1. Introduction
Forests of the Pacific Northwest (PNW) can be highly productive [
1,
2,
3,
4], and as temperate forests they may store more biomass carbon (C) per unit area than most other ecosystems [
1,
5,
6,
7,
8]. Second-growth forest productivity can also be highly variable, and understanding reasons for this variability is an important research priority with implications for ecosystem C dynamics and global C cycles. While a large portion of variability in forest C flux (both above- and belowground) occurs in response to abiotic variation [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19], biotic gradients in plant community composition and diversity may be especially important as drivers of patterns in C cycling and productivity even at fine scales [
4,
7,
20,
21,
22,
23]. Stand diversity (richness, evenness, and diversity of dominant species), species biomass dominance, and presence/absence of soil modifying organisms (such as nitrogen [N]-fixing plants) could all represent major biotic influences on ecosystem C flux. A better understanding of how ecosystem C dynamics are affected by biotic factors such as these could lead to a better understanding of C dynamics of the global climate system [
24].
Productivity-diversity and diversity-ecosystem function experiments (especially in grassland systems), generally show that more diverse plots are more productive [
4,
25,
26], and have suggested positive relationships between ecosystem C-uptake and species diversity [
25,
26,
27,
28], and recent research spanning PNW forests also suggest positive relationships between productivity and diversity in natural forested ecosystems [
4]. In fact, studies across productivity gradients have suggested the reverse relationship where productivity predicts forest tree diversity in large-scale regional datasets [
16,
29]. Forests may exhibit positive productivity-diversity relationships even though peaks in forest diversity across gradients in site quality have often shown intermediate productivity [
16,
21,
30]. However, when productivity and diversity are related within a site, it is unclear whether productivity-diversity relationships are due to the influence of hyper-productive species (
i.e., sampling effects) or complementarity in resource acquisition [
21,
31,
32]. There is also a general need for more studies examining biodiversity-ecosystem function patterns in natural systems [
33] where site-quality variation is constrained.
Undoubtedly, presence of dominant plant species can play a major role in determining the C flux in ecosystems [
34]. Although biomass stand dominance by abundant species can influence productivity [
4], species differences in functional traits are also important as drivers of ecosystem productivity and are well-recognized as important controls on ecosystem processes (e.g., [
35,
36]). It is also possible that different species achieve similar net C uptake rates via different trait suites. For example,
Alnus rubra Bong., a deciduous species in the PNW with a symbiotic association with an N-fixing bacteria, may maintain high productivity during the regular growing season that is matched by consistently lower (comparatively) productivity by coniferous evergreen species like
Pseudotsuga menziesii (Mirb.) Franco., which accumulate biomass over a greater portion of the year [
37]. Thus it has been hypothesized that mixtures of these N-fixing and non-N-fixing species could yield more productive forests [
38,
39,
40]. Presence of
A. rubra may be especially important in increasing forest productivity (e.g., [
16,
38,
39,
40,
41,
42,
43,
44]) via N additions to soils [
37,
45,
46,
47]. This is important because low levels of soil N can limit growth in many PNW, USA forests (but see Binkley
et al. [
44]).
Aboveground C stocks and aboveground productivity (especially aboveground net primary productivity (ANPP)) have been thoroughly investigated using numerous metrics in multiple experimental systems [
48], but soil CO
2 efflux responses to biotic variation in forest ecosystems are less well-studied [
8,
17,
49]. Significant variation in belowground C flux exists alongside variation in dominant plant diversity [
18], and this could be driven by the influence of hyper-productive (or nutrient-cycle altering) species (e.g., [
21,
32,
49]).
In this study, we hypothesized that stand diversity, species biomass dominance, and presence/absence of an N-fixing tree species were all major biotic influences related to ecosystem C flux. We focused on three key measurable C flux measures in a natural second-growth PNW forest: net changes in aboveground tree C pools (hereafter; aboveground biomass increment (ABI), aboveground net primary productivity of trees (ANPPtree)), and net soil CO2 efflux. While not an exhaustive list of potential C fluxes in forests, these variables (ABI, ANPPtree, and soil CO2 efflux) may be indicative of major above- and belowground shifts in C flux associated with biotic variation in forests. All three of these variables can be responsive to both biotic and abiotic changes, and are important to quantify in order to understand whole ecosystem C flux. Our primary goal was to identify variation in aboveground C flux and net soil CO2 efflux co-varying with stand diversity at a relatively homogeneous site. We examined C flux with regard to influences of: (1) overstory richness and diversity; (2) biomass-based stand dominance of overstory tree species; and (3) soil nutrient pools.
