Synergy Effect of Nanocrystalline Cellulose for the Biosensing Detection of Glucose
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Synthesis of the PPy/CNC Nanocomposite
2.4. Preparation of Chemical Solutions
2.5. Preparation of Modified Electrodes
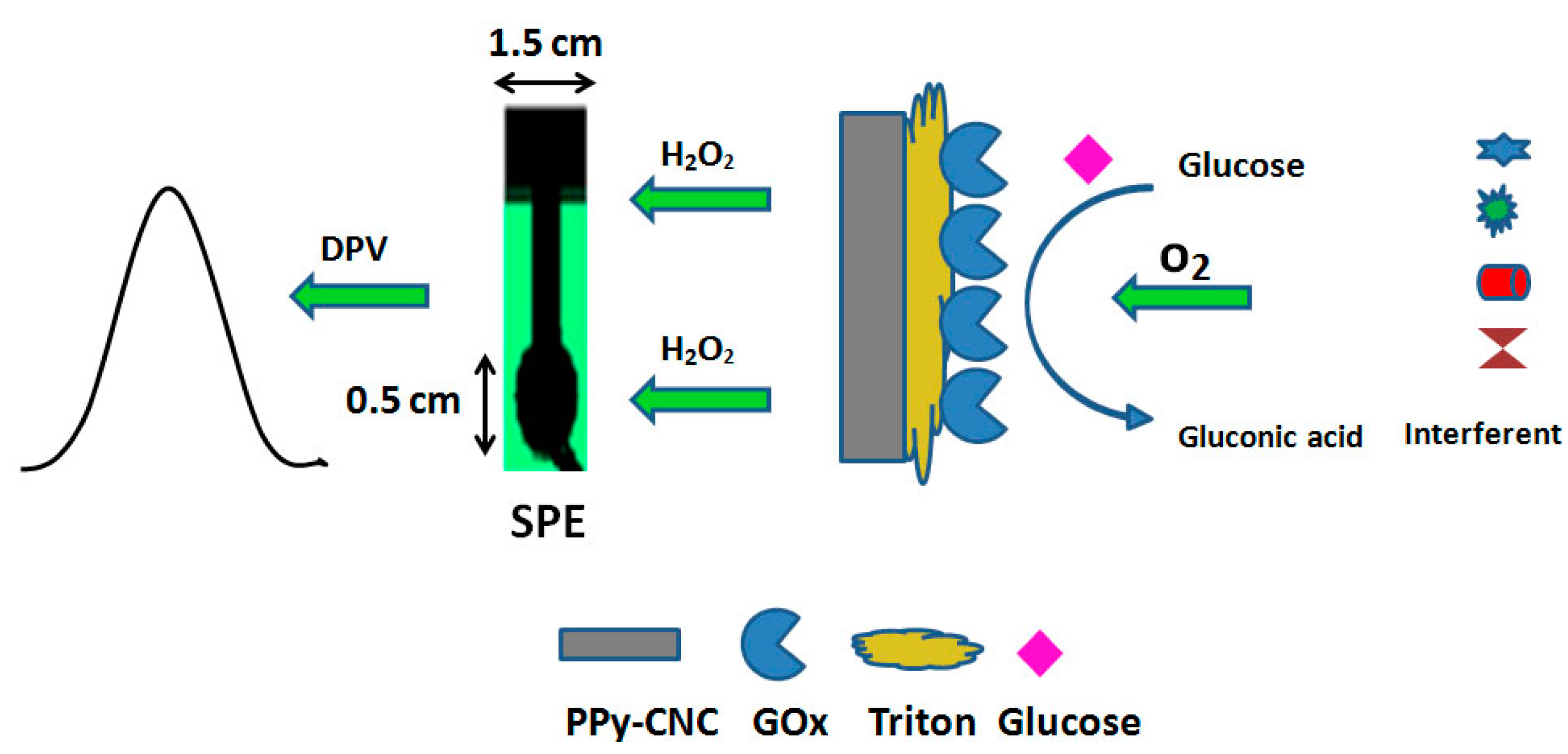
3. Results and Discussion
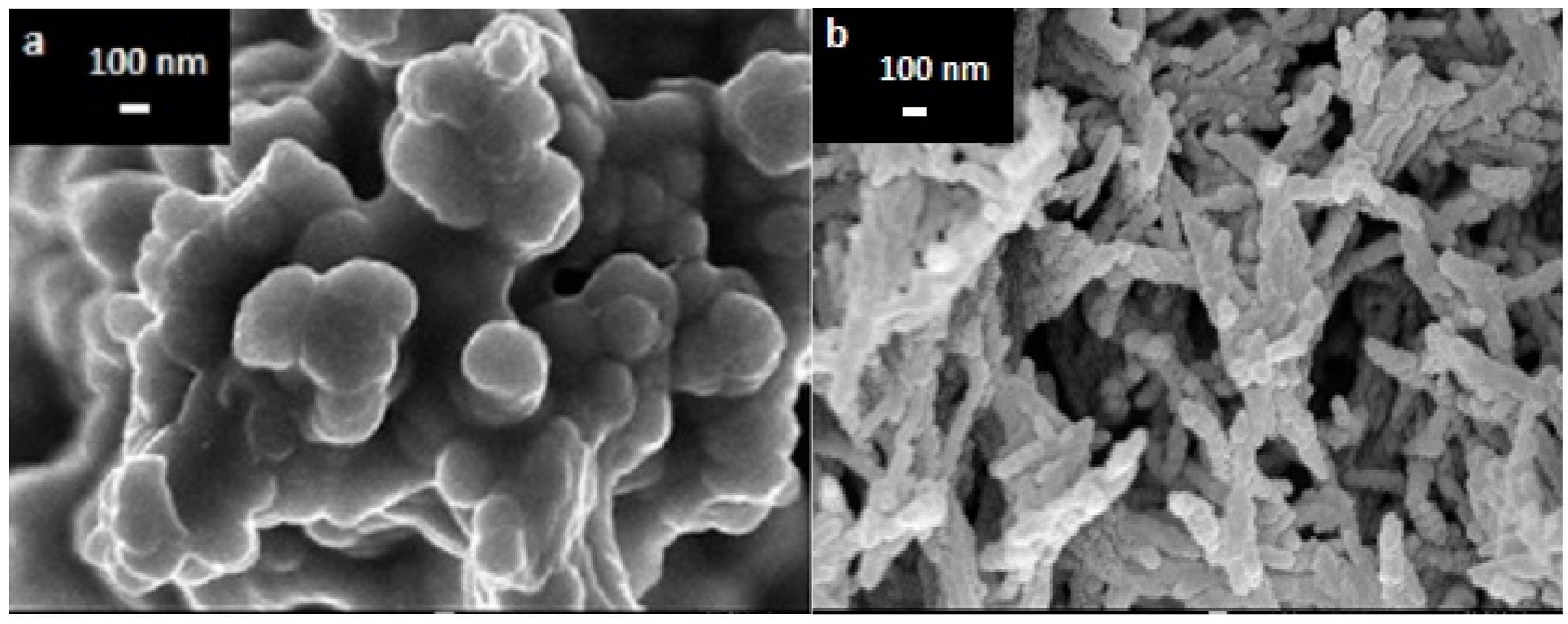
3.1. The Morphology of PPy and PPy/CNC Nanocomposites
3.2. Electrochemical Characterization
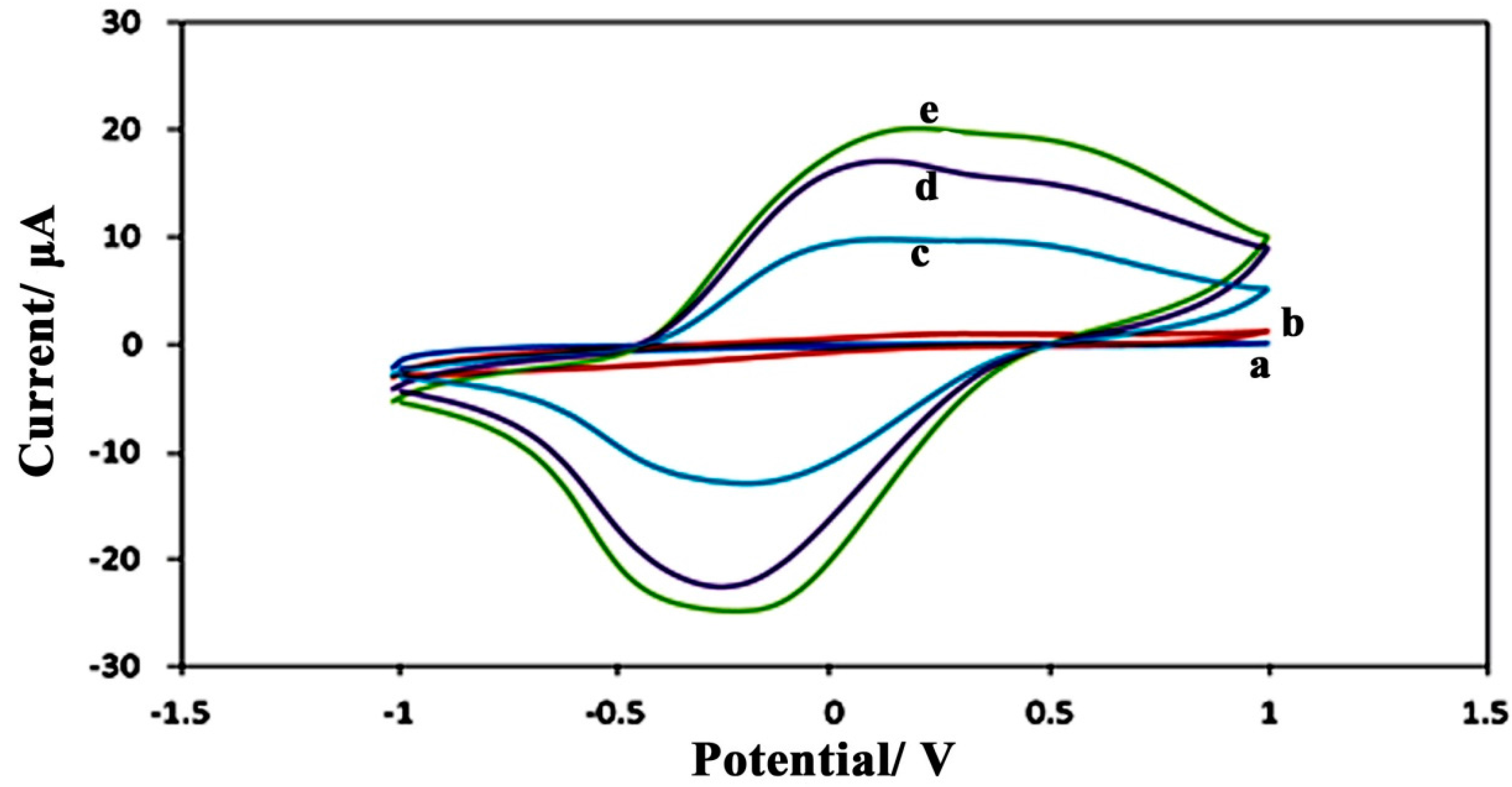
3.2.1. Voltammetric Studies
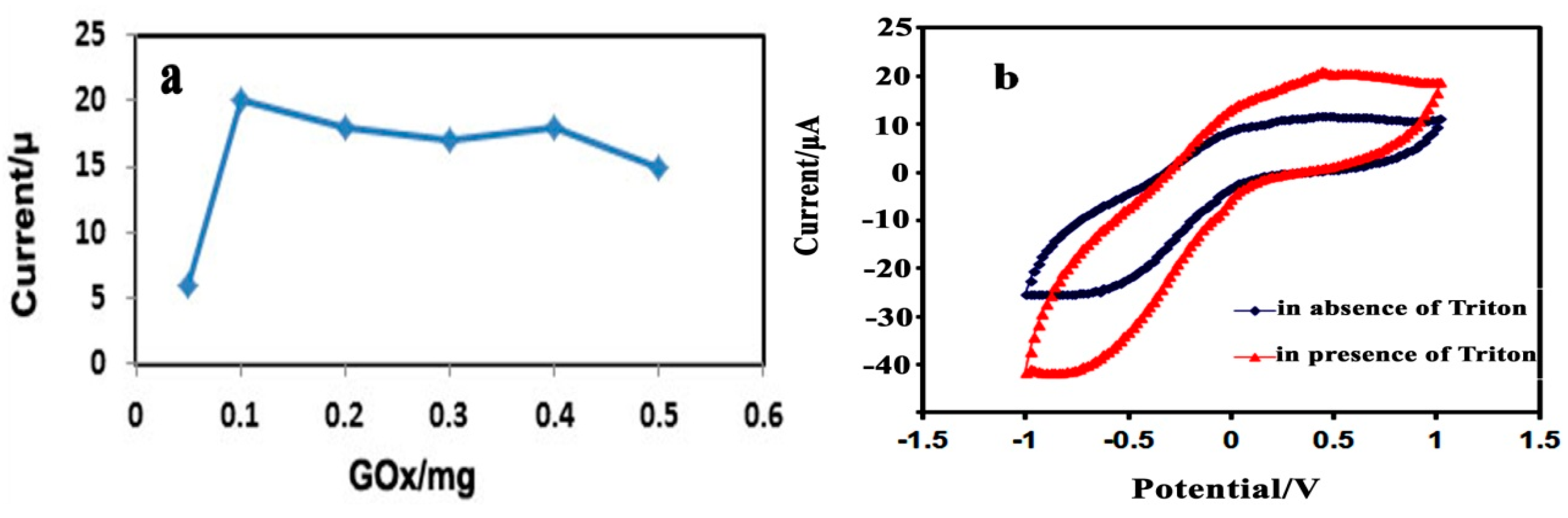
3.2.2. Effect of the Immobilized GOx, Triton X-100, and PPy-CNC Concentration
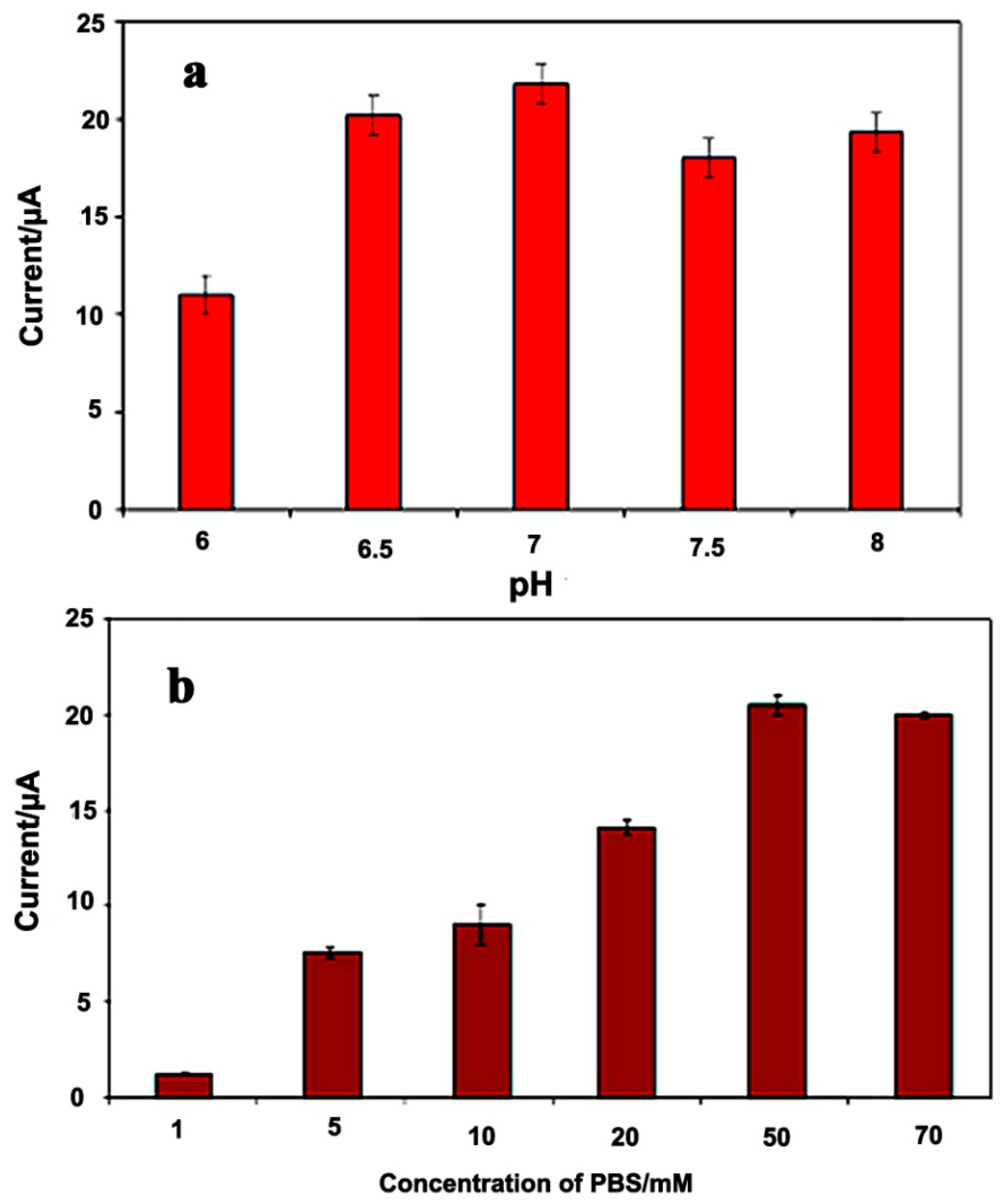
3.2.3. Effect of pH and Buffer Capacity
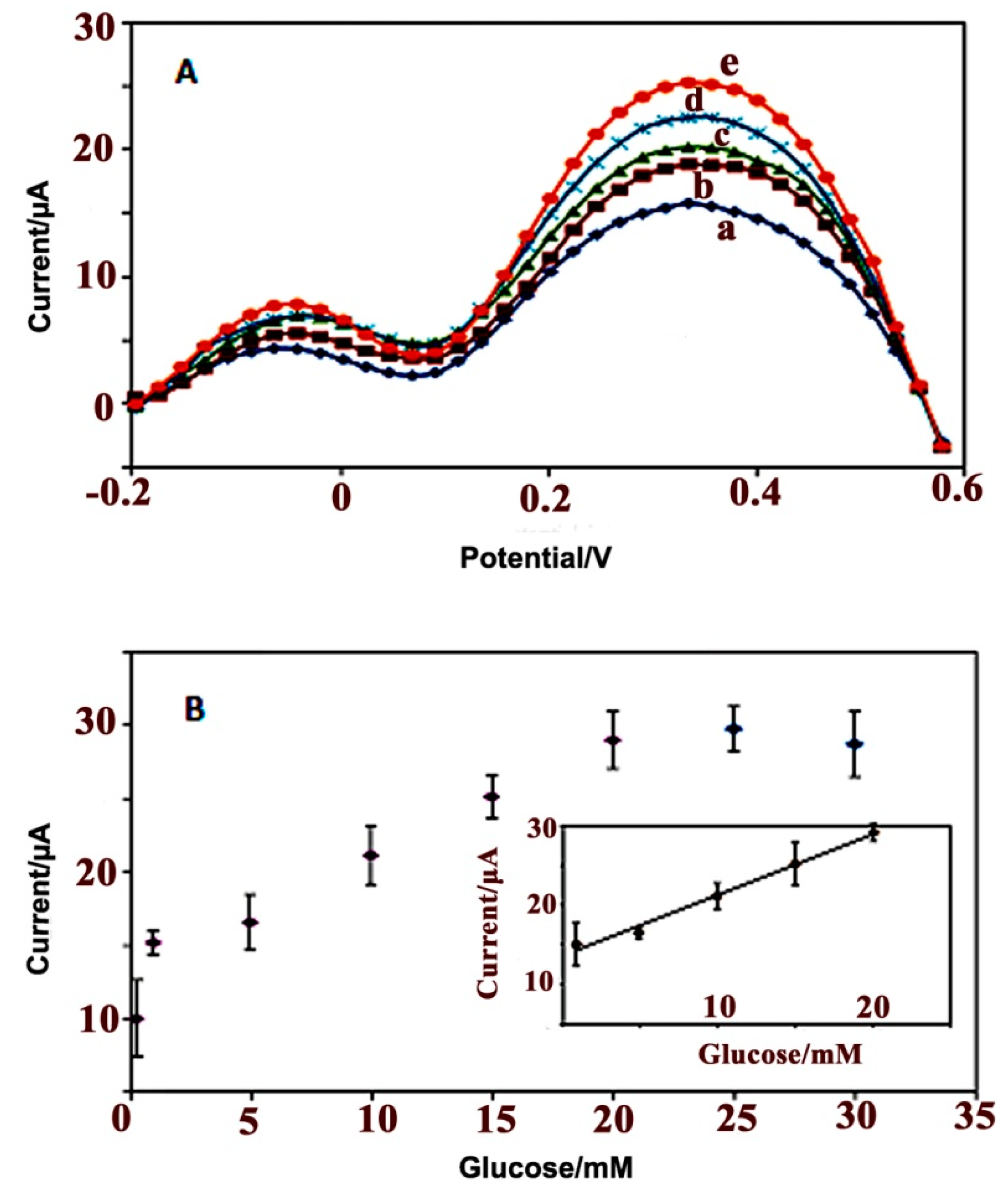
3.2.4. Electrochemical Response Studies of the Glucose Biosensor
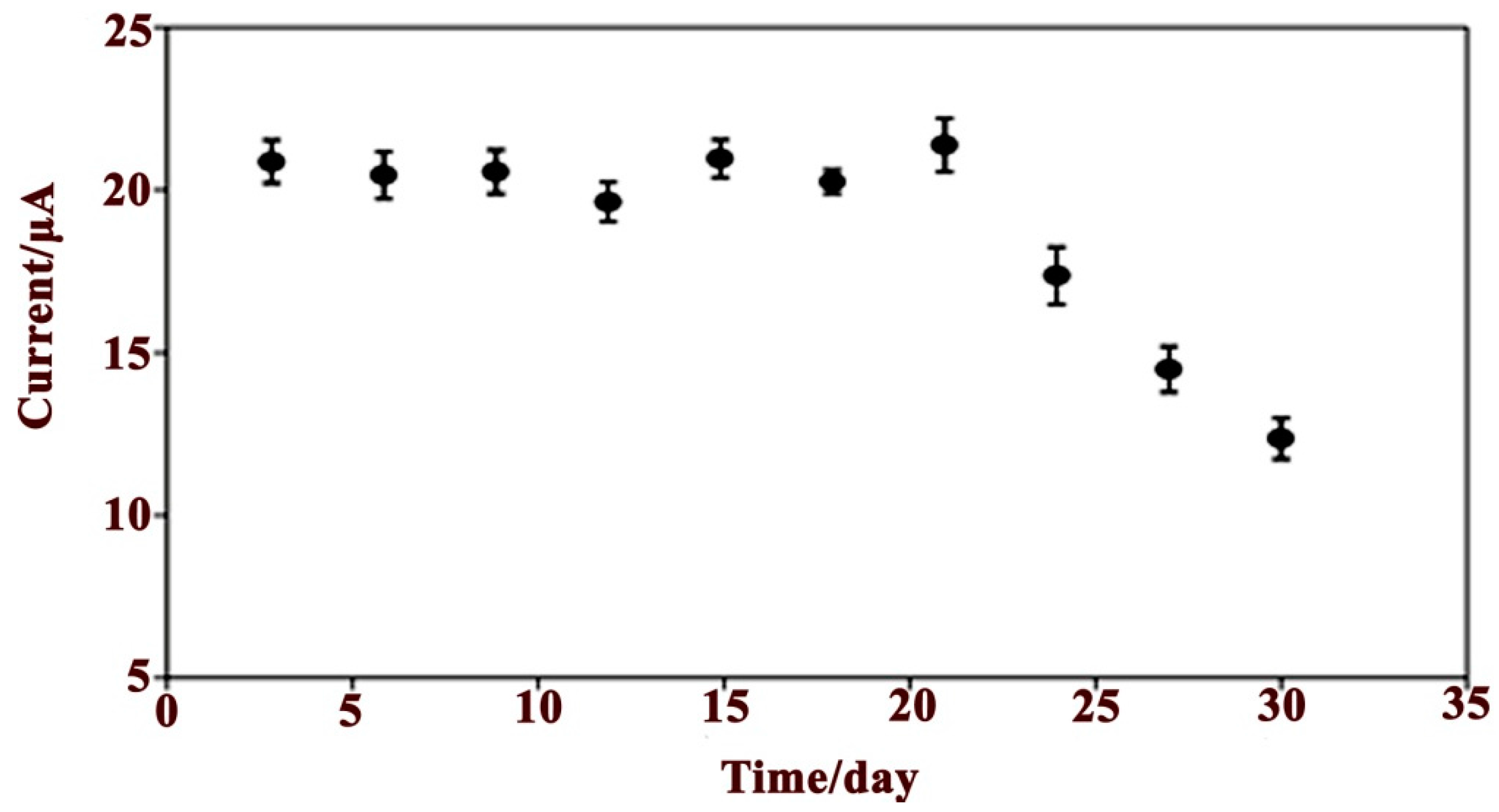
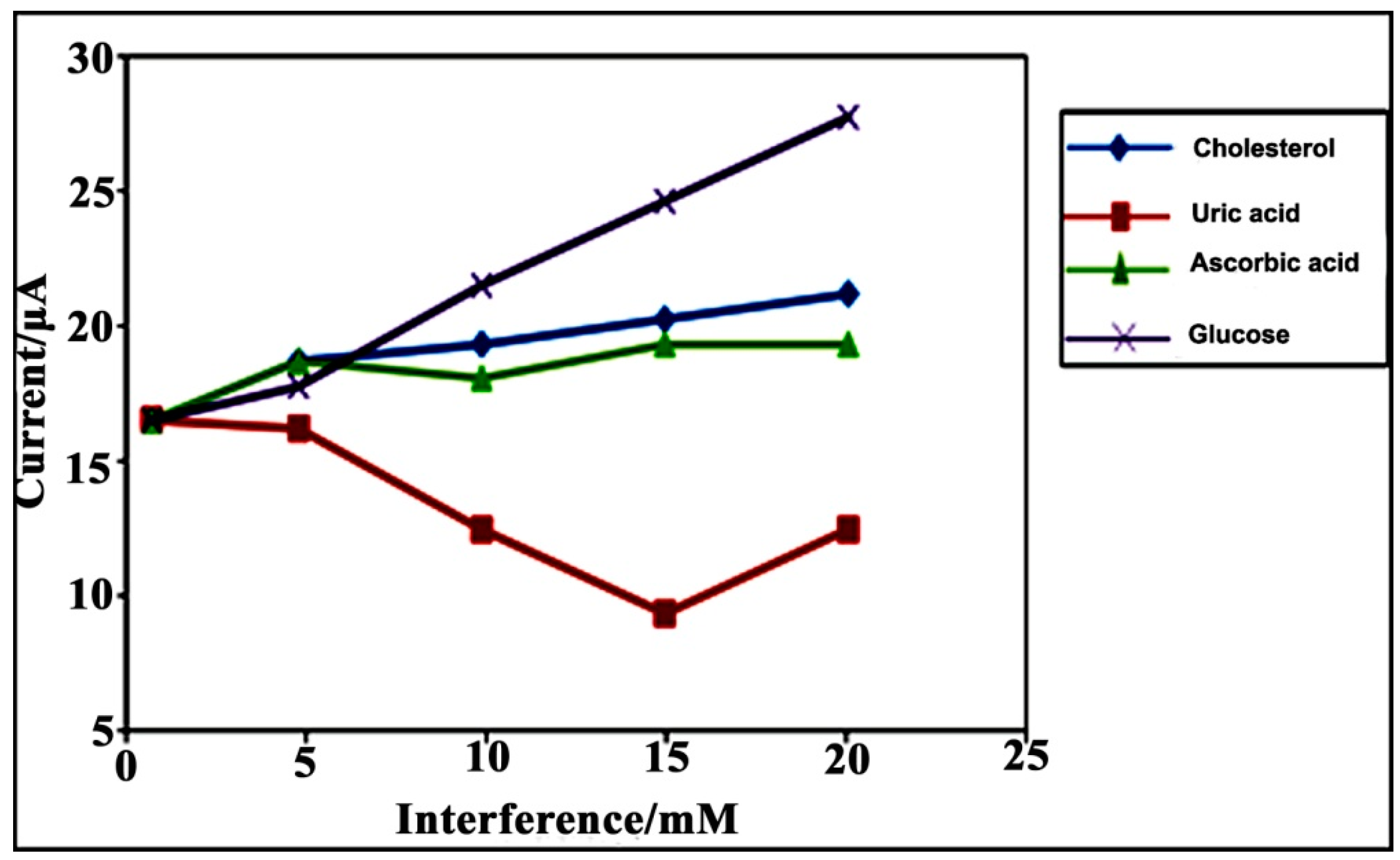
3.2.5. Reproducibility, Stability and Interference Studies
| Electrode | Detection Methods | Linear Range (mM) | LOD (mM) | Long-Term Stability (Days) | References |
|---|---|---|---|---|---|
| Py/CNC | DPV | 1–20 | 0.05 | 21 | Present work |
| Graphene/CdS nanocrystals | CV and impedance spectroscopy | 2–16 | 0.7 | - | [54] |
| Hydrophilic cellulose paper | Amperometric | 1–5 | 0.18 | 110 | [55] |
| Graphene/AuNPs/Chitosan nanocomposites | CV | 2–14 | 0.18 | 15 | [41] |
| silica sol–gel | Fluorescence | 0.1–5 | 0.06 | 30 | [56] |
| ZnO nanorods | Amperometric | 0.01–3.45 | 0.01 | 7 | [57] |
| Colloidal gold modified carbon paste electrode | CV | 0.04–0.28 | 0.01 | - | [58] |
| PPy/CNT | Amperometric | 0–50 | 0.2 | 75 min | [59] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdi, M.M.; Abdullah, L.C.; Tahir, P.M.; Zaini, L.H. Handbook of Green Materials: Processing Technologies, Properties and Applications; World Scientific Publishing: Singapore, 2014. [Google Scholar]
