Ecological Impact on Nitrogen and Phosphorus Cycling of a Widespread Fast-growing Leguminous Tropical Forest Plantation Tree Species, Acacia mangium
Abstract
: Symbiotic nitrogen fixation is one of the major pathways of N input to forest ecosystems, enriching N availability, particularly in lowland tropics. Recently there is growing concern regarding the wide areas of fast-growing leguminous plantations that could alter global N2O emissions. Here, we highlight substantially different N and phosphorus utilization and cycling at a plantation of Acacia mangium, which is N2-fixing and one of the major plantation species in tropical/subtropical Asia. The litterfall, fresh leaf quality and fine-root ingrowth of A. mangium were compared to those of non-N2-fixing Swietenia macrophylla and coniferous Araucaria cunninghamii in wet tropical climates in Borneo, Malaysia. The N and P concentrations of the A. mangium fresh leaves were higher than those of the other two species, whereas the P concentration in the leaf-litterfall of A. mangium was less than half that of the others; in contrast the N concentration was higher. The N:P ratio in the A. mangium leaf was markedly increased from fresh-leaf (29) to leaf-litterfall (81). Although the N flux in the total litterfall at the A. mangium plantation was large, the fine-root ingrowth of A. mangium significantly increased by applying both N and P. In conclusion, large quantities of N were accumulated and returned to the forest floor in A. mangium plantation, while its P resorption capacity was efficient. Such large N cycling and restricted P cycling in wide areas of monoculture A. mangium plantations may alter N and P cycling and their balance in the organic layer and soil on a stand level.1. Introduction
The amount of tropical plantations is increasing due to the demand for woody biomass. Acacia is one of the major plantation genera which is planted as frequently as Eucalyptus. Acacia mangium has been planted in more than 2 million ha around the world, which is nearly a quarter of the total planted area of all Acacias [1]. A. mangium is one of the major fast-growing species which yields 31–128 kg ha−1 year−1 of nitrogen (N) [2,3], and which grows well in the wet tropics even under acidic and poor nutrient conditions [4]. However, such fast-growing plantations have been criticized because of their resource consumption, such as of water and nutrients, their lower biodiversity and vulnerability to pests and disease [5]. In addition to these problems, A. mangium is also criticized for extracting large nitrous oxide (N2O) from soil [6,7].
Symbiotic nitrogen fixation is one of the major pathways of N input to forest ecosystems enriching N availability, particularly in lowland tropics. Tropical forests generally are large N2O and NO sources compared to other forests in upper latitudes, because of higher N availability [8]. As compared to carbon dioxide (CO2), N2O is a 300-fold stronger green house gas, and is considered the most dominant ozone depleting substance of the 21st century [9]. Arai et al. [6] reported that A. mangium plantations produced 8-fold larger N2O emissions compared to adjacent secondary forests during wet seasons and these results were supported by Konda et al. [7]. They attributed the reasons for the larger emissions to dinitrification [6] and N mineralization [7], due to higher N availability under A. mangium soil, in addition to sufficient soil water conditions. Previous studies on N input through litterfall and N mineralization in A. mangium plantations have reported a large N availability [10-13]. In addition to standing plantations, logging of tropical forests can increase N2O emissions by promoting soil N mineralization [14]. Therefore such large areas of short-rotation A. mangium plantations can be a crucial source of N2O, which may reduce CO2 mitigation effects by accumulating C in their biomass.
In contrast to the large N cycling in A. mangium plantations, phosphorus (P) cycling is unclear. Some tropical N2-fixing trees exhibit specific P acquisition abilities by producing larger extracellular phosphatase [15]. This phosphatase production was larger in soils under A. mangium than those under secondary forests [16]. Houlton et al. [17] suggested that N2-fixing plants could thrive under P-deficit conditions, such as those encountered in the wet tropics, in part by enhanced phosphatase production using the abundant N resources. A. mangium growing in solution cultures and pots were found to use P efficiently under P-deficit conditions [18]. Hence, the phosphorus cycling in A. mangium plantations is expected to be different from that of others.