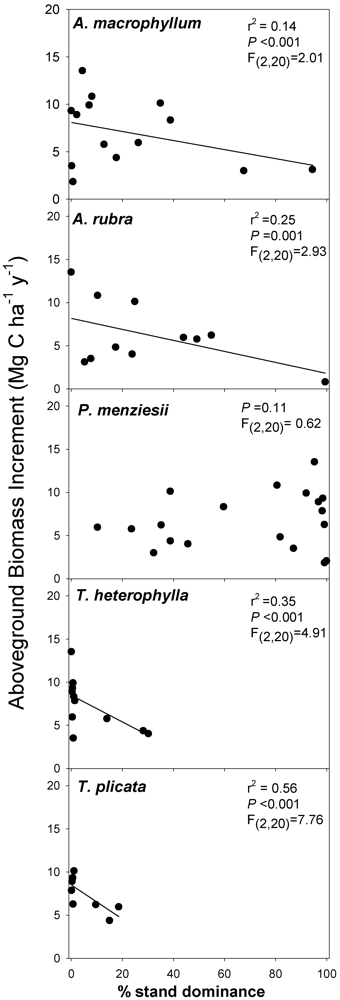
We prioritized these examinations in regards to four, more specific, hypotheses. First, we hypothesized aboveground productivity (ABI and ANPPtree) would increase with an increase in forest tree richness and diversity. Second, we hypothesized stands containing the N-fixing species, A. rubra, would have higher aboveground productivity (ABI, and ANPPtree) driven by high values in inorganic N pools. We expected lower productivity in sites dominated by slow-growing coniferous species such as Thuja plicata Donn ex D. Don and Tsuga heterophylla (Raf.) Sarg. Third, we hypothesized stands with higher stand diversity would also have higher soil CO2 efflux. And finally, if C flux predictably varies with stand diversity, then we should expect total standing C stocks to be higher where aboveground productivity is higher, as long as aboveground C uptake is not overcome by high soil CO2 efflux, and all sites have had a similar recovery time post-disturbance. This is important because sites with similar recovery times since disturbance are likely to have similar establishment dates and may be similarly aged. Thus with similarly ages stands we hypothesized C pools would increase with stand diversity and A. rubra presence.
2. Materials and Methods
2.1. Study Area
This study was conducted adjacent to the Puget Sound, Washington, USA in the Evergreen Ecological Observation Network, a long-term permanent plot network of 44 permanent ecological monitoring plots located throughout a 380 ha forest reserve owned and managed by The Evergreen State College. The ecosystem is a second-growth temperate forest that was clear-cut in 1937-39 using cable techniques. Our site has an average temperature of 10 °C and receives approximately 100 cm of annual rainfall [
50]. All plots were located on similar Alderwood gravelly loam soils [
51].
The forest overstory is dominated by a mixed canopy of
P.