- Oksman, K.; Mathew, A.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Thielemans, W.; Warbey, C.R.; Walsh, D.A. Permselective nanostructured membranes based on cellulose nanowhiskers. Green Chem. 2009, 11, 531–537. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Coll. Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Butun, S.; Ince, F.G.; Erdugan, H.; Sahiner, N. One-step fabrication of biocompatible carboxymethyl cellulose polymeric particles for drug delivery systems. Carbohydr. polym. 2011, 86, 636–643. [Google Scholar] [CrossRef]
- Ren, X.; Chen, D.; Meng, X.; Tang, F.; Du, A.; Zhang, L. Amperometric glucose biosensor based on a gold nanorods/cellulose acetate composite film as immobilization matrix. Coll. Surf. B Biointerfaces 2009, 72, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, S.K.; Walus, K.; Stoeber, B. Paper as a Platform for Sensing Applications and Other Devices: A Review. ACS Appl. Mater. Interfaces 2015, 7, 8345–8362. [Google Scholar] [PubMed]
- Chen, S.; Seidel, A. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Dubitsky, Y.A.; Zhubanov, B.A. Polypyrrole-poly (vinyl chloride) and polypyrrole-cellulose acetate conducting composite films by opposite-diffusion polymerization. Synth. Metals. 1993, 53, 303–307. [Google Scholar] [CrossRef]
- Xian, Y.; Hu, Y.; Liu, F.; Xian, Y.; Wang, H.; Jin, L. Glucose biosensor based on Au nanoparticles—Conductive polyaniline nanocomposite. Biosens. Bioelectron. 2006, 21, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Ramanavičius, A.; Kaušaitė, A.; Ramanavičienė, A. Polypyrrole-coated glucose oxidase nanoparticles for biosensor design. Sens. Actuators B Chem. 2005, 111, 532–539. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A.; Acaite, J.; Malinauskas, A. Redox enzyme—Glucose oxidase—Initiated synthesis of polypyrrole. Synth. Metals 2006, 156, 409–413. [Google Scholar] [CrossRef]