In this study, we reviewed the difference in N and P retranslocation and in N and P demand in the case of the A. mangium plantation compared with two other plantations of Swetenia macrophylla and Araucaria cunninghamii; as well as litterfall data from adjacent primary forests [19]. We also discussed how A. mangium plantations can influence the regional and global N and P cycling and nutrient conditions for other plants.
2. Materials and Methods
This study was conducted at the Gum Gum Forest Reserve in Sandakan, Sabah, Malaysia (5°52′N, 117°54′E). The average annual precipitation was 2,572 mm, and the average annual temperature was 27.9 °C during the measurement period. This area has a wet tropical climate, with no month receiving a long-term average of less than 100 mm of rain. Fluctuations in the mean monthly temperature are less than ±1.5 °C, and no obvious long droughts have occurred. The research sites were on gently sloping alluvial plains at an altitude of less than 40 m above sea level. Litterfall, fresh leaves and fine-root ingrowth by nutrient applications were examined at three plantations: (i) 20-year-old Acacia mangium Willd., (ii) 34-year-old S. macrophylla King, and (iii) 27-year-old Araucaria cunninghamii Sweet., as of 2002, located within 1 km of each other. A. mangium is a N2-fixing evergreen tree from northern Australia and Papua New Guinea, S. macrophylla is a non-N2-fixing evergreen tree from Central and South America, and Ar. cunninghamii is a conifer from Australia and Papua New Guinea, respectively. The forest conditions of each plot are described by Inagaki et al. [20]. The soil is Haplic Alisol, and the chemical conditions in the surface soil (0–5 cm, 5–15 cm, and 15–30 cm) were virtually the same among the sites, particularly in the sub-surface soil [21].
All litterfall data was obtained from Inagaki et al. [13]. Litterfall samples were collected from March 2002 to February 2005 (3 years), twice per month on the 1st and 15th days, using 1 m × 1 m square litter traps. The litter traps were randomly placed at each site, corresponding to the density of one trap per 0.01 ha. Litter samples were dried and separated into five fractions: species leaves, other leaves, fine wood (<20 mm in diameter), reproductive parts, and miscellaneous. Large wood samples (≥20 mm) were omitted from the analysis because of large fluctuations in values. Fresh leaf data was obtained from Inagaki et al. [22]. Fresh leaf samples were collected in July 2008 using slingshots from as high as possible on five different trees of each species. All samples were dried at 70 °C to a constant weight, and were ground before chemical analyses. Total C and N concentrations were measured using the dry combustion method (NC-22F, Sumitomo chemical, Tokyo, Japan). For P analyses, samples were tested on an ICP-AES (Optima 4300 DV, PerkinElmer, Waltham, MA, USA) using the wet combustion method.
The root-ingrowth core method was used for evaluating the N and P limitations at the plantations [20]. Expanded vermiculite was immersed in a 0.1 M NH4NO3 for N application and in a 0.1 M KH2PO4 solution for P application for 24 h. For combined treatments, a 0.1 M mixed solution of both NH4NO3 and KH2PO4 was used. As the control, vermiculite was immersed in water. We filled 1-mm-mesh polyethylene bags with approximately 100 g of moist vermiculite, that is called as root-ingrowth core [23,24]. Six replicates were prepared for each treatment, and 24 cores were placed at each site. We installed the cores on October 2002. Soil monolyths of 5 cm in diameter and 10 cm in depth (ca. 200 mL) were excavated vertically from the soil surface using augers, and the cores were positioned in the soil surface void. The depth was almost equivalent to the Ah horizon, where most fine roots were found to be distributed. The in-growth cores were collected after 5 months. Penetrating roots were carefully cut away from the cores on site using scissors. At the laboratory, vermiculite and roots were separated using tweezers. The root samples were dried at 70 °C for 3 days and then measured for dry weight.
