Theoretical Calculation of the Gas-Sensing Properties of Pt-Decorated Carbon Nanotubes
Abstract
: The gas-sensing properties of Pt-decorated carbon nanotubes (CNTs), which provide a foundation for the fabrication of sensors, have been evaluated. In this study, we calculated the gas adsorption of Pt-decorated (8,0) single-wall CNTs (Pt-SWCNTs) with SO2, H2S, and CO using GGA/PW91 method based on density functional theory. The adsorption energies and the changes in geometric and electronic structures after absorption were comprehensively analyzed to estimate the responses of Pt-SWCNTs. Results indicated that Pt-SWCNTs can respond to the three gases. The electrical characteristics of Pt-SWCNTs show different changes after adsorption. Pt-SWCNTs donate electrons and increase the number of hole carriers after adsorbing SO2, thereby enhancing its conductivity. When H2S is adsorbed on CNTs, electrons are transferred from H2S to Pt-SWCNTs, converting Pt-SWCNTs from p-type to n-type sensors with improved conductivity. However, Pt-SWCNTs obtain electrons and show decreased conductivity when reacted with CO gas.1. Introduction
Carbon nanotubes (CNTs) have structures with abundant pores, large surface-to-volume ratios, and strong adsorption and desorption capabilities for gases. Gas molecules that adsorb on the surface of CNTs change the shape of CNTs and trigger redistribution of electrons, leading to a macroscopic change in resistance [1]. Kong et al. [2] used chemical vapor deposition (CVD) to fabricate single-wall CNTs (SWCNTs) on SiO2/Si substrates to detect NO2 (2 ppm to 200 ppm) and NH3 (0.1% to 1%) diluted in air or Ar. Results showed that the conductivity of SWCNTs decreases threefold after adsorbing NH3, whereas the conductivity increases threefold after adsorbing NO2. Unlike traditional gas sensors, CNT gas sensors exhibit faster response, higher sensitivity, smaller size, and lower working temperatures [3,4]. These advantages make CNTs suitable for application in industries, the medical field, and environmental protection. Recently, the CNT gas sensor has received extensive research attention and achieved certain interesting results. It is significant to study the gas sensitive properties of CNTs, which is the foundation of sensor design. In this paper, the gas sensitive property of CNTs is studied mainly through theoretical calculation analysis. Intrinsic CNTs can only detect several strong oxidizing and reducing gases, while other gases are only weakly adsorbed with low sensitivity because of the structure and chemical properties of intrinsic CNTs [5–7]. To overcome this limitation, some researchers have proposed various physical and chemical modifications, such as introduction of new active sites on the surface of CNTs. The authors in [8] showed that polar groups (COOH, NH2, NO2 and H2PO3) are promising candidates for enhancing CO2 and CH4 adsorption capacity by strengthening adsorption and activating exposed edges and terraces to introduce additional binding sites. Peng et al. [9] found B-doped CNT gas sensors have a good sensitivity to CO and H2O. Besides, transition metals are rich in d-electrons and empty orbitals, wherein small gas molecules can bond strongly to the metal when adsorbed on the surface [10,11]. Studies [12] indicated that compared with intrinsic CNTs, CNTs with metal depositions have better sensitivity. For instance, Pt- and Au-functionalized CNTs are more sensitive by an order of magnitude for NO2 and NH3 detection than intrinsic CNTs. The response characteristics of sensors largely depend on the number of active sites, which can strengthen the response of sensors [11]. Pt can adsorb small molecules [13–16], and Pt has a good catalytic activity. CNTs have excellent physical and chemical properties and unique structures that can be used as a supporting material that influences the activity of Pt catalysts [17]. Conversely, the catalytic activity of Pt can improve the gas sensing properties of CNTs. Therefore the present study introduces Pt as a new active site. The adsorptive processes and properties of Pt-SWCNTs for SO2, H2S, and CO are calculated. These gases are highly toxic to the human body, making this research significant by providing theoretical support and a good foundation for the fabrication of suitable CNT-based sensors.
2. Computation Model and Methods
We built (8,0) SWCNTs and gas molecule models using Materials Studio (Accelrys, San Diego, CA, USA), a molecular dynamics simulation software. The geometries and properties of the system were derived using the quantum mechanics program DMol3 code (Accelrys). We adopted GGA to treat the electronic exchange and correlation effects, as described by PW91 [18]. Pt is a heavy metal with an atomic number of 78; therefore, DFT semi-core pseudopotentials [19] were used to manage the interactions between the nucleus and the valence electron. To ensure accuracy, the energy threshold and self-consistent field convergence criteria were set to 2.72 × 10−4 and 2.72 × 10−5 eV, respectively. The space orbital cutoff radius was set to 0.40 nm, whereas the Brillouin zone k-point sampling was performed in a 1 × 1 × 2 [20,21] Monkhorst–Pack mesh. A 2.50 nm × 2.50 nm × 0.85 nm periodic boundary was adopted to avoid the interaction between adjacent cells.