menziesii and four primary codominant species:
Acer macrophyllum Pursh,
A. rubra,
T. heterophylla and
T. plicata, with an understory dominated primarily by
Polystichum munitum (Kaulf.) C. Presl and
Gaultheria shallon Pursh. The plot network was established in 2005 using a systematic 250 m spaced grid placed with a random start point using circular 20 m-diameter plots (
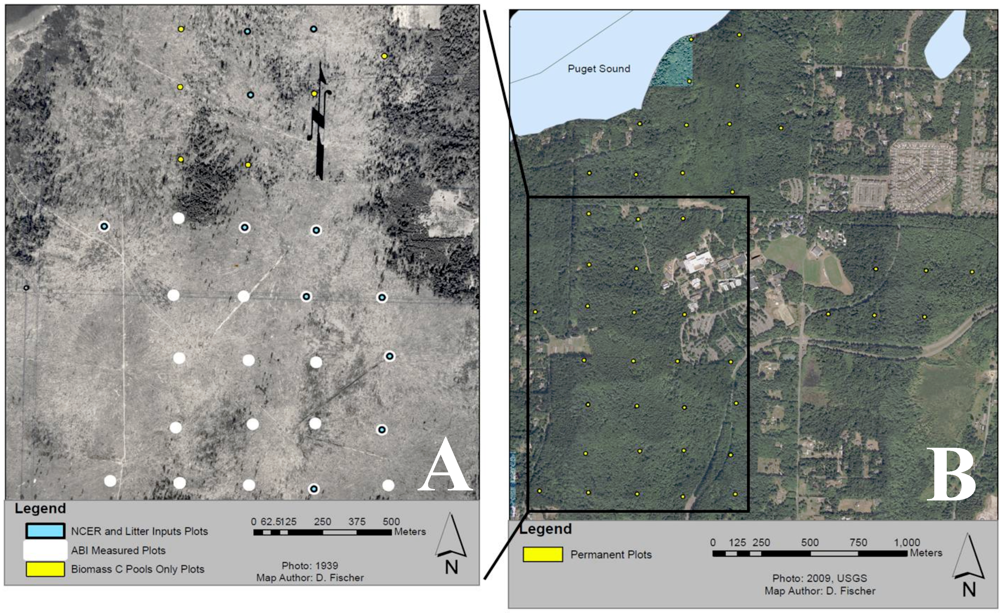
Figure 1).
Figure 1.
Map of study area depicting circular 10 m radius permanent plots along a 250 m grid with a random starting point: (A) Historical (1939) photo of the study area showing part of a clear-cut which took place from 1937-39. Blue dots depict the subset of 11 plots measured for soil CO2 efflux and leaf litter, white dots depict the subset of 21 plots measured for aboveground biomass increment (ABI), and yellow dots depict 6 of the 44 plots measured for aboveground biomass pools only. (B) Locations of all 44 permanent plots measured for aboveground biomass pools only. For reference The Evergreen State College is located at the center of the image.
Figure 1.
Map of study area depicting circular 10 m radius permanent plots along a 250 m grid with a random starting point: (A) Historical (1939) photo of the study area showing part of a clear-cut which took place from 1937-39. Blue dots depict the subset of 11 plots measured for soil CO2 efflux and leaf litter, white dots depict the subset of 21 plots measured for aboveground biomass increment (ABI), and yellow dots depict 6 of the 44 plots measured for aboveground biomass pools only. (B) Locations of all 44 permanent plots measured for aboveground biomass pools only. For reference The Evergreen State College is located at the center of the image.
For this study we utilize 44 plots to estimate C pools in live and dead standing biomass, coarse woody debris (CWD), and understory vegetation. A subset of 21 plots were used to measure aboveground biomass increment (ABI), sampled once in 2006 and again in 2008. These years were expected to be average growth years based on data from an on-site weather station [
50]. A smaller subset of 11 plots was intensively measured between 2006–2008 for estimates of leaf litterfall and aboveground net primary productivity of trees (ANPP
tree). Another subset of 10 intensive plots was independently selected in 2008 for the measurement of fine woody debris (FWD), and net soil CO
2 efflux (see
Section 2.7;
Net Soil CO2 Efflux Rate). All intensive plots were chosen haphazardly from a subset of plots that could be determined to have similar site histories (similarly cleared) based on a 1939 orthophoto showing the last logging operation to take place at our study site.
2.2. Soil Nutrients
Variation in soil nutrient status could underlie any observed co-variation in forest diversity and C flux. In order to address potential variation in plot nutrient status, we analyzed cation exchangeable pools of mineral soil PO
43-, K
+, Ca
2+, NH
4+, NO
3- from a spring 2011 survey of the intensively measured plots. Briefly, for measurement of soil cation exchangeable PO
43-, K
+, and Ca
2+, we collected soils at 10 cm depths on north and south borders of each plot. Sieved (4 mm) soil samples were freeze-dried and powdered in a SPEX ball mill. Aliquots of 0.2 to 0.5 g of each soil were leached in 5 mL of 1M NH
4Cl solution for 20–24 h at room temperature in an agitator to separate the cation-exchangeable fraction of ions (
sensu Nezat
et al. [
52]). Solutions were centrifuged to separate the supernatant and the remainder of the sample was rinsed 2 times in DI water to quantitatively collect the NH
4Cl leach. Leach solutions were analyzed for PO
43-, K
+, and Ca
2+, by Inductively-coupled Plasma Mass Spectrometry (ICP-MS; Perkin-Elmer DRC-e). For inorganic soil N (NH
4+, NO
3–), a 10 g aliquot of sieved (4 mm) soil was extracted in 100 ml of 2M KCl and hand-agitated (2 min) prior to leaching overnight (12 h;
sensu Robertson
et al. [
53]). Suspensions were vacuum-filtered (grade 50 cellulose filter paper) and stored at 4°C until analyzed by University of Idaho Analytical Sciences Laboratory for colorimetric analysis utilizing flow injection analysis.