- Abdi, M.M.; Abdullah, L.C.; Sadrolhosseini, A.R.; Yunus, W.M.M.; Moksin, M.M.; Tahir, P.M. Surface plasmon resonance sensing detection of mercury and lead ions based on conducting polymer composite. PLoS ONE 2011, 6, 24578. [Google Scholar]
- Virji, S.; Kaner, R.B.; Weiller, B.H. Hydrogen sensors based on conductivity changes in polyaniline nanofibers. J. Phys. Chem. B 2006, 110, 22266–22270. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Jang, J. Controlled amine functionalization on conducting polypyrrole nanotubes as effective transducers for volatile acetic acid. Biomacromolecules 2007, 8, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, C.; Ghasemi, M.; Heng, L.Y.; Hassan, S.H.; Abdi, M.M.; Daud, W.R.W.; Ilbeygi, H.; Ismail, A.F. Synthesis and application of polypyrrole/carrageenan nano-bio composite as a cathode catalyst in microbial fuel cells. Carbohydr. Polym. 2014, 114, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoseini, S.H.; Entezami, A.A. Graft copolymer of polystyrene and polypyrrole and studies of its gas and vapor sensing properties. Iran. Polym. J. 2005, 14, 101–110. [Google Scholar]
- Ma, H.; Zhou, B.; Li, H.S.; Li, Y.Q.; Ou, S.Y. Green composite films composed of nanocrystalline cellulose and a cellulose matrix regenerated from functionalized ionic liquid solution. Carbohydr. Polym. 2011, 84, 383–389. [Google Scholar] [CrossRef]