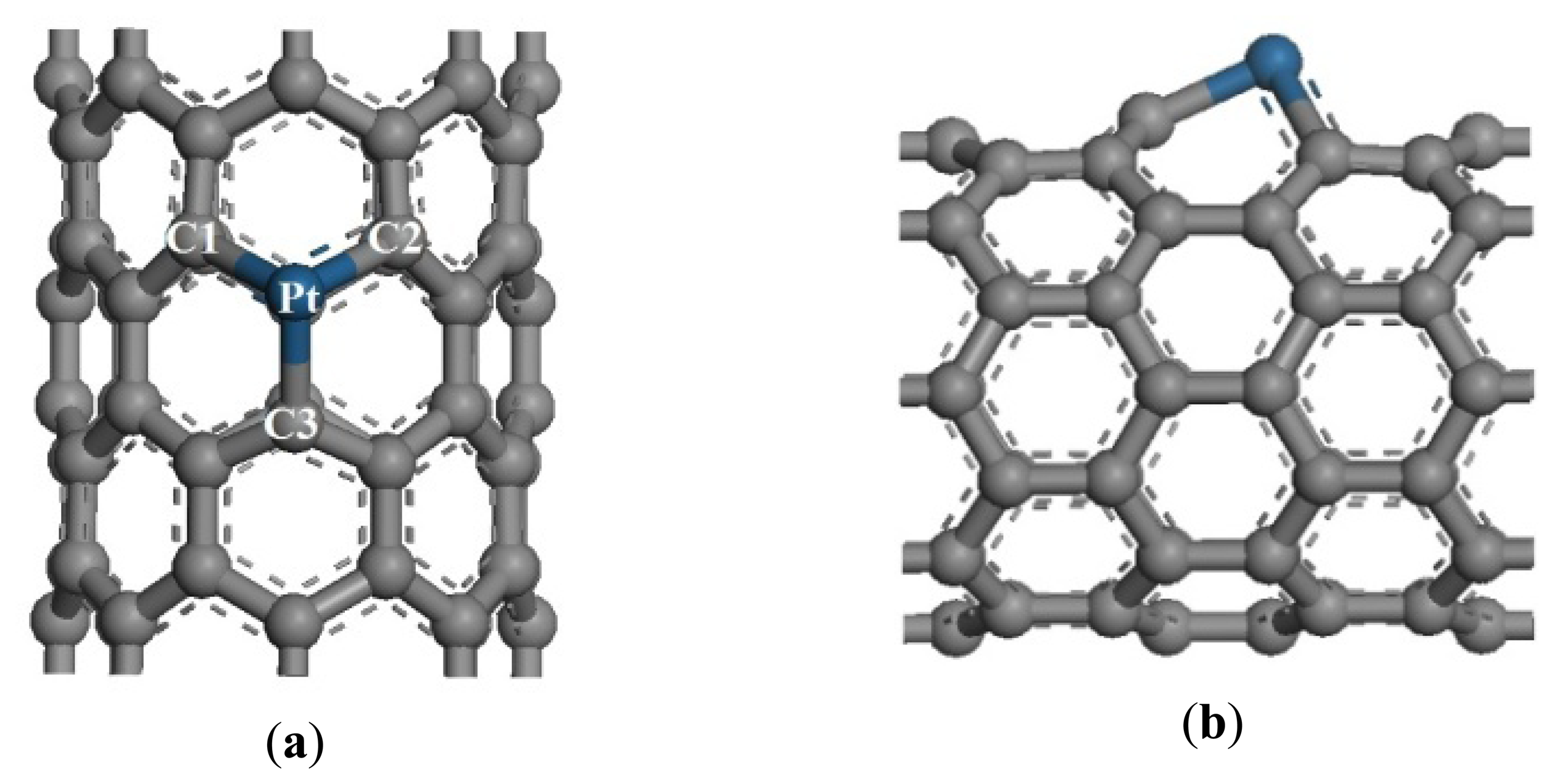
Two SWCNT unit cells were selected as intrinsic CNTs to build Pt-SWCNTs. According to references [11,22], Pt can easily adsorb on the vacancy defects of SWCNTs, and its adsorption energy is 6.400 eV, which is greater than that of Pt adsorbed on perfect crystal surface (2.750 eV). Accordingly, the present research selected this Pt-SWCNT model with geometry optimization structure, as shown in Figure 1.
3. Results and Discussion
The radius of a Pt atom is 0.183 nm, which is greater than that of a C atom (0.070 nm). Thus, Pt is highlighted on the CNT surface. The bond lengths between the Pt atom and three adjacent C atoms changed from 0.142 nm to 0.199, 0.199, and 0.189 nm for Pt-C1, Pt-C2, and Pt-C3, respectively. These results are consistent with the reference [22].
The quantum chemical energies of Pt-SWCNTs and gas molecules, as well as the optimized structure of the adsorption systems (EPt-SWCNTs, Egas, and Egas-Pt-SWCNTs) were calculated. The adsorption energy (Eb) between a gas molecule and CNTs can be calculated by the following formula:
At Eb < 0, the energy of the absorption system is less than the total energy of gas molecules and Pt-SWCNTs. Therefore, the reaction is exothermic and spontaneous. Greater adsorption energy releases more energy during the reaction process. However, when Eb > 0 it is relatively difficult for the reaction to continue because of the energy required.
In actual practice, the gas-sensitive response of the sensor is evaluated by the changes in electrical characteristics (e.g., resistance) of sensors. Therefore, we also calculated and analyzed the electronic structure of Pt-SWCNTs, gas molecules, and adsorption system. EHOMO and ELUMO represent the highest occupied molecular orbital (HOMO) energy and the lowest unoccupied orbital (LUMO) energy, respectively. Eg is the difference of ELUMO and EHOMO, and Q is the net charge of the system. The parameters are defined as follows:
3.1. SO2
SO2 is colorless, corrosive, and has a strong pungent odor. Moreover, when SO2 is dissolved in bodies of water, sulfurous acid rain is generated, which is harmful to the environment. SO2 can also form sulfuric acid when dissolved in water, which can irritate the mucous membrane of the eyes and nose.
The full geometric optimization of the Pt-SWCNTs and SO2 adsorption model is shown in Figure 2. An oxygen atom O1 points to Pt, with Pt-O1 and Pt-S distances of 0.212 and 0.245 nm, respectively. The reaction adsorption energy is –1.225 eV (Table 1), which denotes an exothermic and spontaneous reaction. By contrast, the reaction adsorption energy of intrinsic SWCNTs is –0.830 eV, so Pt-doping enhances the interaction between SO2 and SWCNTs. Pt is not only a sensing element of Pt-SWCNTs, but also an active site. Strong interaction with gas molecules adsorbed on the surface results in deformation of Pt-SWCNTs and elongation of the Pt-C bond.
The frontier orbital energy difference of SO2 and Pt-SWCNTs is EH-L ≪ EL-H. A Pt-SWCNT electron only needs to overcome a 0.158 eV energy barrier to transfer to SO2, whereas a SO2 electron needs to overcome a 3.818 eV energy barrier to transfer to Pt-SWCNTs. Therefore, Pt-SWCNTs provide electrons to SO2 in the adsorption process. A portion of electrons fill the anti-bonding orbital of S-O1, changing the bond length from 0.143 nm to 0.165 nm. O2 is far from the CNT surface, so the interaction is small, allowing only a small change in the bond length of S-O2 (0.150 nm).
According to the respective Mulliken charge populations, SWCNTs of Pt-SWCNTs have 0.147 positive charge and Pt has 0.147 negative charge before adsorption. After the adsorption process, SWCNTs have 0.509 positive charge, whereas Pt has 0.116 negative charge. SO2 obtains 0.393 electrons during the adsorption reaction with Pt-SWCNTs, which is 4.6 times than intrinsic SWCNTs (Table 2). Charge variation (ΔQSWCNTs, ΔQPt) of SWCNTs and Pt are 0.362 and 0.031, respectively (Table 3). Therefore, SO2 obtains electrons mainly from SWCNTs, whereas the Pt exhibits a small charge change.
