Sulfonated Polyaniline Coated Mercury Film Electrodes for Voltammetric Analysis of Metals in Water
Abstract
:Introduction
Experimental
Reagents and sample collection
Equipament
Methods
Preparation of the SPAN-coated mercury thin film electrode (SPANME)
Results and Discussion
Electrochemical behavior
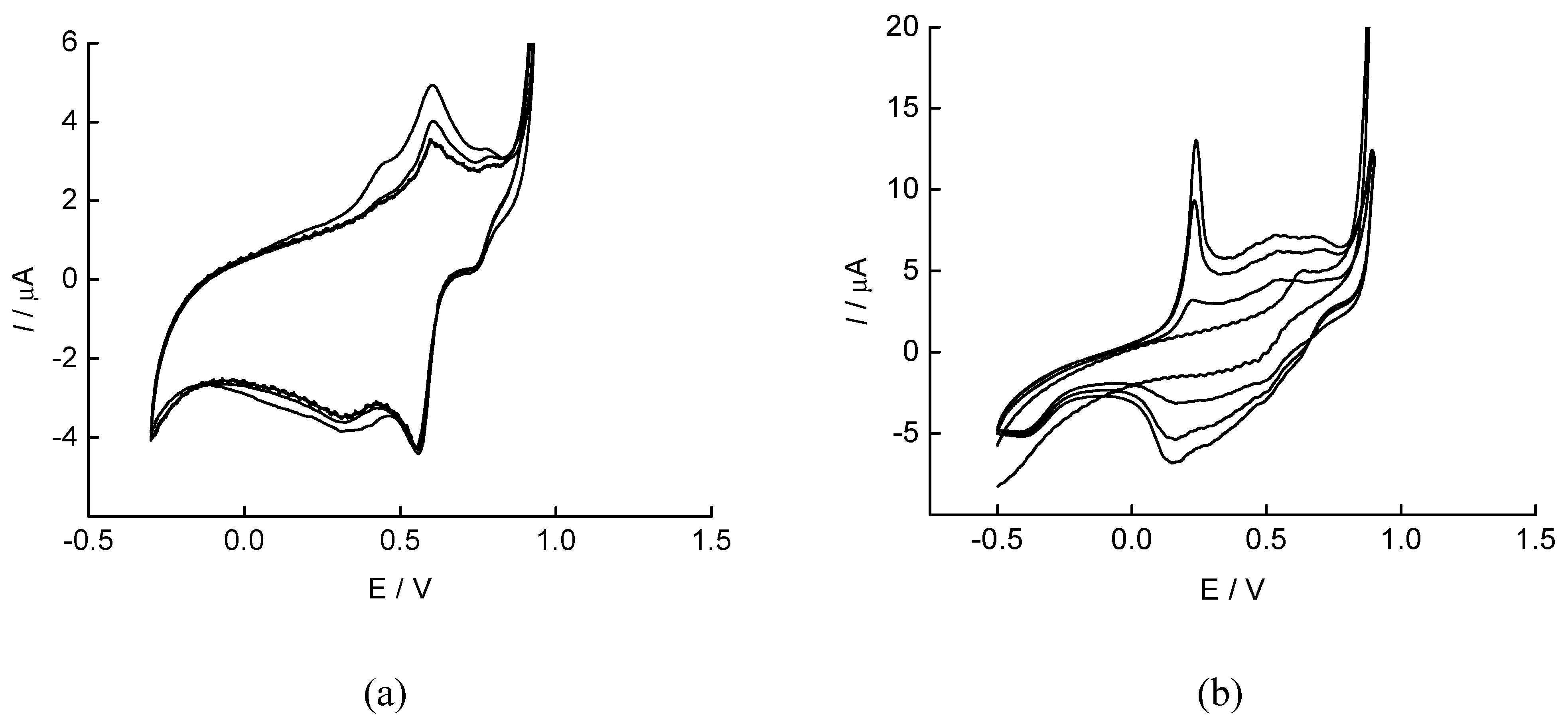
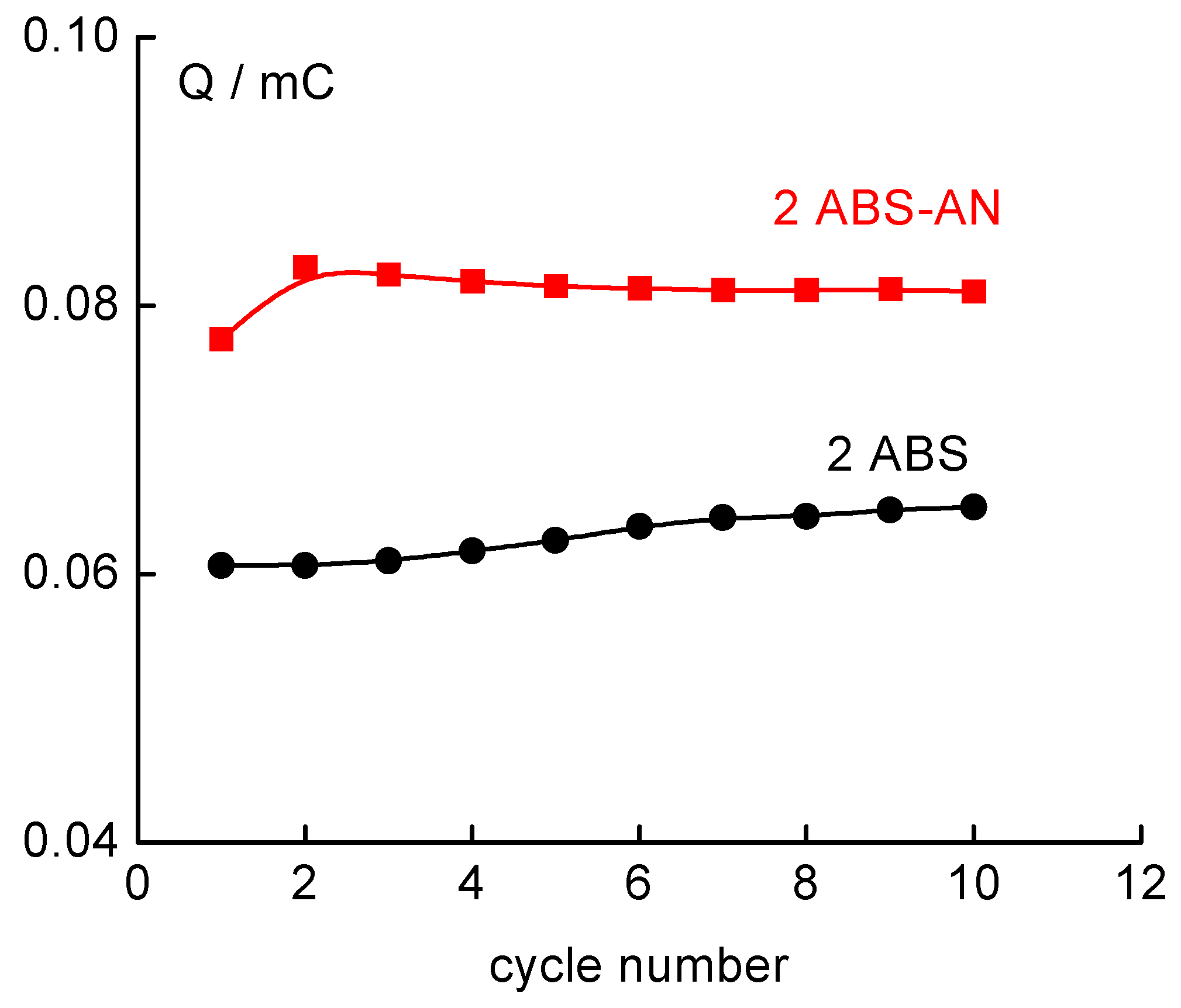
SWASV response characteristics of SPANME
| SPANME | ρip (%) | ||
|---|---|---|---|
| Zn | Cd | Pb | |
| 2 ABS | 200 | 62 | 232 |
| 2 ABS-AN | - 65 | 6 | 11 |
| Metal | Slope (μA nmol L-1) | Intercept (μA) | Correlation coefficient (n = 6) (%) |
|---|---|---|---|
| Cd | 0.067 | -0.03 | 99.3 |
| Pb | 0.033 | 0.03 | 99.3 |
| Zn | 0.048 | 0.13 | 99.5 |
Analysis of environmental samples
| Analyte | Samples | ||
|---|---|---|---|
| river water | drinking water | rain water | |
| Zn | nd | 1.2 ± 3.7 % | 20.7 ± 1.9 % |
| Cd | nd | nd | 18.1 ± 3.6 % |
| Pb | 132.0 ± 3.1 % | 5.7 ± 2.9 % | 96.0 ± 2.7 % |
Conclusions
Acknowledgements
References
- Yue, J.; Wang, Z. H.; Cromack, K. R.; Epstein, A.J.; Macdiarmid, A. G. Effect of sulfonic acid group on polyaniline backbone. J. Am. Chem. Soc. 1991, 113, 2665–2671. [Google Scholar] [CrossRef]
- Wei, X.-L.; Wang, Y. Z.; Long, S. M.; Bobeczko, C.; Epstein, A. J. Synthesis and physical properties of highly sulfonated polyaniline. J. Am. Chem. Soc. 1996, 118, 2545–2555. [Google Scholar] [CrossRef]
- Yue, J.; Epstein, A. J. Synthesis of self-doped conducting polyaniline. J. Am. Chem. Soc. 1990, 112, 2800–2801. [Google Scholar] [CrossRef]
- Wei, X.-L.; Epstein, A. J. Synthesis of highly sulfonated polyaniline. Synth. Metals 1995, 74, 123–125. [Google Scholar] [CrossRef]
- Wang, X. H.; Li, J.; Wang, L. X.; Jing, X. B.; Wang, F. S. Structure and properties of self-doped polyanilines. Synth. Metals 1995, 69, 147–148. [Google Scholar] [CrossRef]