- Razaq, A.; Mihranyan, A.; Welch, K.; Nyholm, L.; Strømme, M. Influence of the type of oxidant on anion exchange properties of fibrous Cladophora cellulose/polypyrrole composites. J. Phys. Chem. B 2008, 113, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, B.D. Fabrication and characterization of a surface plasmon resonance based fiber optic sensor using gel entrapment technique for the detection of low glucose concentration. Sens. Actuators B Chem. 2013, 177, 589–595. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Lin, Y.; Zhu, N.; Ma, Y.; Mao, L. Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew. Chem. 2010, 122, 4910–4914. [Google Scholar] [CrossRef]
- Moschou, E.A.; Sharma, B.V.; Deo, S.K.; Daunert, S. Fluorescence glucose detection: Advances toward the ideal in vivo biosensor. J. fluoresc. 2004, 14, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, E.; Bas, S.Z.; Gulce, H.; Yildiz, S. Glucose biosensor based on immobilization of glucose oxidase in electropolymerized poly (o-phenylenediamine) film on platinum nanoparticles-polyvinylferrocenium modified electrode. Electrochim. Acta 2014, 123, 93–102. [Google Scholar] [CrossRef]
- Ammam, M.; Easton, E.B. High-performance glucose sensor based on glucose oxidase encapsulated in new synthesized platinum nanoparticles supported on carbon Vulcan/Nafion composite deposited on glassy carbon. Sens. Actuators B Chem. 2011, 155, 340–346. [Google Scholar] [CrossRef]
- Esmaeili, C.; Heng, L.Y.; Ling, T.L. Nile Blue chromoionophore-doped kappa-carrageenan for a novel reflectometric urea biosensor. Sens. Actuators B Chem. 2015, 221, 969–977. [Google Scholar] [CrossRef]
- Lee, A.D.H. Conductive Nanocrystalline Cellulose Polymer Composite Film as a Novel Mediator in Biosensor Applications. Ph.D. Thesis, University of Toronto, Toronto, Ontario, Canada.
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis 2001, 13, 983. [Google Scholar] [CrossRef]