2.3. Changes in Aboveground Standing Carbon
To estimate net changes in aboveground C in forest plots, we rely on tree biomass estimation equations, and do not include sapling or understory biomass in changes of aboveground carbon stocks because trees dominate the system and aboveground portions of trees tend to reliably represent the majority of plant mass in most temperate forest ecosystems [
48,
54]. Changes in CWD C were also not included in calculation of aboveground C stocks as changes in CWD C are usually measured over decades rather than years (but see
Section 2.4;
Coarse and Fine Woody Debris). Death of trees and recruitment of new trees were accounted for on an individual tree basis as suggested in Clark
et al. [
48].
Aboveground tree biomass was estimated using independent allometric relationships based on stem diameter at 1.37 m (diameter at breast height; DBH) and tree height (HT). All trees within plot boundaries with DBH ≥ 5 cm were tagged and measured. We measured tree DBH in 2006 and again in 2008 at a tagged location on the tree trunk. Tree HT measurements were taken in 2007 and applied to both 2006 and 2008 allometric biomass equation estimates of tree mass. Measurement of tree HT was obtained using a laser range finder and clinometer, and validated using estimates of tree HT generated from aerial LiDAR and processed in the program FUSION [
55,
56]. For mass estimation, we used species-specific biomass estimation equations from published studies compiled in the BIOPAK software package [
57,
58]. We primarily used biomass equations from Standish
et al. [
57] with the exception of the equation for
A. macrophyllum biomass. For this species we compiled an equation from Gholz [
59] using summation of individual equations for five separate components of the tree: total foliage biomass, stem wood biomass, live branch biomass, dead branch biomass, and stem bark biomass. In instances where a tree species did not have an associated biomass equation, the biomass equation for
A. rubra [
57] was substituted since it produced allometric predictions intermediate to other equations. This substitution was applied to the species
Salix scouleriana Barratt ex Hook.,
Frangula purshiana (DC.) Cooper,
Corylus cornuta Marsh.,
Cornus nuttallii Audubon ex Torr. & A. Gray, and
Ilex aquifolium L. Across all species, aboveground C was assumed to be 50% of plant biomass, which is a common assumption [
48].
We analyzed changes in aboveground tree C pools using two distinct metrics that each account for ecosystem C flux with different limitations. First, ABI measures aboveground tree biomass increment C and was estimated using repeat measures of biomass (2006, 2008) according to the equation:
ABI = (Bt2 – Bt1)/T (1)
where Bt1 is the total aboveground tree biomass at time 1 (2006), Bt2 is total aboveground tree biomass at time 2 (2008), and T refers to the number of years over which data were taken (2). This metric focuses on live trees, relies on estimation of foliar mass based on stem dimensions, and does not account for foliar loss due to abscission (i.e., it only accounts for net changes in standing C without accounting for replacement of foliage produced and shed to the forest floor). This metric was available for most plots in our study because it relies on relatively simple field measurements.
Second, for the subset of 11 more intensively measured plots, we estimated ANPPtree by summing estimates of ABI with estimates of litterfall from litter traps. This metric provides a combined estimate of ANPP in trees through estimates of changes in aboveground tree woody increment, new foliar production, and foliar production that replaced foliage lost through litterfall. We collected litterfall using 50 cm diameter litter traps suspended 20 cm above the forest floor at plot center. Litterfall was collected monthly from November 2006 through November 2007. Following collection, litter was immediately dried at 70 °C for 72 h, sorted by species, and weighed to the nearest mg. While ABI was calculated as the average over two years (2006–2008), litterfall was estimated based on the 2006–2007 collection. Thus, ANPPtree estimates represent ABI averaged over two years and litterfall from the single year it was measured.