3. Results and Discussion
3.1. Litterfall
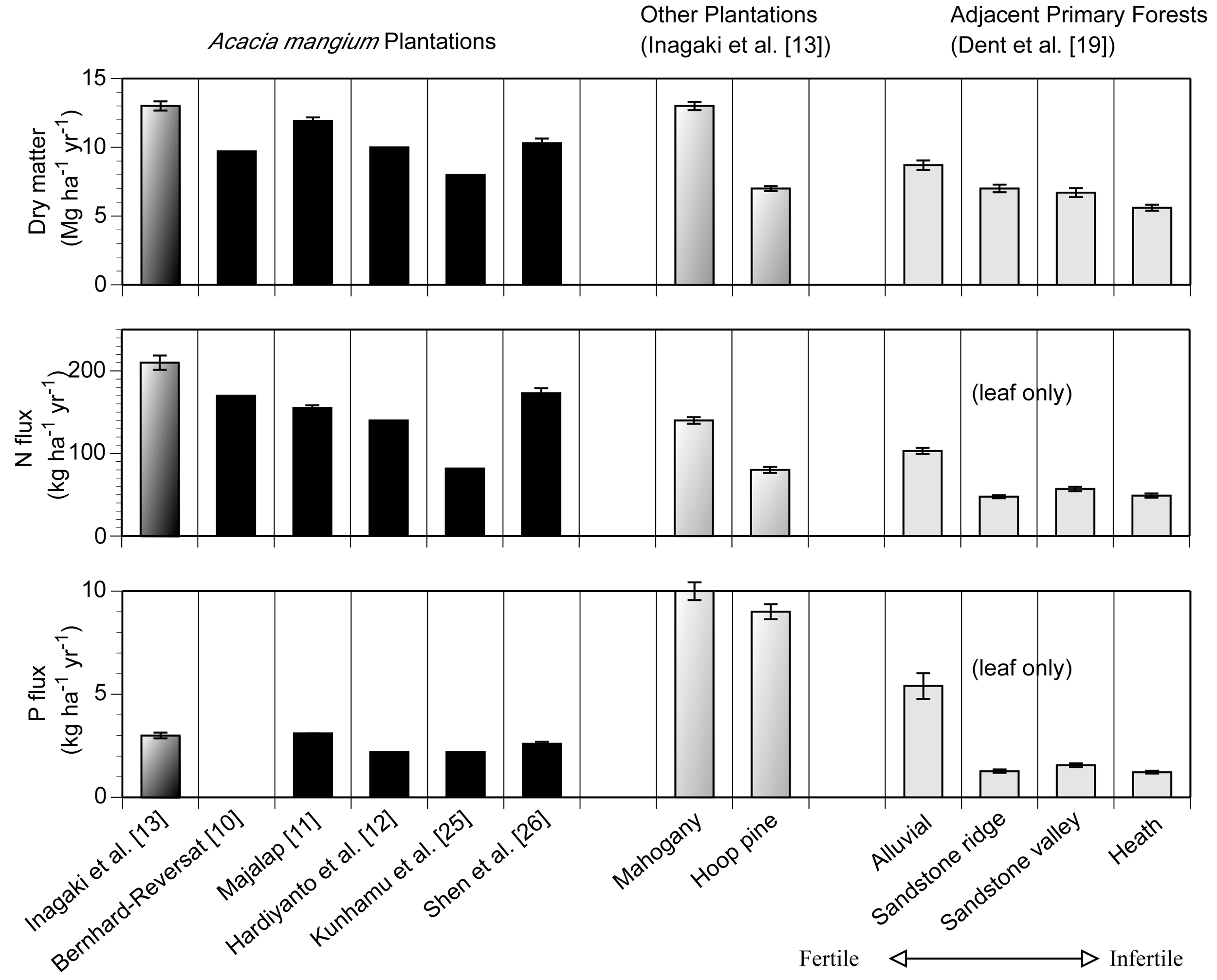
The annual litterfall mass in the A. mangium and S. macrophylla plantations exceeded 12 Mg ha−1 year−1 (Figure 1). These amounts were about 1.6-fold higher than that in the adjacent primary forest under nutrient rich alluvial soil [19], which is located about 5 km away from this study site. The mean annual N flux via litterfall of A. mangium (217 kg ha−1 year−1) exceeded that of S. macrophylla (137 kg ha−1 year−1) because the N concentrations in the litterfall of A. mangium were higher at all fractions than those of S. macrophylla. Across the studies, the annual N flux ranged between 88–228 kg ha−1 year−1 [10-13,25,26]. In contrast to the N flux, the mean annual P flux via litterfall of A. mangium (3.3 kg ha−1 year−1) was considerably smaller than at other plantations and the primary forest at the alluvial site. The P fluxes of A. mangium plantations exhibited a narrower range (2.7–3.3 kg ha−1 year−1) than the N flux. A. mangium plantations likely move larger N and smaller P via litterfall than other forests. Leaf litterfall of A. mangium accounted for 7.1 Mg ha−1 year−1, which corresponded to 54 % of total litterfall. N and P fluxes through leaf litterfall were 123 and 1.14 kg ha−1 year−1, which corresponded to 57 and 35 % of total litterfall, respectively.