- Manesh, K.; Kim, H.T.; Santhosh, P.; Gopalan, A.; Lee, K.P. A novel glucose biosensor based on immobilization of glucose oxidase into multiwall carbon nanotubes–polyelectrolyte-loaded electrospun nanofibrous membrane. Biosens. Bioelectron. 2008, 23, 771–779. [Google Scholar] [CrossRef]
- Zhao, H.; Ju, H. Multilayer membranes for glucose biosensing via layer-by-layer assembly of multiwall carbon nanotubes and glucose oxidase. Anal. Biochem. 2006, 350, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, Y.; Li, J.; Zhang, Y.; Hu, X. Facile synthesis of tetragonal columnar-shaped TiO2 nanorods for the construction of sensitive electrochemical glucose biosensor. Biosens. Bioelectron. 2014, 54, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Kamyabi, M.A.; Hajari, N.; Turner, A.P.F.; Tiwari, A. A high-performance glucose biosensor using covalently immobilised glucose oxidase on a poly(2,6-diaminopyridine)/carbon nanotube electrode. Talanta 2013, 116, 801–808. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Pikul, J.H.; King, W.P.; Pak, J.J. Zinc oxide inverse opal enzymatic biosensor. Appl. Phys. Lett. 2013, 102, 253103. [Google Scholar] [CrossRef]
- Nenkova, R.; Ivanova, D.; Vladimirova, J.; Godjevargova, T. New amperometric glucose biosensor based on cross-linking of glucose oxidase on silica gel/multiwalled carbon nanotubes/polyacrylonitrile nanocomposite film. Sens. Actuators B Chem. 2010, 148, 59–65. [Google Scholar] [CrossRef]
- Xue, K.; Zhou, S.; Shi, H.; Feng, X.; Xin, H.; Song, W. A novel amperometric glucose biosensor based on ternary gold nanoparticles/polypyrrole/reduced graphene oxide nanocomposite. Sens. Actuators B Chem. 2014, 203, 412–416. [Google Scholar] [CrossRef]
- Sekar, N.C.; Shaegh, S.A.M.; Ng, S.H.; Ge, L.; Tan, S.N. A paper-based amperometric glucose biosensor developed with Prussian Blue-modified screen-printed electrodes. Sens. Actuators B Chem. 2014, 204, 414–420. [Google Scholar] [CrossRef]