2.4. Coarse and Fine Woody Debris
Although not used for estimates of changes in C stocks over time, we measured CWD mass to estimate total plot aboveground C stocks (
Table 1). A survey of coarse woody debris (CWD) was conducted in all plots between 2006–2008. We separate CWD into two categories, where all downed logs ≥10 cm in diameter are considered downed woody debris (DWD) and all standing dead tress ≥5 cm DBH are considered snags. All CWD falling into these two categories were tagged and measured in all plots [
60] for estimation of total plot non-living C pool. Length (L) was measured on each piece of DWD and diameter was recorded at three points: base (A
b), middle (A
m) and top (A
t) of each piece of DWD. When DWD extended beyond the plot, end measurements were made at the plot boundary. Decay classes for each species were estimated using a five class decomposition scale [
61]. Dimension measurements were used with Newton’s formula to obtain volume for each piece of DWD [
60]. We then used volumetric estimates combined with estimates of density and C content, based on decay stage, in order to provide estimates of DWD C. Density values were obtained from a table of mean density for decay classes of CWD in the Cascade-Temperate region [
60] for the following species:
P. menziesii,
T. plicata, and
T.
heterophylla. Where a decay-class-sensitive density estimate could not be found for a species, a substitute density was used by selecting densities from decay classes of another species whose live tree bole densities closely matched published live tree bole density estimates for the species of concern.
Table 1.
Stand characteristics and carbon (C) pools for measured plots *.
Table 1.
Stand characteristics and carbon (C) pools for measured plots *.
| S | n | TPH | SPH | DWDPH | Tree C | Snag C | DWD C | Sap C | FWD C1 | US C | Plot C |
|---|
| 1 | 2 | 525.48 | 143.31 | 302.55 | 148.05 | 12.01 | 14.86 | 0.06 | 8.62 (2) | 7.31 | 182.29 |
| 2 | 5 | 343.95 | 343.95 | 407.64 | 148.79 | 15.37 | 12.8 | 0.16 | 9.15 (1) | 5.8 | 182.92 |
| 3 | 13 | 443.41 | 210.68 | 440.96 | 236.95 | 5.45 | 22.86 | 0.18 | 8.81 (4) | 3.89 | 269.33 |
| 4 | 16 | 491.64 | 222.93 | 423.97 | 287.21 | 5.84 | 21.72 | 0.17 | 8.01 (4) | 3.97 | 318.91 |
| 5 | 7 | 509.55 | 200.18 | 414.01 | 286.55 | 4.72 | 16.78 | 0.19 | n/a | 5.06 | 321.792 |
| 6 | 1 | 668.79 | 127.39 | 95.54 | 154.35 | 13.2 | 1.25 | 0.32 | n/a | 5.08 | 182.692 |
| Weighted | Ave. | 469.02 | 223.65 | 412.57 | 247.18 | 7.08 | 19.48 | 0.17 | 8.52 | 4.51 | 286.91 |
Snags were measured for DBH and HT. A five class decomposition score (similar to the CWD scale) was also estimated for all snags measured, and used as above for DWD. The Huber formula was used for snag volume [
60]. Biomass C of each snag was calculated using species- and decay-class-specific density values similar to those used for calculation of DWD.
An estimation of fine woody debris (FWD; any woody debris <10 cm in diameter) was produced from locations adjacent to each of a subset of 10 intensive plots in the fall of 2008. Four 0.25 m
2 sampling frames were placed just outside plot boundaries (to reduce plot disturbance) in four cardinal directions to determine C content of FWD of the forest floor. Woody debris <10 cm in diameter was collected, oven-dried at 70 °C for 72h, sorted, and weighed. As stated in
Section 2.2, 50% of dry mass was assumed to be C. The four subplot values were averaged for an estimation of plot FWD C.
2.5. Saplings
A sapling survey was conducted in all plots to determine C storage of young trees and shrubs. Tree saplings <5 cm DBH and ≥1 m in HT were counted, while all shrubs <5 cm DBH and ≥2 m tall were counted. Trees and shrubs not meeting these minimum criteria were considered to be part of the understory community and were included in understory community sampling (see
Section 2.6;
Understory Community). Saplings were recorded for species and abundance. A random sampling of 40 saplings yielded a mean stem basal diameter at the litter surface (DBA) of approximately 2.5 cm. Based on this sampling each sapling was given a DBA of 2.5 cm for calculation of biomass using equations from the BIOPAK database [
58]. While this assumption of similar biomass based on average sapling mass may be a source of error, sapling abundance contributed minimally to whole plot C estimates (<0.05%). Species that did not have an associated biomass equation were assigned a species closely related by genus, family, or morphology.
