Wang, J.X.; Xu, M.T. A highly sensitive resonance light scattering probe for Alzheimer’s amyloid-β peptide based on Fe3O4@Au composites. Talanta 2015, 131, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Weaver, W.; Kittur, H.; Dhara, M.; Carlo, D.D. Research highlights: Microfluidic point-of-care diagnostics. Lab Chip 2014, 14, 1962–1965. [Google Scholar] [CrossRef]
- Gervais, L.; Rooij, N.; Delamarche, E. Microfluidic chips for point of care immunodiagnostics. Adv. Mater. 2011, 23, H151–H176. [Google Scholar] [CrossRef] [PubMed]
- Nashida, N.; Satoh, W.J.; Suzuki, H. Electrochemical immunoassay on a microfluidic device with sequential injection and flushing functions. Biosens. Bioelectron. 2007, 22, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, X.; Wang, H.Y.; Woolley, A.T. Integrated microfluidic device for serum biomarker quantitation using either standard addition or a calibration curve. Anal. Chem. 2009, 81, 8230–8235. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, K.; Kim, K.H.; Kwang, W.O.; Choo, J. SERS-based immunoassay using a gold array-embedded gradient microfluidic chip. Lab Chip 2012, 12, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Michalzik, M.; Wendler, J.; Rabe, J.; Büttgenbach, S.; Bilitewski, U. Development and application of a miniaturised quartz crystal microbalance (QCM) as immunosensor for bone morphogenetic protein-2. Sens. Actuators B Chem. 2005, 105, 508–515. [Google Scholar] [CrossRef]
- Orelma, H.; Teerinen, T.; Johansson, L.S.; Holappa, S.; Laine, J. CMC-modified cellulose biointerface for antibody conjugation. Biomacromolecules 2012, 13, 1051–1058. [Google Scholar] [CrossRef]
- Uludag, Y.; Tothill, I.E. Cancer biomarker detection in serum samples using surface plasmon resonance and quartz crystal microbalance sensors with nanoparticle signal amplification. Anal. Chem. 2012, 84, 5898–5904. [Google Scholar] [CrossRef] [PubMed]
- Teller, C.; Halámek, J.; Žeravík, J.; Stöcklein, W.F.; Scheller, F.W. Development of a bifunctional sensor using haptenized acetylcholinesterase and application for the detection of cocaine and organophosphates. Biosens. Bioelectron. 2008, 24, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Michalzik, M.; Wilke, R.; Büttgenbach, S. Miniaturized QCM-based flow system for immunosensor application in liquid. Sens. Actuators B Chem. 2005, 111–112, 410–415. [Google Scholar] [CrossRef]
- Ohlsson, G.; Axelsson, P.; Henry, J.; Petronis, S.; Svedhem, S.; Kasemo, B. A miniatureized flow reaction chamber for use in combination with QCM-D sensing. Microfluid. Nanofluid. 2010, 9, 705–716. [Google Scholar] [CrossRef]
- Martin, S.J.; Granstaff, V.E.; Frye, G.C. Characterization of a quartz crystal microbalance with simultaneous mass and liquid loading. Anal. Chem. 1991, 63, 2272–2281. [Google Scholar] [CrossRef]
- Xie, Q.J.; Zhang, Y.Y.; Xu, M.; Li, Z.; Yuan, Y.; Yao, S.Z. Combined quartz crystal impedance and electrochemical impedance measurements during adsorption of bovine serum albumin onto bare and cysteine-or thiophenol-modified gold electrodes. J. Electroanal.Chem. 1999, 478, 1–8. [Google Scholar] [CrossRef]
- Xie, Q.J.; Zhang, Y.Y.; Yuan, Y.; Guo, Y.; Wang, X.; Yao, S.Z. An electrochemical quartz crystal impedance study on cystine precipitation onto an Au electrode surface during cysteine oxidation in aqueous solution. J. Electroanal. Chem. 2000, 484, 41–54. [Google Scholar] [CrossRef]
- Xie, Q.J.; Wang, J.; Zhou, A.; Zhang, Y.Y.; Liu, H.; Xu, Z. A study of depletion layer effects on equivalent circuit parameters using an electrochemical quartz crystal impedance system. Anal. Chem. 1999, 71, 4649–4656. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Tang, D.; Que, X.; Zhuang, J.; Fu, L.; Chen, G. Enzyme-catalyzed silver deposition on irregular-shaped gold nanoparticles for electrochemical immunoassay of alpha-fetoprotein. Anal. Chim. Acta 2012, 755, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- Stenberg, M.; Nygren, H. Kinetics of antigen-antibody reactions at solid-liquid interfaces. J. Immunol. Methods 1988, 113, 3–15. [Google Scholar] [CrossRef]
- Chandekar, A.; Sengupta, S.K.; Whitten, J.E. Thermal stability of thiol and silane monolayers: A comparative study. Appl. Surf. Sci. 2010, 256, 2742–2749. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, W.; Xie, Q.; Wang, H.; Ke, S.; Lin, P.; Zeng, X. Integration of a Miniature Quartz Crystal Microbalance with a Microfluidic Chip for Amyloid Beta-Aβ42 Quantitation. Sensors 2015, 15, 25746-25760. https://doi.org/10.3390/s151025746
Tao W, Xie Q, Wang H, Ke S, Lin P, Zeng X. Integration of a Miniature Quartz Crystal Microbalance with a Microfluidic Chip for Amyloid Beta-Aβ42 Quantitation. Sensors. 2015; 15(10):25746-25760. https://doi.org/10.3390/s151025746
Chicago/Turabian StyleTao, Wenyan, Qingji Xie, Hairui Wang, Shanming Ke, Peng Lin, and Xierong Zeng. 2015. "Integration of a Miniature Quartz Crystal Microbalance with a Microfluidic Chip for Amyloid Beta-Aβ42 Quantitation" Sensors 15, no. 10: 25746-25760. https://doi.org/10.3390/s151025746