- Rauf, S.; Ihsan, A.; Akhtar, K.; Ghauri, M.; Rahman, M.; Anwar, M.; Khalid, A. Glucose oxidase immobilization on a novel cellulose acetate—polymethylmethacrylate membrane. J. Biotechnol. 2006, 121, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Compton, R.G.; Hallaj, R. Glucose biosensor prepared by glucose oxidase encapsulated sol-gel and carbon-nanotube-modified basal plane pyrolytic graphite electrode. Anal. Biochem. 2004, 333, 49–56. [Google Scholar] [CrossRef]
- You, X.; Pak, J.J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuators B Chem. 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens. Bioelectron. 2010, 25, 1070–1074. [Google Scholar] [CrossRef]
- Cunningham, D.D.; Stenken, J.A. In vivo Glucose Sensing; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- He, C.; Liu, J.; Zhang, Q.; Wu, C. A novel stable amperometric glucose biosensor based on the adsorption of glucose oxidase on poly(methyl methacrylate)—Bovine serum albumin core—Shell nanoparticles. Sens. Actuators B Chem. 2012, 166, 802–808. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Matuszewski, W.; Podsiadła, M. Enzyme entrapped polypyrrole modified electrode for flow-injection determination of glucose. Biosens. Bioelectron. 1990, 5, 149–156. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tan, X.R.; Chen, S.H.; Hu, F.X.; Zhong, H.A.; Zhang, Y. The Construction of Glucose Biosensor Based on Platinum Nanoclusters—Multiwalled Carbon Nanotubes Nanocomposites. Appl. Biochem. Biotechnol. 2012, 166, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Rao, C.R.; Vijayan, M.; Trivedi, D. Biosensing and drug delivery by polypyrrole. Anal. Chim. Acta. 2006, 568, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, X.; Lu, J.; Sun, F.; Yi, F.; Wang, Y.; Tang, Y. A novel glucose biosensor fabricated with electroactive Nb0.95Ti0.95O4 nano-composite film. Int. J. Electrochem. Sci. 2012, 7, 9354–9365. [Google Scholar]