2.6. Understory Community
Understory vegetation (% cover) was measured in 2008 using 10 cm point-line intercepts along four 10 m transects from plot center to plot edge in cardinal directions. The mass of understory plants was determined using equations from the BIOPAK database [
58]. Since understory community measurements were made only once during the study, measurements were used for estimates of plot C pools, but were not used for estimates of net changes in aboveground C pools.
2.7. Net Soil CO2 Efflux Rate
We measured net soil CO2 efflux in a subset of 11 intensively measured plots selected in 2008. Soil respiration was measured on the forest floor using a differential open system infrared gas analyzer with soil chamber attachment (ADC Bioscientific Ltd., Hertz, UK). In four evenly-stratified subplots per plot, 5-min measurements were taken monthly for a full year between January 2008 and January 2009. During spring and summer, 24-h measurements were conducted for assessment of diel patterns in soil CO2 efflux. For each 24-hour measurement, two plots were sampled every three hours. These measurements suggested a mild diel pattern with peak soil CO2 efflux from ~10:00–16:00, and all regular measurements were taken between these times for consistent measurement of soil CO2 efflux within daily temperature and moisture regimes.
2.8. Overstory Richness and Diversity
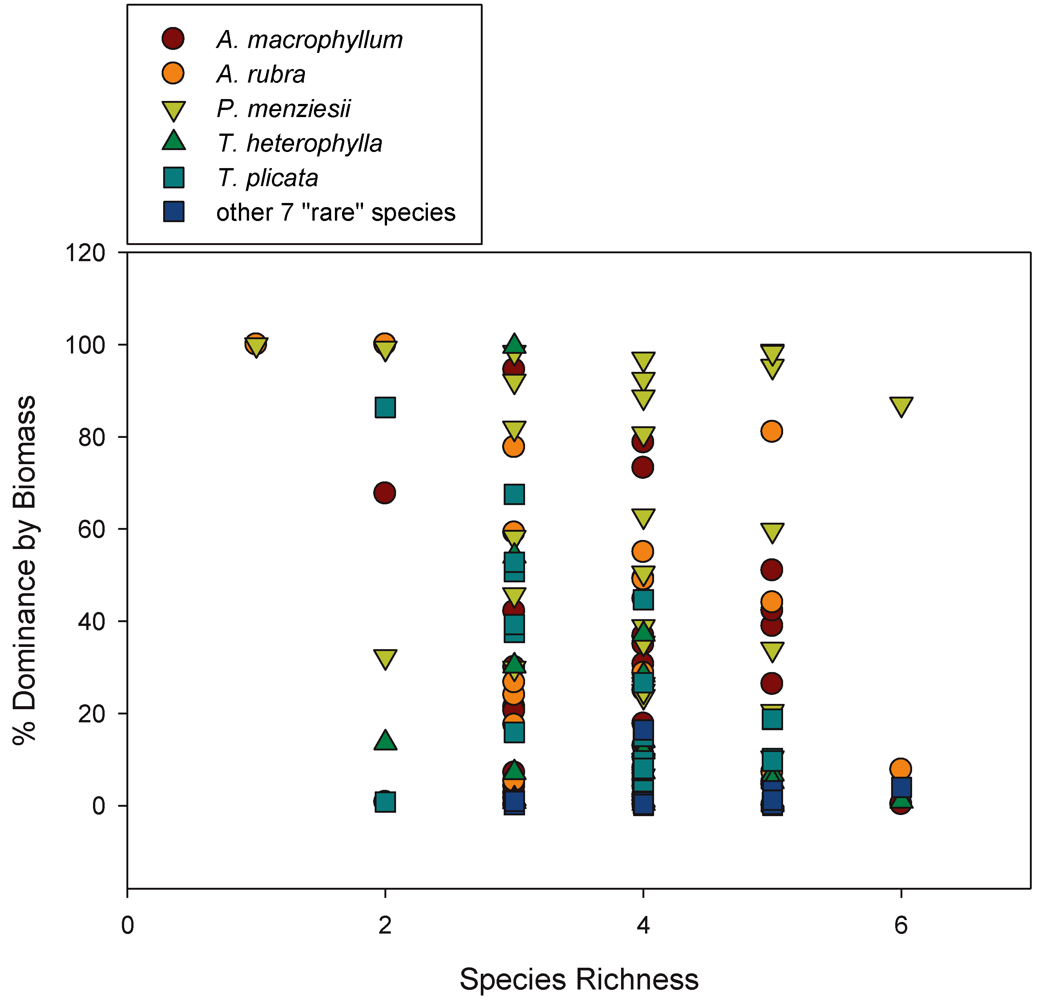
We classified species richness within plots in two ways: (1) Using just the five most dominant overstory species (
P.