3.2. N and P Demand under the Root Ingrowth-core Experiment
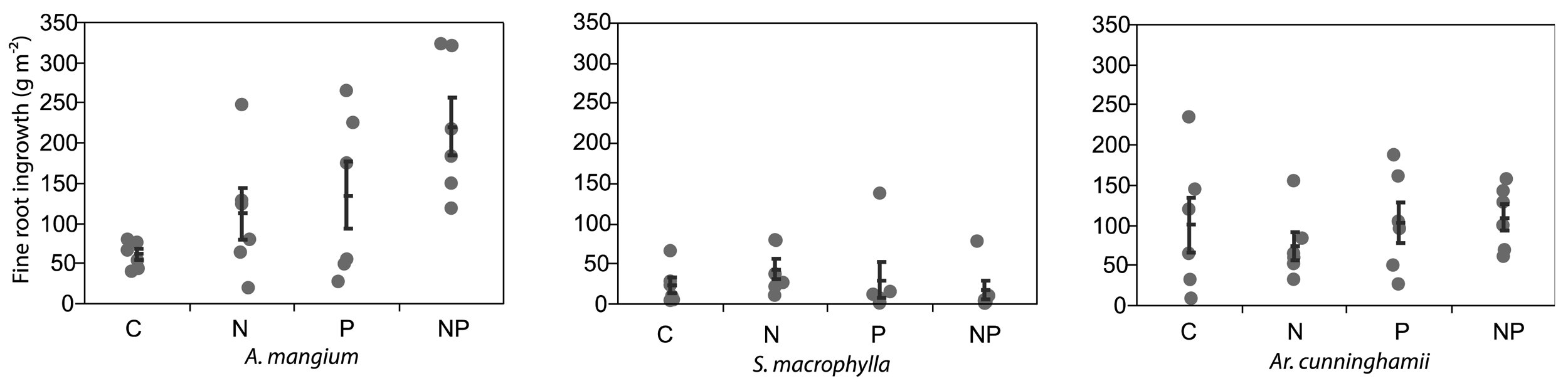
After 5 months from the installation of the root ingrowth-cores, the fine roots of A. mangium in the cores were significantly increased by N + P application (ANOVA and Dunnett's step-down test; P < 0.05; Figure 2). The averages of fine root growth in the cores were doubled by both N and P application. The fine roots of the other two plantations did not increase by N or P applications. Raich et al. [24] reported that root-growth by nutrient applications using the root-ingrowth core method had significant positive correlation with aboveground growth under fertilized conditions. Although the nitrogen availability at the A. mangium site was likely high from the results of litterfall, we considered that A. mangium indicated significant demand for both N and P.
3.3. N and P Resorption and N:P Ratio
We compared the N and P concentrations in fresh leaves collected in July 2008 and the three year averages of July data in leaf litterfall from 2002 to 2004 (Table 1). The N and P concentrations in fresh leaves were highest for A. mangium. In the leaf litterfall, the N concentration was highest but the leaf litterfall was lowest in A. mangium. To express the differences in the degree of N and P resorption, we used an N to P (N:P) ratio. The N:P ratio of A. mangium increased drastically from fresh leaves to leaf litterfall. In the other two species, such a drastic increase did not occur. Therefore, we considered that A. mangium selectively retranslocated high amounts of P, whereas it retranslocated less N.
Killingbeck [27] reported that N2-fixing trees had lower N resorption efficiencies than non-N2-fixing trees, but P resorption efficiency did not differ between both groups. Australian sclerophyll species, developed under P limited soils, tended to have lower P concentrations in senesced leaves [28]. Our results supported the findings of those studies, but A. mangium showed a drastic P resorption, while P was not limiting for two other species (Figure 2).