- Dzyadevich, S.V.; Arkhipova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Shul’ga, A.A. Glucose conductometric biosensor with potassium hexacyanoferrate (III) as an oxidizing agent. Anal. Chim. Acta 1998, 374, 11–18. [Google Scholar] [CrossRef]
- Soldatkin, A.; El’Skaya, A.; Shul’ga, A.; Jdanova, A.; Dzyadevich, S.; Jaffrezic-Renault, N.; Martelet, C.; Clechet, P. Glucose sensitive conductometric biosensor with additional Nafion membrane: Reduction of influence of buffer capacity on the sensor response and extension of its dynamic range. Anal. Chim. Acta 1994, 288, 197–203. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Palanisamy, S.; Chen, S.M. A simple electrochemical approach to fabricate a glucose biosensor based on graphene—glucose oxidase biocomposite. Biosens. Bioelectron. 2013, 39, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Brânzoi, V.; Pilan, L.; Branzoi, F. Amperometric glucose biosensor based on electropolymerized carbon nanotube/polypyrrole composite film. Rev. Roum. Chim. 2009, 54, 783–789. [Google Scholar]
- Yang, P.; Wang, L.; Wu, Q.; Chen, Z.; Lin, X. A method for determination of glucose by an amperometric bienzyme biosensor based on silver nanocubes modified Au electrode. Sens. Actuators B Chem. 2014, 194, 71–78. [Google Scholar] [CrossRef]
- Saeedfar, K.; Heng, L.Y.; Ling, T.L.; Rezayi, M. Potentiometric Urea Biosensor Based on an Immobilised Fullerene-Urease Bio-Conjugate. Sensors 2013, 13, 16851–16866. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Q.; Guan, Q.M.; Wu, J.; Li, H.N.; Yan, J.J. Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosens. Bioelectron. 2011, 26, 2252–2257. [Google Scholar] [CrossRef] [PubMed]