menziesii, A. macrophyllum A. rubra, T. heterophylla, T. plicata), referred to as ‘Overstory Richness 5’ in tables and figures. This was done to observe patterns associated with abundant species that have a significant physical presence in plots, and to avoid patterns in richness associated with small, rare individuals. The five most dominant overstory species comprise 95.5% of all tagged trees and account for 99% of total tree biomass. Thus, dominant species were assumed to drive a majority of the productivity-diversity relationship; (2) Using all 12 overstory species that occur within plots (also including
S. scouleriana,
F. purshiana,
I. aquifolium,
Abies grandis [Douglas ex D. Don] Lindl.,
C. cornuta,
C.
nuttallii, and
Picea sitchensis [Bong] Carrière], referred to as ‘Overstory Richness All’ in tables and figures. This was done to classify true plot tree richness so data could be analyzed with the influence of rare species. Any patterns related to richness or diversity in this study can be assumed to be based on random sampling, as this plot network is located throughout an even-aged forest using a stratified-random plot design. We included all 12 overstory species for both Shannon’s (H’) and Simpson’s (D) diversity indices calculated using the program PC-Ord [
62]. We also constructed an index of community similarity among plots based on non-metric multidimensional scaling (NMS) ordination of our tree data using a single axis solution of ordinated data and 500 max iterations [
62]. The final stress of this ordination was 47.7, with instability of 0.0005. This single axis-ordination gives a score (hereafter NMS community similarity) where communities that are more similar exhibit a similar score.
2.9. Statistical Analysis
Our work analyzing tree diversity and C can be summarized by three sets of analyses: (1) We conducted linear regression analyses between species diversity indices and the variables ABI, ANPP
tree, and soil CO
2 efflux; (2) Because hyper-productive species (e.g., the N-fixing species
A. rubra) could be responsible for productivity-diversity relationships, we also assessed individual co–variation with C flux by conducting separate linear regressions of C flux/pool measures and individual biomass-based stand dominance estimates for each tree species in each plot. Our regressions examined individual tree species stand dominance relationships with ABI, ANPP
tree, and soil CO
2 efflux; (3) Finally, in order to further clarify which factors were better predictors than others, we conducted model selection analysis to compare single-factor models using ranked model selection criteria [
63]. For this analysis, we compared models where C flux was predicted from each diversity index, % biomass dominance of all species individually, a model that combined all species, NMS community similarity, inorganic N measures, or an intercept only (null model). Briefly, our approach used Akaike’s Information Criterion, (adjusted for small sample size; AICc), model likelihood, computed weights of evidence (w
i), and an “evidence ratio” computed from these variables, to rank multiple models. Each measure provided an index of the best model given the data, and the “evidence ratio” gives a “gambler’s odds” of the top model being the best model compared to other models. Models in the same set whose AICc differed by less than 2.0 where not considered statistically distinguishable, as is common [
63]. This approach is considered less biased and less prone to error compared to other model ranking approaches like stepwise regression [
63]. The approach favors more parsimonious models, and thus single factor models are generally selected over multivariate predictor models. Because we included soil N variables, this analysis was limited to only the plots in which soil N was measured (n = 8–11).
For exploratory examination of all other correlations among variables we used Pearson’s product-moment correlation analysis. All data which did not meet normality assumptions were transformed using log transformations, or arcsine square-root transformations in the case of percentage data. All analyses were conducted in JMP 8.0 (SAS Institute Inc. Cary, NC). An α = 0.05 was used to determine statistical significance.