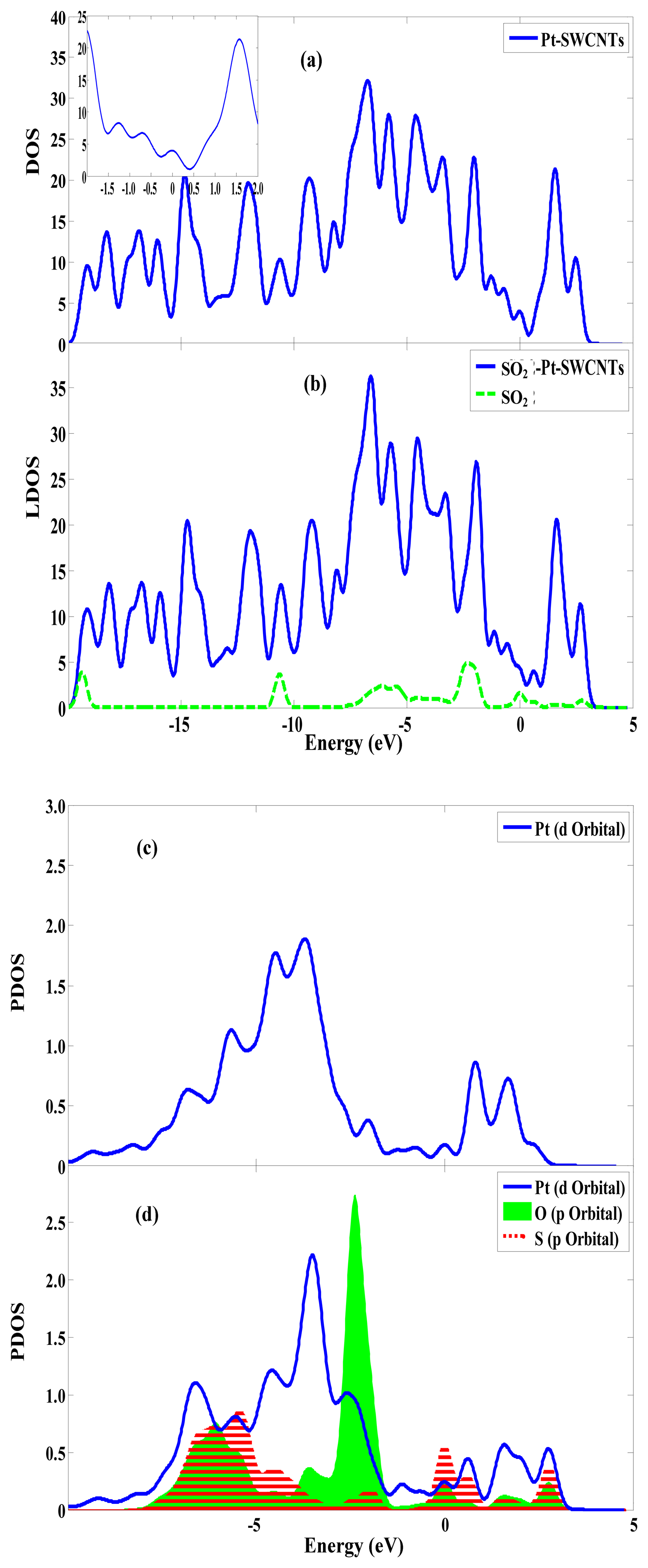
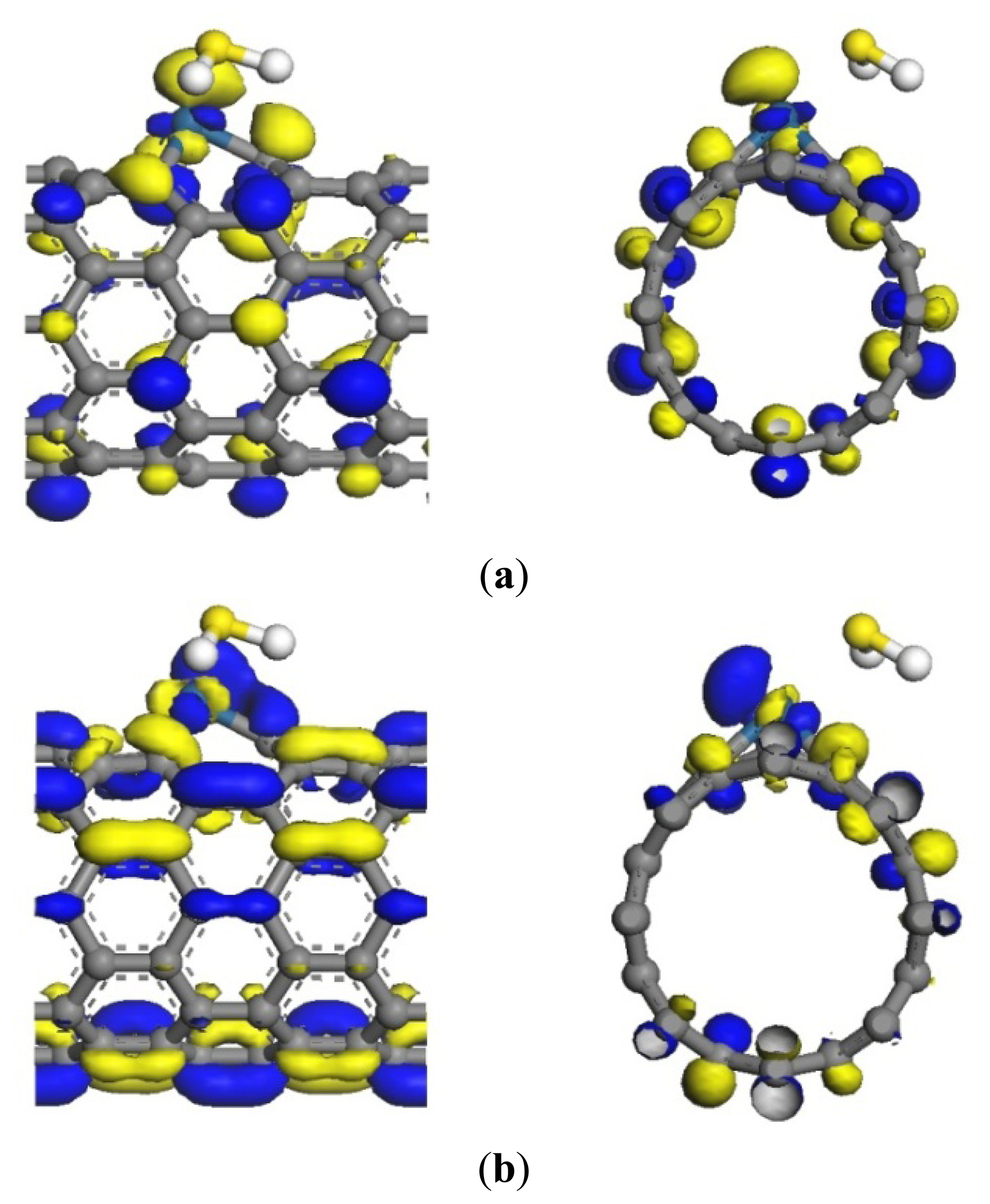
The transfer of a large number of electrons during adsorption causes the redistribution of system charges. The density of states (DOS) near the Fermi level appears to be impure, for example there is a peak in –0.5eV. And the DOS between HOMO and LUMO changes. Figure 3 shows that these impure states are caused by SO2 adsorption. The p orbitals of S and O atoms have a large overlap with the d orbitals of Pt atom, and it demonstrates that SO2 can strongly hybridize with Pt [23]. This has significant effect on the frontier orbital of the adsorption system, which changes the HOMO and LUMO orbital formation, causing the change in the DOS. Figure 4a shows that the p orbitals of C1 and C3 form σ bond with S. In Figure 4b, the d orbitals of Pt and the p orbitals of S are hybridized. The frontier orbital energy gap Eg of the system is 0.285 eV after adsorbing SO2, which is reduced by 0.047 eV compared with that in the non-adsorbed SO2. This is beneficial for the transfer of electrons between HOMO and LUMO, thereby enhancing conductivity.