- Brett, C. M. A.; Thiemann, C. Synthesis and properties of self-doped polyanilines. In 47th Meeting of the ISE, Veszprém-Balatonfured, Hungary; 1996. [Google Scholar]
- Pasquali, M.; Pistoia, G.; Rosati, R. Storage characteristics of polyanilines. An investigation by cyclic voltammetry and impedance spectroscopy. Synth. Metals 1993, 58, 1–15. [Google Scholar] [CrossRef]
- Kitani, A.; Satoguchi, K.; Tang, H. Q.; Ito, S.; Sasaki, K. Electrosynthesis and properties of self-doped polyaniline. Synth. Metals 1995, 69, 129–130. [Google Scholar] [CrossRef]
- Barbero, C.; Kötz, R. Electrochemical formation of a self-doped conductive polymer in the absence of a supporting electrolyte. The copolymerization of o-aminobenzenesulfonic acid and aniline. Adv. Mat. 1984, 6, 577–580. [Google Scholar] [CrossRef]
- Dao, L. H.; Leclerc, M.; Guay, J.; Chevalier, J. W. Synthesis and characterization of substituted poly(anilines). Synth. Metals 1989, 29, E377–E382. [Google Scholar] [CrossRef]
- Lee, J. Y.; Cui, C. Q.; Su, X. H. Modified polyaniline through simultaneous electrochemical polymerization of aniline and metanilic acid. J. Eletroanal. Chem. 1993, 360, 177–197. [Google Scholar] [CrossRef]
- Karyakin, A. A.; Maltsev, I. A.; Lukachova, L. V. The influence of defects in polyaniline structure on its electroactivity: optimization of ‘self-doped’ polyaniline synthesis. J. Electroanal. Chem. 1996, 402, 217–219. [Google Scholar] [CrossRef]
- Karyakin, A. A.; Strakhova, A. K.; Yatsimirsky, A. K. Self-doped polyanilines electrochemically active in neutral and basic aqueous solutions. J. Electroanal. Chem. 1994, 371, 259–265. [Google Scholar] [CrossRef]
- Lee, J. Y.; Su, X. H.; Cui, C. Q. Characterization of electrodeposited copolymers of aniline and metanilic acid. J. Eletroanal. Chem. 1994, 367, 71–78. [Google Scholar] [CrossRef]