3.4. Effect of A. Mangium Plantations on Global and Regional N and P Cycling
A. mangium produces a large amount of N in litterfall and enhances the N availability at the forest floor. The N demand of A. mangium is likely high under such N rich conditions. Conversely, A. mangium selectively retranslocates a great deal of P before leaf-fall. N and P utilization by A. mangium differed substantially. These characteristics of A. mangium would be an advantage when growing in the wet tropics, where in general, N availability is high and P availability is low. A. mangium is only competitive under high light conditions [29] and such N and P utilization by A. mangium is likely to be associated with higher photosynthetic production. Large areas of mono-culture A. mangium plantations may alter the cycling of both nutrients. While not only exhibiting the general problems of mono-culture plantation, A. mangium plantations can also cause a potential nutrient imbalance in ecosystems, particularly at the forest floor.
Here we tried to estimate how much N and P flux is altered by A. mangium plantations in the world. The global averages of N and P concentrations in tropical senesced-leaf were 12.5 and 0.4 mg g−1, respectively [30]. Given that N and P concentrations in the leaf-litterfall of tropical forests were equal, the N and P fluxes via litterfall of A. mangium accounted for 140 % of N and only 40% of P compared to average tropical forests. This estimation is concluded from our results of three years averages of N and P concentrations in A. mangium leaf litterfall (17.4 and 0.16, respectively; [22]). Given that all tropical leaf-litterfall was equal to the mean annual leaf-litterfall for A. mangium (7.1 Mg ha−1 year−1), A. mangium plantations in the world (2.1 million ha) resulted in an increase of 73 Gg N year−1 and a reduction of 3.6 Gg P year−1 in litterfall flux. The leaf (needle) litterfall of S. macrophylla and Ar. cunninghamii were 6.4 and 8.0 Mg ha−1 year−1, respectively, and the range of 3 plots was narrower than total litterfall. So this estimation is conservative but contains less uncertainty compared to using the total litterfall data. Other litterfall fractions will increase these differences because leaf-litterfall accounts for only 54 % of total litterfall in an A. mangium forest [13], although the contributions from these fractions are difficult to estimate because the global averages are uncertain. Also, the area of A. mangium plantation could be wider because the species name of Acacia was not certain in the original data source from some countries [1]. This estimation is very rough because the N concentrations of A. mangium varied from 6–17 mg g−1 across the studies [13], but this estimation can show the potential alteration of N and P flux by single species mono-culture plantations. In addition, a part of excess nitrogen through N2-fixation and litterfall can contribute to 4.5 Gg N of N2O flux using the data from Arai et al. [6] (2.23 kg N ha−1 year−1).
Although N2-fixing trees, including A. mangium, are expected to be used as fertilizer trees or protection trees, we recommend mixed planting with other plants or the application of a mixed litter for mulching with lower N:P ratios, e.g., conifers [22]. Moreover, forest researchers and managers should pay more attention to the nutrient conditions of monoculture A. mangium plantations. Further studies are needed to assess the effect of nutrient imbalance at the forest floor of A. mangium plantations regarding litter decomposing soil fauna and microbial communities, for the better understanding and management of fast-growing tropical plantations. Because they could be regulated by stociometric ratios in their habitat [31].
| Nitrogen | Phosphorus | N:P ratio | ||||
|---|---|---|---|---|---|---|
| Site | Fresh leaves (mg g−1) | Leaf litterfall (mg g−1) | Fresh leaves (mg g−1) | Leaf litterfall (mg g−1) | Fresh leaves | Leaf litterfall |
| A. mangium | 29.4 | 21.8 | 1.01 | 0.27 | 29 | 81 |
| S. macrophylla | 19.2 | 16.1 | 0.82 | 0.68 | 23 | 24 |
| Ar. cunninghamii | 12.3 | 14.2 | 0.71 | 1.20 | 17 | 12 |
Modified from Inagaki et al. [22].
Acknowledgments
This study was funded by the Ministry of Agriculture, Forestry and Fisheries of Japan, and supported by the FFPRI Encouragement Model in Support of Researchers with Family Responsibilities.