- Kuek Lawrence, C.S.; Tan, S.N.; Floresca, C.Z. A “green” cellulose paper based glucose amperometric biosensor. Sens. Actuators B Chem. 2014, 193, 536–541. [Google Scholar] [CrossRef]
- Chang, G.; Tatsu, Y.; Goto, T.; Imaishi, H.; Morigaki, K. Glucose concentration determination based on silica sol—Gel encapsulated glucose oxidase optical biosensor arrays. Talanta 2010, 83, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Sun, X.W.; Wang, J.; Lei, Y.; Cai, X.; Li, C.M.; Dong, Z.; Huang, W. Enzymatic glucose biosensor based on ZnO nanorod array grown by hydrothermal decomposition. Appl. Phys. Lett. 2006, 89, 123902. [Google Scholar] [CrossRef]
- Liu, S.; Ju, H. Reagentless glucose biosensor based on direct electron transfer of glucose oxidase immobilized on colloidal gold modified carbon paste electrode. Biosens. Bioelectron. 2003, 19, 177–183. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon-nanotubes doped polypyrrole glucose biosensor. Anal. Chim. Acta 2005, 539, 209–213. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaeili, C.; Abdi, M.M.; Mathew, A.P.; Jonoobi, M.; Oksman, K.; Rezayi, M. Synergy Effect of Nanocrystalline Cellulose for the Biosensing Detection of Glucose. Sensors 2015, 15, 24681-24697. https://doi.org/10.3390/s151024681
Esmaeili C, Abdi MM, Mathew AP, Jonoobi M, Oksman K, Rezayi M. Synergy Effect of Nanocrystalline Cellulose for the Biosensing Detection of Glucose. Sensors. 2015; 15(10):24681-24697. https://doi.org/10.3390/s151024681
Chicago/Turabian StyleEsmaeili, Chakavak, Mahnaz M. Abdi, Aji P. Mathew, Mehdi Jonoobi, Kristiina Oksman, and Majid Rezayi. 2015. "Synergy Effect of Nanocrystalline Cellulose for the Biosensing Detection of Glucose" Sensors 15, no. 10: 24681-24697. https://doi.org/10.3390/s151024681