SO2 adsorption on the surface of Pt-SWCNTs has large adsorption energy and can form a stable structure. The p-type Pt-SWCNTs [11] donate electrons and increase the number of hole carriers, reducing the frontier orbital energy, diminishing energy gap Eg, and enhancing conductivity. Pt-SWCNTs are highly responsive to SO2, the doped Pt effectively improved the adsorption sensitivity of SWCNTs to SO2.
3.2. H2S
H2S which is the simplest hydride of sulfur, is a colorless toxic gas that smells like rotten eggs and is strongly corrosive. It is also harmful to human health. The S in H2S is at the lowest valence state, so it is strongly reducible.
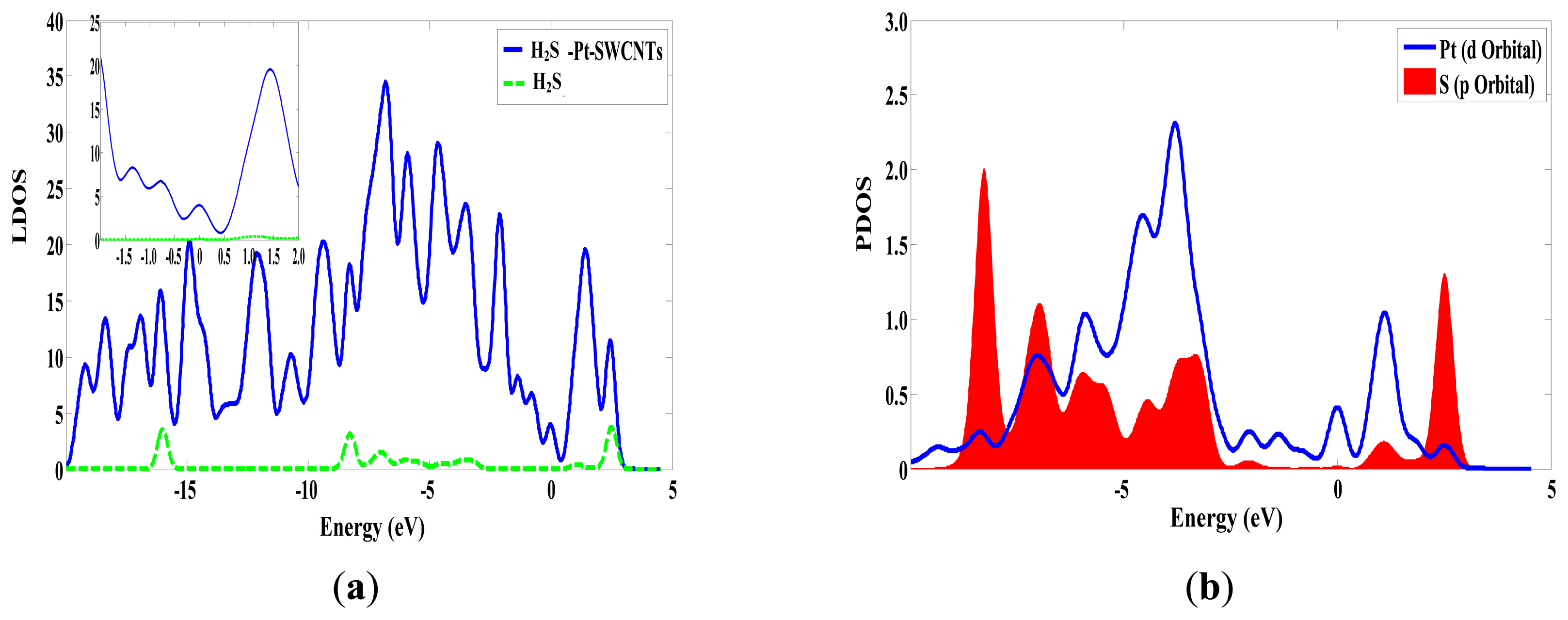
The adsorption reaction of Pt-SWCNTs and H2S is also exothermic, with Eb of –0.977 eV, more than intrinsic SWCNTs (–0.591eV in Table 2). The frontier orbital energy differences are EH-L = 4.438 eV and EL-H = 1.519 eV, therefore H2S provides electrons to Pt-SWCNTs in this reaction. Mulliken charge analysis (Table 3) shows that H2S donates 0.285 electrons almost 22 times more than intrinsic SWCNTs. Pt and SWCNTs have 0.019 and 0.266 electrons, respectively, after H2S is adsorbed on Pt-SWCNTs (Figure 5). A large number of electron transformations convert Pt-SWCNTs from p-type to n-type. Eg of the adsorption system is 0.283 eV, which is reduced by 0.049 eV compared that with non-adsorbed H2S, thus enhancing conductivity. H2S-Pt-SWCNT frontier orbitals concentrate on Pt-SWCNTs, and H2S is not involved in the composition of HOMO and LUMO orbitals. Figure 6 shows that the DOS of H2S is not distributed between the HOMO and LUMO, and the DOS near the Fermi level is basically the same as that of Pt-SWCNTs, which is consistent with the results of frontier orbitals (Figure 7). The p orbitals of S have a large overlap with the d orbitals of Pt, and the strong interaction of them enhances the adsorption between H2S and the nanotube surface.
From the comparison results of adsorption energy and transfer charge, it can be seen that doped Pt obviously improves the adsorption ability of the instrinsic SWCNTs to H2S. H2S adsorbs on the surface of Pt-SWCNTs and donates substantial electrons to Pt-SWCNTs, which converts CNTs from p-type to n-type. The frontier orbital energy is increased, while the conductivity is enhanced because of the decrease in the frontier orbital energy gap.