- Lee, J. Y.; Cui, C. Q. Electrochemical copolymerization of aniline and metanilic acid. J. Eletroanal. Chem. 1996, 403, 109–116. [Google Scholar] [CrossRef]
- Mav, I.; Zigon, M.; Sebenik, A.; Vohlidal, J. Sulfonated polyanilines prepared by copolymerization of 3-aminobenzenesulfonic acid and aniline: The effect of reaction conditions on polymer properties. J. Polym. Sci. Part A: Polym. Chem. 2000, 38, 3390–3398. [Google Scholar] [CrossRef]
- Fan, J. H.; Wan, M.; Zhu, D. Synthesis and characterization of water-soluble conducting copolymer poly(aniline-co-o-aminobenzenesulfonic acid). J. Polym. Sci. Part A: Polym. Chem. 1998, 36, 3013–3019. [Google Scholar] [CrossRef]
- Kitani, A.; Satoguchi, K.; Iwai, K.; Ito, S. Electrochemical behaviors of polyaniline/polyaniline-sulfonic acid composites. Synth. Metals 1999, 102, 1171–1172. [Google Scholar] [CrossRef]
- Pistoia, G.; Montesperelli, G.; Nuziante, P. Polyaniline derivatives: an investigation by ciclic voltammetry and impedance spectroscopy. J. Mol. Electron. 1990, 6, 89–94. [Google Scholar]
- Zotti, G.; Cattarin, S.; Comisso, N. Electrodeposition of polythiophene, polypyrrole and polyaniline by cyclic potential sweep method. J. Eletroanal. Chem. 1987, 235, 259–273. [Google Scholar] [CrossRef]
- Nunziante, P.; Pistoia, G. Factors affecting the growth of thick polyaniline films by the cyclic voltammetry technique. Electrochim. Acta 1989, 34, 223–228. [Google Scholar] [CrossRef]
- Stiwell, D.E.; Park, S.M. Electrochemistry of conductive polymers. V. In situ spectro-electrochemical studies of polyaniline films. J. Electrochem. Soc. 1989, 136, 427–433. [Google Scholar]
- Sasaki, K.; Kaya, M.; Yano, J.; Kitani, A.; Kunai, A. Growth-mechanism in the electropolymerization of aniline and p-aminodiphenylamine. J. Eletroanal. Chem. 1986, 215, 401–407. [Google Scholar] [CrossRef]
- Zotti, G.; Cattarin, S.; Comisso, N. Cyclic potential sweep electropolymerization of aniline. The role of anions in the polymerization mechanism. J. Eletroanal. Chem. 1988, 239, 387–396. [Google Scholar] [CrossRef]
