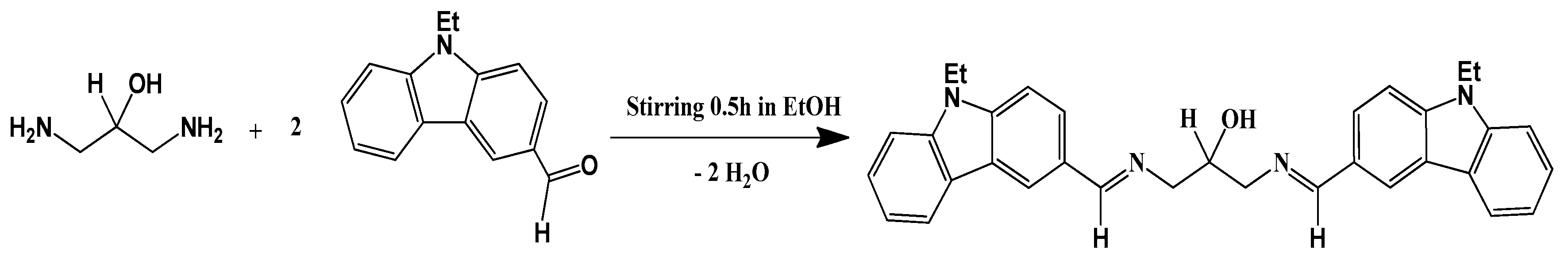
1,3-Bis{[(E)-(9-ethyl-9H-carbazol-3-yl)methylene]amino}propan-2-ol
Abstract
:1. Introduction
2. Results
2.1. Electronic Transfer
2.2. Optimization, Mulliken, and MPE Analysis
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schiff, H.H. The syntheses and characterization of Schiff base. Ann. Chem. Suppl. 1864, 3, 343–349. [Google Scholar]
- Warad, I.; Abedalrazeq, H.; Amer, N.; Al-Nuri, M.; Al Ali, A.; Al-Zaqri, N.; Shivalingegowda, N. 1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol. Molbank 2017, 2017, M971. [Google Scholar] [CrossRef]
- Locke, J.M.; Griffith, R.; Bailey, T.D.; Crumbie, R.L. Competition between cyclisation and bisimine formation in the reaction of 1,3-diaminopropanes with aromatic aldehydes. Tetrahedron 2009, 65, 10685–10692. [Google Scholar] [CrossRef]
- Mukherjee, T.; Pessoa, J.C.; Kumar, A.; Sarkar, A.R. Synthesis, structure, magnetic properties and biological activity of supramolecular copper(II) and nickel(II) complexes with a Schiff base ligand derived from vitamin B6. Dalton Trans. 2013, 42, 2594–2607. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, H.; Fan, X.; Su, Q.; Gao, W.; Mu, Y. Synthesis, structure and electroluminescent properties of Schiff-base boron complex with anilido-imine ligand. J. Phys. Chem. Solids 2009, 70, 92–96. [Google Scholar] [CrossRef]
- Lee, S.A.; You, G.R.; Choi, Y.W.; Jo, H.Y.; Kim, A.R.; Noh, I.; Kim, S.J.; Kim, Y.; Kim, C. A new multifunctional Schiff base as a fluorescence sensor for Al3+ and a colorimetric sensor for CN− in aqueous media: An application to bioimaging. Dalton Trans. 2014, 43, 6650–6659. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, W.; Al Mohanna, N. Membrane sensors based on Schiff bases as chelating ionophores—A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Nwankwo, H.U.; Onwudiwe, D.C. Experimental and theoretical studies of (Z)-N-(2-chlorobenzylidene) naphthalen-1-amine and (Z)-N-(3-nitrobenzylidene)naphthalen-1-amine, and their corrosion inhibition properties. J. Mol. Struct. 2018, 1155, 123–132. [Google Scholar] [CrossRef]
- Jeevadason, A.W.; Murugavel, K.K.; Neelakantan, M.A. Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- Gong, D.; Wang, B.; Jia, X.; Zhang, X. The enhanced catalytic performance of cobalt catalysts towards butadiene polymerization by introducing a labile donor in a salen ligand. Dalton Trans. 2014, 43, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Khan, M.; Ahmad, P.; Al-Nuri, M.; Husein, A.; Haddad, S.; Hammouti, B.; et al. Structural studies on Cd(II) complexes incorporating di-2-pyridyl ligand and the X-ray crystal structure of the chloroform solvated DPMNPH/CdI2 complex. Inorg. Chem. Commun. 2014, 43, 155–161. [Google Scholar] [CrossRef]
- Azam, M.; Warad, I.; Al-Resayes, S.; Alzaqri, Z.; Khan, M.; Pallepogu, R.; Dwivedi, S.; Musarrat, J.; Shakir, M. Synthesis and structural characterization of Pd(II) complexes derived from perimidine ligand and their in vitro antimicrobial studies. J. Mol. Struct. 2013, 1047, 48–54. [Google Scholar] [CrossRef]
- Resayes, S.; Warad, I.; Choudhary, M.; Wahab, A.; Rasheed, S. Heterocyclic Schiff’s Bases as Novel and New Antiglycation Agents. USA Patent 2014/0221429A1, 7 August 2014. [Google Scholar]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Haddad, S. Design and structural studies of diimine/CdX2 (X = Cl, I) complexes based on 2,2-dimethyl-1,3-diaminopropane ligand. J. Mol. Struct. 2014, 1062, 167–173. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Warad, I.; Al-Demeri, Y.; Al-Nuri, M.; Suleiman, M.; Al-Ali, A.; Amereih, S. N-[(1E)-(3-Bromophenyl)methylene]-N-(2-piperidin-1-ylethyl)amine. Molbank 2016, 2016, M903. [Google Scholar] [CrossRef]
- Warad, I.; Al-Noaimi, M.; Haddad, S.; Al-Demeri, Y.; Hammouti, B.; Ben Hadda, T. Rac-(E,E)-N,N′-Bis(2-chlorobenzylidene)-cyclohexane-1,2-diamine. Acta Cryst. 2013, 69, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Abdoh, M.; Warad, I.; Naveen, S.; Lokanath, N.; Salghi, R. Crystal structure of (1E,1’E)-N,N′-(ethane-1,2-diyl)bis[(pyridin-2-yl)-methanimine]. Acta Cryst. 2015, 71, 431–435. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warad, I.; Amer, N.; Abedalrazeq, H.; Al Ali, A.; Al-Zaqri, N.; Elmsellem, H.; Zarrouk, A. 1,3-Bis{[(E)-(9-ethyl-9H-carbazol-3-yl)methylene]amino}propan-2-ol. Molbank 2018, 2018, M986. https://doi.org/10.3390/M986
Warad I, Amer N, Abedalrazeq H, Al Ali A, Al-Zaqri N, Elmsellem H, Zarrouk A. 1,3-Bis{[(E)-(9-ethyl-9H-carbazol-3-yl)methylene]amino}propan-2-ol. Molbank. 2018; 2018(1):M986. https://doi.org/10.3390/M986
Chicago/Turabian StyleWarad, Ismail, Nisreen Amer, Huda Abedalrazeq, Anas Al Ali, Nabil Al-Zaqri, Hicham Elmsellem, and Abdelkader Zarrouk. 2018. "1,3-Bis{[(E)-(9-ethyl-9H-carbazol-3-yl)methylene]amino}propan-2-ol" Molbank 2018, no. 1: M986. https://doi.org/10.3390/M986