3.3. CO
CO is a colorless, non-irritating gas. However when it enters the human body, CO combines with blood hemoglobin, which prevents the union of hemoglobin and oxygen, leading to body tissue hypoxia and even suffocation. The C atom in CO has +2 valence electrons and that can be further oxidized to +4. Accordingly, CO is a reducing gas that provides electrons in reactions.
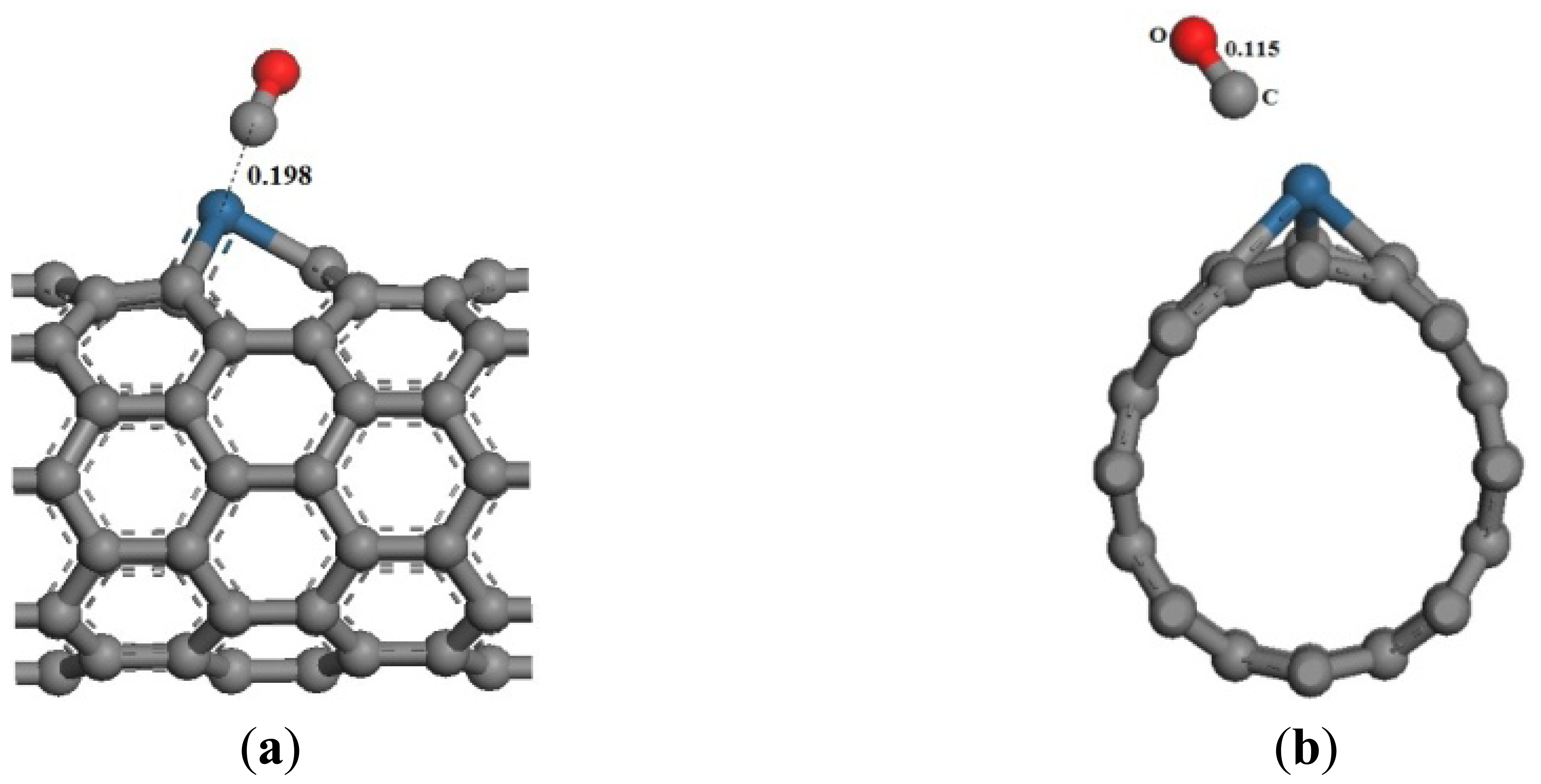
The optimized adsorption structure of CO adsorbed on Pt-SWCNTs is shown in Figure 8, which is consistent with reference [24], C atoms point to Pt, whereas O atoms point away from the CNT surface. The adsorption reaction is exothermic. High adsorption energy results in a tight bond between gases and CNTs, as well as an interaction distance of 0.198 nm.