References
- FAO. Global Planted Forests Thematic Study: Results and Analysis; FAO: Rome, Italy, 2006; p. 168. [Google Scholar]
- Bouillet, J.P.; Laclau, J.P.; Gonçalves, J.L.D.M.; Moreira, M.Z.; Trivelin, P.C.O.; Jourdan, C.; Silva, E.V.; Piccolo, M.C.; Tsai, S.M.; Galiana, A. Mixed-species plantations of Acacia mangium and Eucalyptus grandis in Brazil 2: Nitrogen accumulation in the stands and biological N2 fixation. For. Ecol. Manag. 2008, 255, 3918–3930. [Google Scholar]
- Mercado, A.R.; van Noordwijk, M.; Cadisch, G. Positive nitrogen balance of Acacia mangium woodlots as fallows in the Philippines based on 15N natural abundance data of N2 fixation. Agrofor. Syst. 2011, 81, 221–233. [Google Scholar]
- Norisada, M.; Hitsuma, G.; Kuroda, K.; Yamanoshita, T.; Masumori, M.; Tange, T.; Yagi, H.; Nuyim, T.; Sasaki, S.; Kojima, K. Acacia mangium, a nurse tree candidate for reforestation on degraded sandy soils in the Malay Peninsula. For. Sci. 2005, 51, 498–510. [Google Scholar]
- Cossalter, C.; Pye-Smith, C. Fast-Wood Forestry-Myths and Realities; CIFOR: Jakarta, Indonesia, 2003; p. 54. [Google Scholar]
- Arai, S.; Ishizuka, S.; Ohta, S.; Ansori, S.; Tokuchi, N.; Tanaka, N.; Hardjono, A. Potential N2O emissions from leguminous tree plantation soils in the humid tropics. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Konda, R.; Ohta, S.; Ishizuka, S.; Heriyanto, J.; Wicaksono, A. Seasonal changes in the spatial structures of N2O, CO2, and CH4 fluxes from Acacia mangium plantation soils in Indonesia. Soil Biol. Biochem. 2010, 42, 1512–1522. [Google Scholar]
- Matson, P.; Vitousek, P.M. Ecosystem approach to a global nitrous oxide budget. Bioscience 1990, 40, 667–672. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar]
- Bernhard-Reversat, F. Nitrogen cycling in tree plantations grown on a poor sandy savanna soil in Congo. Appl. Soil Ecol. 1996, 4, 161–172. [Google Scholar]
- Majalap, N. Effects of Acacia Mangium on Soils in Sabah. Ph.D. Dissertation, The University of Aberdeen, Aberdeen, UK, 1999; p. 323. [Google Scholar]
- Hardiyanto, E.; Wicaksono, A. Inter-rotation site management, stand growth and soil properties in Acacia mangium plantations in South Sumatra, Indonesia. Site Management and Productivity in Tropical Plantation Forests: Proceedings of Workshops in Piracicaba (Brazil) 22&26 November 2004 and Bogor (Indonesia) 6&9 November 2006; Nambiar, E., Ed.; CIFOR: Jakarta, Indonesia, 2008; pp. 107–122. [Google Scholar]
- Inagaki, M.; Kamo, K.; Titin, J.; Jamalung, L.; Lapongan, J.; Miura, S. Nutrient dynamics through fine litterfall in three plantations in Sabah, Malaysia, in relation to nutrient supply to surface soil. Nutr. Cycl. Agroecosyst. 2010, 88, 381–395. [Google Scholar]
- Yashiro, Y.; Kadir, W.; Okuda, T.; Koizumi, H. The effects of logging on soil greenhouse gas (CO2, CH4, N2O) flux in a tropical rain forest, Peninsular Malaysia. Agric. For. Meteorol. 2008, 148, 799–806. [Google Scholar]
- Khanna, P.K. Nutrient cycling under mixed-species tree systems in southeast Asia. Agrofor. Syst. 1998, 38, 99–120. [Google Scholar]
- Lee, Y.K.; Lee, D.K.; Woo, S.Y.; Park, P.S.; Jang, Y.H.; Abraham, E.R.G. Effect of Acacia plantations on net photosynthesis, tree species composition, soil enzyme activities, and microclimate on Mt. Makiling. Photosynthetica 2006, 44, 299–308. [Google Scholar]