- Jovic, V. D.; Trisovi, T.; Jovic, B. M.; Vojnovic, M. The morphology of different metals electrodeposited onto polyaniline films. J. Electroanal. Chem. 1996, 408, 149–155. [Google Scholar] [CrossRef]
- Dalangin, R. R.; Gunasingham, H. Mercury (II) acetate-Nafion modified electrode for anodic stripping voltammetry of lead and copper with flow-injection analysis. Anal. Chim. Acta 1994, 291, 81–87. [Google Scholar] [CrossRef]
- Brett, C. M. A.; Fungaro, D. A.; Morgado, J. M.; Gil, M. H. Novel polymer-modified electrodes for batch injection sensors and application to environmental analysis. J. Electroanal. Chem. 1999, 468, 26–33. [Google Scholar] [CrossRef]
- Brett, C. M. A.; Fungaro, D. A. Poly(ester sulphonic acid) coated mercury thin film electrodes: characterization and application in batch injection analysis stripping voltammetry of heavy metal ions. Talanta 2000, 50, 1223–1231. [Google Scholar] [CrossRef]
- Sample Availability: Available from the author.
© 2001 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Fungaro, D.A. Sulfonated Polyaniline Coated Mercury Film Electrodes for Voltammetric Analysis of Metals in Water. Sensors 2001, 1, 206-214. https://doi.org/10.3390/s10600206
Fungaro DA. Sulfonated Polyaniline Coated Mercury Film Electrodes for Voltammetric Analysis of Metals in Water. Sensors. 2001; 1(6):206-214. https://doi.org/10.3390/s10600206
Chicago/Turabian StyleFungaro, Denise Alves. 2001. "Sulfonated Polyaniline Coated Mercury Film Electrodes for Voltammetric Analysis of Metals in Water" Sensors 1, no. 6: 206-214. https://doi.org/10.3390/s10600206
APA StyleFungaro, D. A. (2001). Sulfonated Polyaniline Coated Mercury Film Electrodes for Voltammetric Analysis of Metals in Water. Sensors, 1(6), 206-214. https://doi.org/10.3390/s10600206