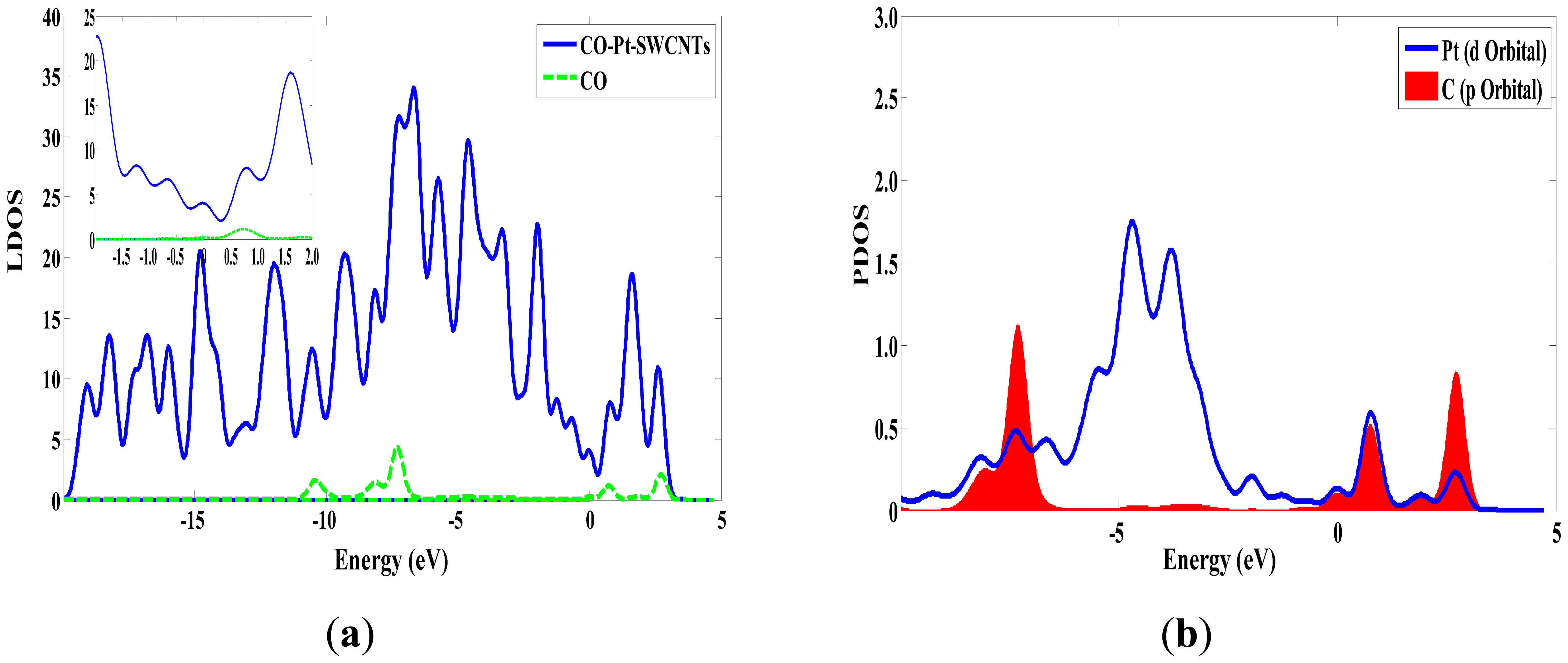
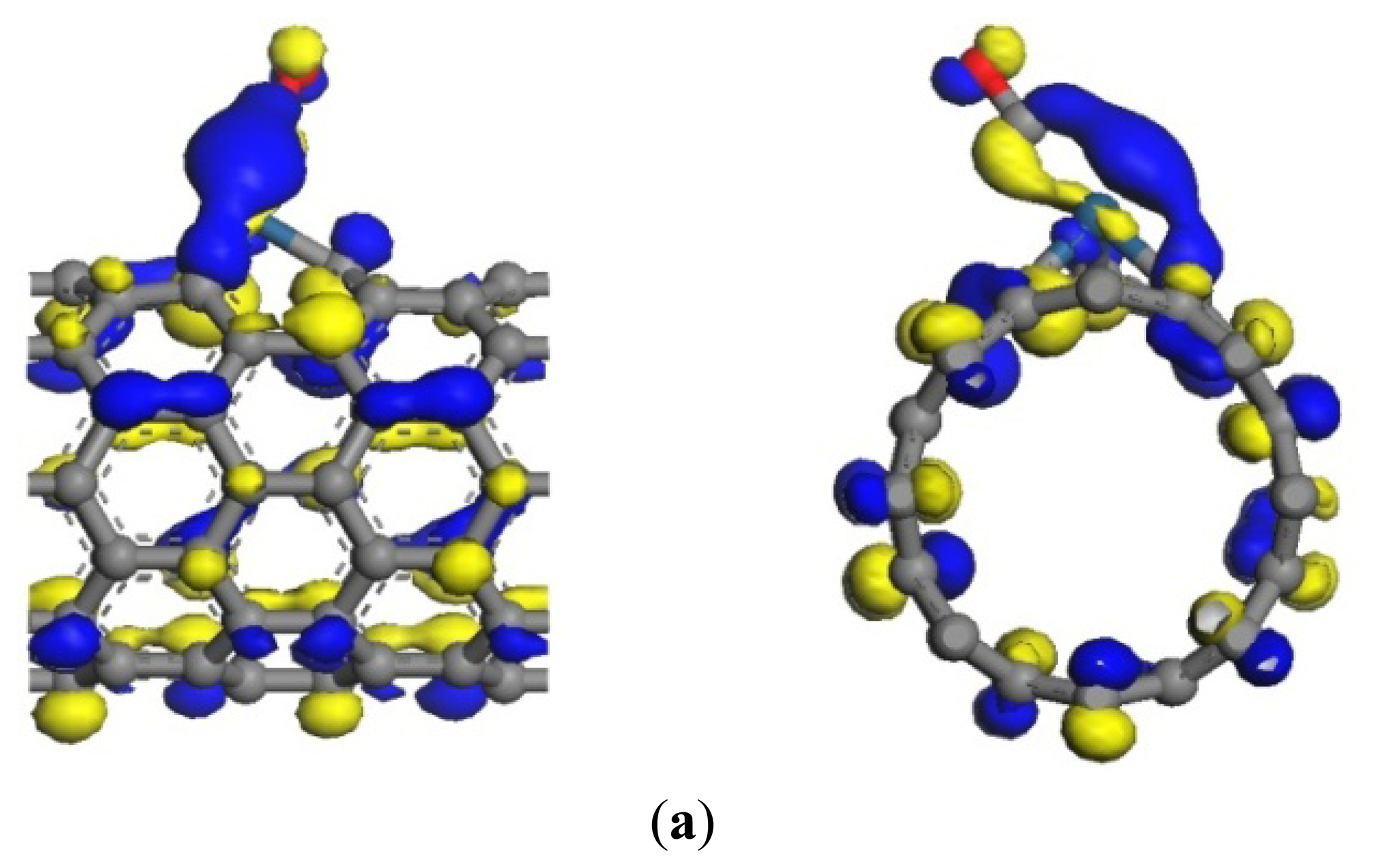
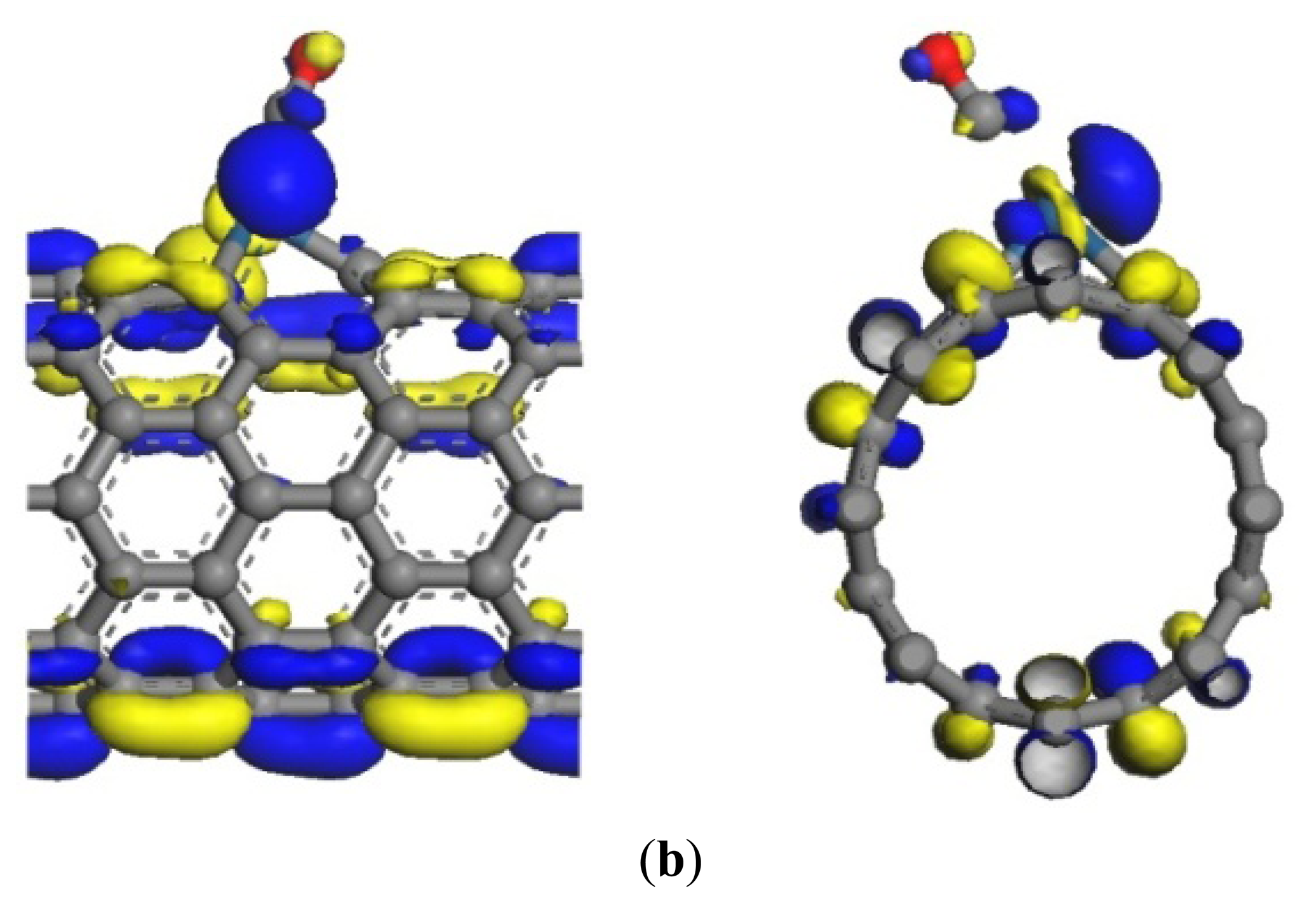
The same as the previous two gases, the adsorption energy and the transfer charge between Pt-SWCNTs and CO are increased obviously, enhancing the adsorption. Figure 9 shows that the p orbitals of C in CO have an overlap with the d orbitals of Pt, especially near the Fermi level. The DOS near the Fermi level is changed, and the peak at –7 eV is split, which is related to CO adsorption. The contributions of CO to HOMO and LUMO are mainly on the p orbitals of C and O atoms (Figure 10), which change the system configuration of frontier orbitals, changing the DOS of the system. During adsorption, CO provides 0.181 electrons (Table 3), p-type CNTs obtain electrons, and the number of hole carriers decreases. Frontier orbital energy and energy gap Eg increase, decreasing conductivity.
4. Discussion
Pt is a heavy metal with an atomic number of 78. Its outer core 5d orbitals have nine electrons and an unpaired d electron, so it easily absorbs electrons to reach a steady state. Pt doped into SWCNTs obtains electrons from C. In Pt-SWCNTs, Pt has 0.147 electrons, C1, C2, and C3 adjacent to Pt have 0.028, 0.029, and 0.097 electrons, respectively, which form an electron accumulation zone around the Pt atom. Given that Pt easily obtains electrons, when SO2 reacts with Pt-SWCNTs, SWCNTs donate most of the electrons, and a small charge change in Pt (ΔQPt) occurs. On the contrary, when the target gases are H2S and CO, Pt exhibits a large charge change.
5. Conclusions
In this study, the adsorptions of three gases on the surface of Pt-SWCNTs were calculated based on DFT. The gas-sensing properties of Pt-SWCNTs were assessed according to the changes in adsorption energy, geometric structure, and electronic structure during adsorption. The main conclusions are as follows:
The doped Pt effectively improves the adsorption sensitivity of intrinsic SWCNTs to the three kind of gases.
The adsorption energy of the reaction between Pt-SWCNTs and SO2 is large, and numerous electrons are transferred from CNTs to the target gases. The frontier orbital energies (EHOMO and ELUMO) and Eg are decreased, and the electrical conductivity of Pt-SWCNTs is enhanced. Pt-SWCNTs have high sensitivity to SO2.
H2S is reduced when reacted with Pt-SWCNTs. H2S provides a large number of electrons, converting CNTs from p-type to n-type. The frontier orbital energies are increased, whereas Eg is decreased, thereby enhancing conductivity.
When CO is adsorbed on Pt-SWCNTs, CO provides electrons to p-type CNTs, decreasing the number of hole carriers. The frontier orbital energies and Eg are increased, decreasing conductivity.
Results of the theoretical calculation show that Pt-SWCNTs can respond to the three gases. The electrical characteristics of Pt-SWCNTs show different degrees of changes after adsorption of the test gases. As SO2 is adsorbed on Pt-SWCNTs, the CNTs lose electrons, the number of hole carriers is increased, and conductivity is enhanced. As H2S is adsorbed on the surface of CNTs, Pt-SWCNTs receive a large number of electrons and transform from p-type into n-type. The conductivity of Pt-SWCNTs is also enhanced. Comparing their adsorption energies and charge transformations, the sensitivity to SO2 is higher than the sensitivity of Pt-SWCNTs to H2S. Moreover, CO is an electron-donor gas, which reduces hole carriers and weakens conductivity. Therefore, Pt-SWCNTs can be used to fabricate gas sensors in detecting SO2, H2S, and CO gases.
Acknowledgments
We gratefully acknowledge the financial support from Project No. 0213005202042, which is supported by the Fundamental Research Funds for the Central Universities.
Conflict of Interest
The authors declare no conflict of interest.