- Houlton, B.Z.; Wang, Y.P.; Vitousek, P.M.; Field, C.B. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 2008, 454, 327–330. [Google Scholar]
- Ribet, J.; Drevon, J.J. The phosphorus requirement of N2-fixing and urea-fed Acacia mangium. New Phytol 1996, 132, 383–390. [Google Scholar]
- Dent, D.H.; Bagchi, R.; Robinson, D.; Majalap-Lee, N.; Burslem, D.F.R.P. Nutrient fluxes via litterfall and leaf litter decomposition vary across a gradient of soil nutrient supply in a lowland tropical rain forest. Plant Soil 2006, 288, 197–215. [Google Scholar]
- Inagaki, M.; Inagaki, Y.; Kamo, K.; Titin, J. Fine-root production in response to nutrient application at three forest plantations in Sabah, Malaysia: Higher nitrogen and phosphorus demand by Acacia mangium. J. For. Res. 2009, 14, 178–182. [Google Scholar]
- Inagaki, M.; Titin, J. Evaluation of site environments for agroforestry production. In Development of Agroforestry Technology for the Rehabilitation of Tropical Forests; JIRCAS Working Report 60; Gotoh, T., Yokota, Y., Eds.; JIRCAS: Tsukuba, Japan, 2009; pp. 26–31. [Google Scholar]
- Inagaki, M.; Kamo, K.; Miyamoto, K.; Titin, J.; Jamalung, L.; Lapongan, J.; Miura, S. Nitrogen and phosphorus retranslocation and N:P ratios of litterfall in three tropical plantations: Luxurious N and efficient P use by Acacia mangium. Plant Soil 2011, 341, 295–307. [Google Scholar]
- Cuevas, E.; Medina, E. Nutrient dynamics within Amazonian forests II. Fine root growth, nutrient availability and leaf litter decomposition. Oecologia 1988, 76, 222–235. [Google Scholar]
- Raich, J.; Riley, R.; Vitousek, P. Use of root-ingrowth cores to assess nutrient limitations in forest ecosystems. Ecosystems 1994, 24, 2135–2138. [Google Scholar]
- Kunhamu, T.K.; Kumar, B.M.; Viswanath, S. Does thinning affect litterfall, litter decomposition, and associated nutrient release in Acacia mangium stands of Kerala in peninsular India? Can. J. For. Res. 2009, 39, 792–801. [Google Scholar]
- Shen, W.; Lin, Y.; Jenerette, G.D.; Wu, J. Blowing litter across a landscape: Effects on ecosystem nutrient flux and implications for landscape management. Landsc. Ecol. 2011, 26, 629–644. [Google Scholar]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar]
- Osunkoya, O.O.; Othman, F.E.; Kahar, R.S. Growth and competition between seedlings of an invasive plantation tree, Acacia mangium, and those of a native Borneo heath-forest species, Melastoma beccarianum. Ecol. Res. 2005, 20, 205–214. [Google Scholar]
- Yuan, Z.; Chen, H.Y.H. Global trends in senesced-leaf nitrogen and phosphorus. Glob. Ecol. Biogeogr. 2009, 18, 532–542. [Google Scholar]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Inagaki, M.; Ishizuka, S. Ecological Impact on Nitrogen and Phosphorus Cycling of a Widespread Fast-growing Leguminous Tropical Forest Plantation Tree Species, Acacia mangium. Diversity 2011, 3, 712-720. https://doi.org/10.3390/d3040712
Inagaki M, Ishizuka S. Ecological Impact on Nitrogen and Phosphorus Cycling of a Widespread Fast-growing Leguminous Tropical Forest Plantation Tree Species, Acacia mangium. Diversity. 2011; 3(4):712-720. https://doi.org/10.3390/d3040712
Chicago/Turabian StyleInagaki, Masahiro, and Shigehiro Ishizuka. 2011. "Ecological Impact on Nitrogen and Phosphorus Cycling of a Widespread Fast-growing Leguminous Tropical Forest Plantation Tree Species, Acacia mangium" Diversity 3, no. 4: 712-720. https://doi.org/10.3390/d3040712