References
- Zhang, X.-X.; Liu, W.-T.; Tang, J.; Xiao, P. Study on PD detection in SF6 using multi-wall carbon nanotube films sensor. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 838–844. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Kyeongjae, C.; Hongjie, D. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar]
- Zhang, X.-X.; Bing, Y.; Wang, X.-J.; Luo, C.-C. Effect of plasma treatment on multi-walled carbon nanotubes for the detection of H2S and SO2. Sensors 2012, 12, 9375–9385. [Google Scholar]
- Zhang, X.-X.; Bing, Y.; Dai, Z.-Q.; Luo, C.-C. The gas response of hydroxyl modified SWCNTs and carboxyl modified SWCNTs to H2S and SO2. Przeglad Elektrotechniczny 2012, 88, 311–314. [Google Scholar]
- Zhao, Z.; Buldum, A.; Han, J.; Lu, J.P. Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar]
- Goldoni, A.; Larciprete, R.; Petaccia, L.; Lizzit, S. Single-wall carbon nanotube interaction with gases: Sample contaminants and environmental monitoring. J. Am. Chem. Soc. 2003, 124, 11329–11333. [Google Scholar]
- Kemp, K.C.; Seema, H.; Saleh, M.; Le, N.H.; Mahesh, K.; Chandra, V.; Kim, K.S. Environmental applications using graphene composities: Water remediation and gas adsorption. Nanoscale 2013, 5, 3149–3171. [Google Scholar]
- Peng, S.; Cho, K. Ab initio study of doped carbon nanotube sensors. Nano Lett. 2003, 3, 513–517. [Google Scholar]
- Kim, S.J.; Park, Y.J.; Ra, E.J.; Kim, K.K.; An, K.H.; Lee, Y.H.; Choi, J.Y.; Park, C.H.; Doo, S.K.; Park, M.H.; Yang, C.W. Defect-induced loading of Pt nanoparticles on carbon nanotubes. Appl. Phys. Lett. 2007, 90, 023114/1–023114/3. [Google Scholar]
- Pannopard, P.; Khongpracha, P.; Probst, M.; Limtrakul, J. Gas sensing properties of platinum derivatives of single-walled carbon nanotubes: A DFT analysis. J. Mol. Graph. Model. 2009, 28, 62–69. [Google Scholar]
- Penza, M.; Cassano, G.; Rossi, R.; Alvisi, M.; Rizzo, A.; Signore, M.A.; Dikonimos, Th.; Serra, E.; Giorgi, R. Enhancement of sensitivity in gas chemiresistors based on carbon nanotube surface functionalized with noble metal (Au, Pt) nanoclusters. Appl. Phys. Lett. 2007, 90, 173123/1–173123/3. [Google Scholar]
- Wang, Y.; Balbuena, P.B. Roles of proton and electric field in the electroreduction of O2 on Pt (111) surfaces: Results of an ab-initio molecular dynamics study. J. Phys. Chem. B 2004, 108, 4376–4384. [Google Scholar]
- Grabow, L.C.; Gokhale, A.A.; Evans, S.T.; Dumesic, J.A.; Mavrikakis, M. Mechanism of the water gas shift reaction on Pt: First principles, experiments, and microkinetic modeling. J. Phys. Chem. C 2008, 112, 4608–4617. [Google Scholar]
- Zhou, C.; Wu, J.; Nie, A.; Forrey, R.C.; Tachibana, A.; Cheng, H. On the sequential hydrogen dissociative chemisorption on small platinum clusters: A density functional theory study. J. Phys. Chem. C 2007, 111, 12773–12778. [Google Scholar]
- Orita, H.; Inada, Y. DFT investigation of CO adsorption on Pt (211) and Pt (311) surfaces from low to high coverage. J. Phys. Chem. B 2005, 109, 22469–22475. [Google Scholar]
- Tiwari, J.N.; Nath, K.; Kumar, S.; Tiwari, R.N.; Kemp, K.C.; Le, N.H.; Youn, D.H.; Lee, J.S.; Kim, S.K. Stable platinum nanoclusters on genomic DNA–graphene oxide with a high oxygen reduction reaction activity. Nat. Commun. 2013, 4, 1–7. [Google Scholar]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125/1–155125/9. [Google Scholar]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar]
- Srivastava, D.; Menon, M.; Cho, K. Nanoplasticity of single-wall carbon nanotubes under uniaxial compression. Phys. Rev. Lett. 1999, 83, 2973–2976. [Google Scholar]
- Park, Y.; Lahaye, R.J.W.E.; Lee, Y.H. Adsorption of Pt on defective carbon nanotube walls: A DFT approach. Comput. Phys. Commun. 2007, 177, 46–46. [Google Scholar]
- Ganji, M.D.; Sharifi, N.; Ardjmand, M.; Ahangari, M.G. Pt-decorated grapheme as superior media for H2S adsorption: A first-principles study. Appl. Surf. Sci. 2012, 261, 697–704. [Google Scholar]
- Yeung, C.S.; Liu, L.V.; Wang, Y.A. Adsorption of small gas molecules onto Pt-doped single-walled carbon nanotubes. J. Phys. Chem. C 2008, 112, 7401–7411. [Google Scholar]



| SO2 | H2S | CO | |
|---|---|---|---|
| dPt-C1/nm | 0.205 | 0.200 | 0.205 |
| dPt-C2/nm | 0.201 | 0.202 | 0.202 |
| dPt-C3/nm | 0.196 | 0.192 | 0.194 |
| Eb/eV | –1.225 | –0.977 | –1.386 |
| Eb/eV | Qgas/e | |
|---|---|---|
| SO2-SWCNTs | –0.830 | –0.067 |
| H2S-SWCNTs | –0.591 | 0.013 |
| CO-SWCNTs | –0.157 | 0.006 |
| System | QSWCNTs/e | QPt/e | Qgas/e | ΔQSWCNTs/e | ΔQPt/e |
|---|---|---|---|---|---|
| Pt-SWCNTs | 0.147 | –0.147 | |||
| SO2-Pt-SWCNTs | 0.509 | –0.116 | –0.393 | 0.362 | 0.031 |
| H2S-Pt-SWCNTs | –0.019 | –0.266 | 0.285 | –0.166 | –0.119 |
| CO-Pt-SWCNTs | 0.166 | –0.347 | 0.181 | 0.019 | –0.200 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, X.; Dai, Z.; Wei, L.; Liang, N.; Wu, X. Theoretical Calculation of the Gas-Sensing Properties of Pt-Decorated Carbon Nanotubes. Sensors 2013, 13, 15159-15171. https://doi.org/10.3390/s131115159
Zhang X, Dai Z, Wei L, Liang N, Wu X. Theoretical Calculation of the Gas-Sensing Properties of Pt-Decorated Carbon Nanotubes. Sensors. 2013; 13(11):15159-15171. https://doi.org/10.3390/s131115159
Chicago/Turabian StyleZhang, Xiaoxing, Ziqiang Dai, Li Wei, Naifeng Liang, and Xiaoqing Wu. 2013. "Theoretical Calculation of the Gas-Sensing Properties of Pt-Decorated Carbon Nanotubes" Sensors 13, no. 11: 15159-15171. https://doi.org/10.3390/s131115159




