(2-Deoxy-2-{[2-(2-pyridinyl-κN)-4-thiazolecarbonyl-κN3]amino}-α-d-glucopyranose)dichloropalladium(II) Methanol Solvate
Abstract
:1. Introduction
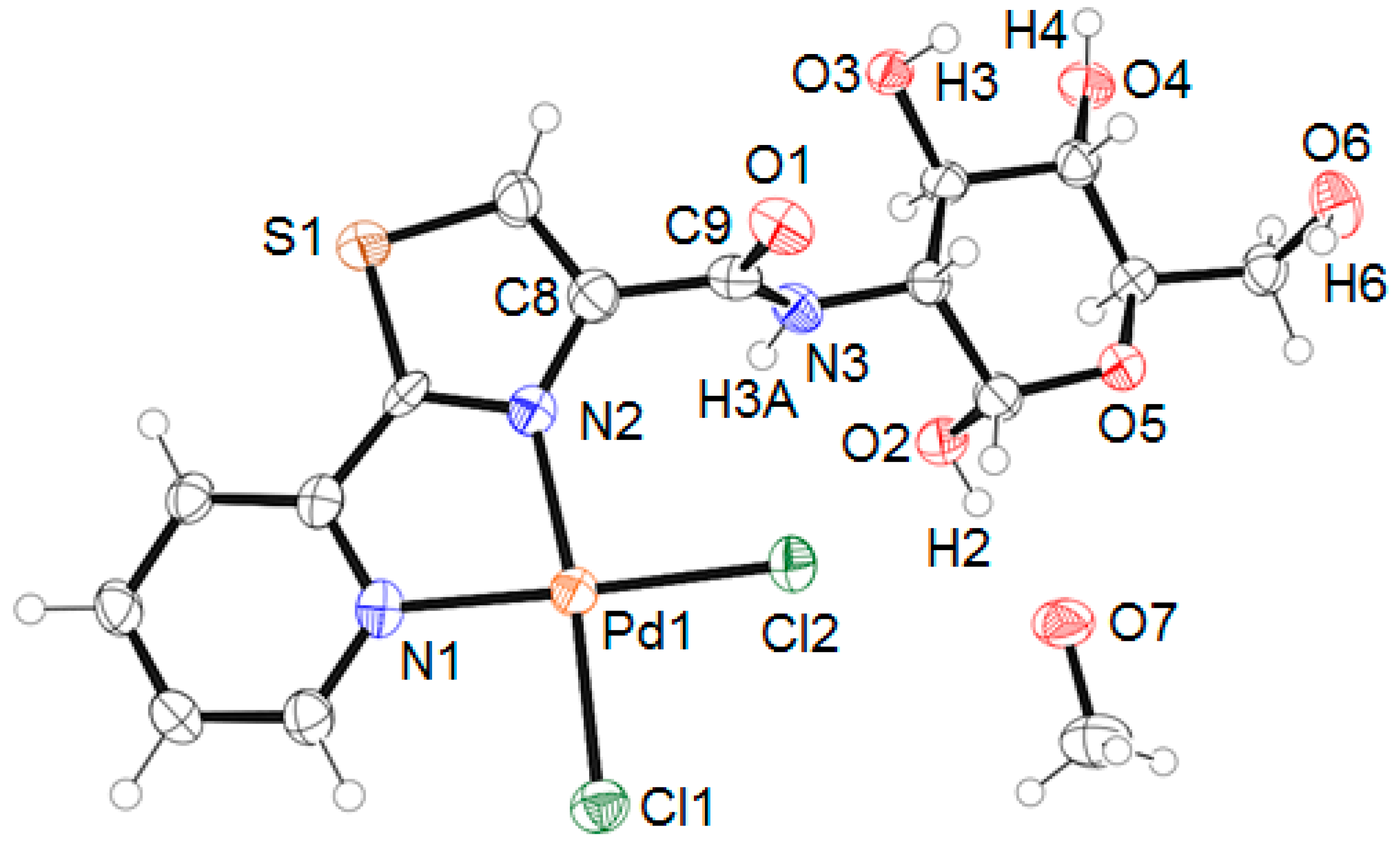
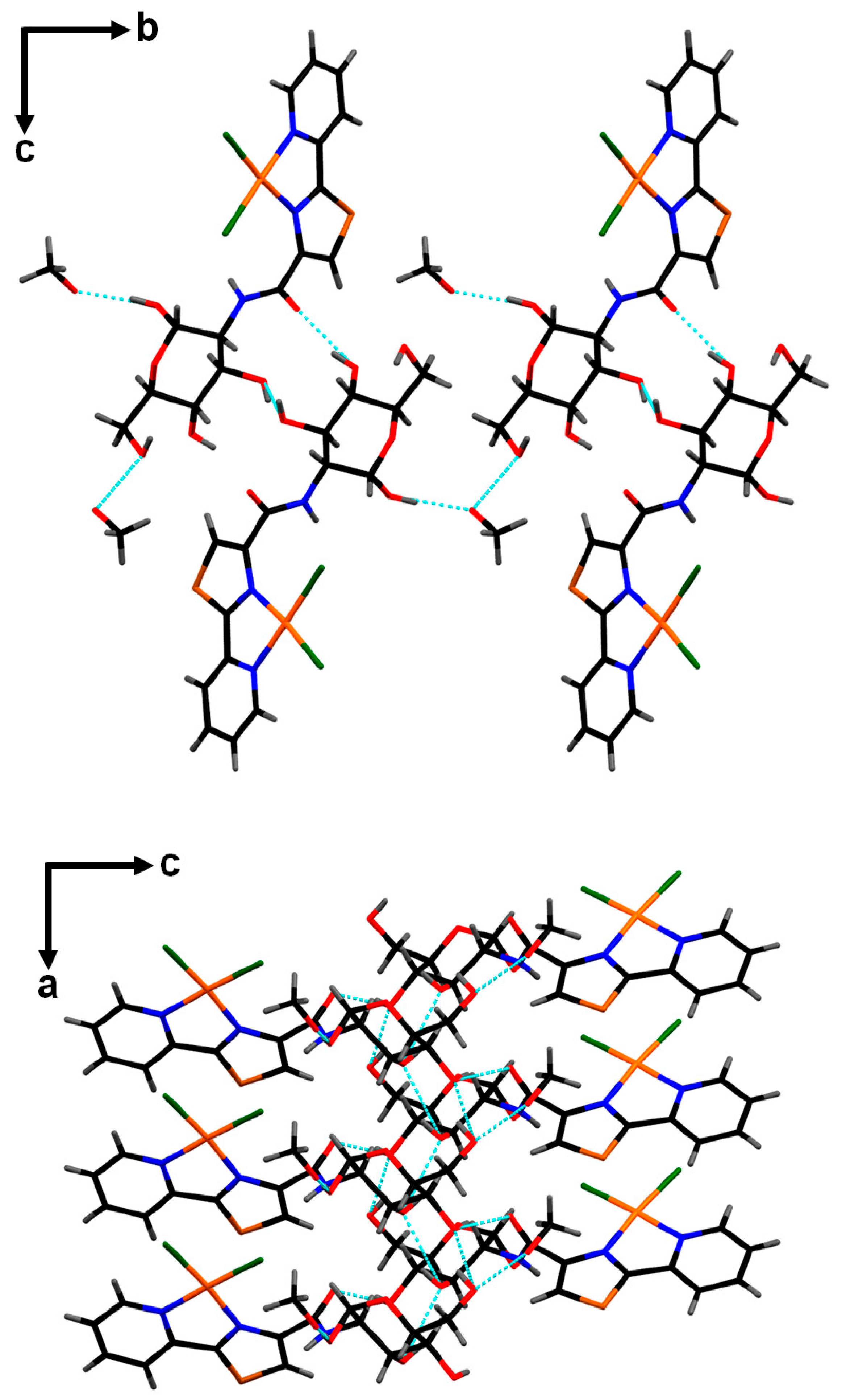
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of Glycoconjugated Palladium(II) Complex 2
3.3. X-ray Diffraction Studies
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yano, S.; Mikata, Y. Recent progress of functional glycoconjugated metal complexes. Bull. Chem. Soc. Jpn. 2002, 75, 2097–2113. [Google Scholar] [CrossRef]
- Storr, T.; Thompson, K.H.; Orvig, C. Design of targeting ligands in medicinal inorganic chemistry. Chem. Soc. Rev. 2006, 35, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Gottschaldt, M.; Schubert, U.S. Prospects of metal complexes peripherally substituted with sugars in biomedicinal applications. Chem. Eur. J. 2009, 15, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.V.S.D.K.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated porphyrins, phthalocyanines, and other porphyrinoids for diagnostics and therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Ohi, H.; Ashizaki, M.; Obata, M.; Mikata, Y.; Tanaka, R.; Nishioka, T.; Kinoshita, I.; Sugai, Y.; Okura, I.; et al. Syntheses, characterization, and antitumor activities of platinum(II) and palladium(II) complexes with sugar-conjugated triazole ligands. Chem. Biodivers. 2012, 9, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Kataoka, H.; Yano, S.; Kikuchi, J.-I.; Tanaka, M.; Nishie, H.; Kinoshita, Y.; Hatano, M.; Nomoto, A.; Ogawa, A.; et al. Anticancer effects of a new aminosugar-conjugated platinum complex agent against cisplatin-resistant gastric cancer. Anticancer Res. 2016, 36, 6005–6010. [Google Scholar] [CrossRef] [PubMed]
- Credico, B.D.; Reginato, G.; Gonsalvi, L.; Peruzzini, M.; Rossin, A. Selective synthesis of 2-substituted 4-carboxy oxazoles, thiazoles and thiazolidines from serine or cysteine amino acids. Tetrahedron 2011, 67, 267–274. [Google Scholar] [CrossRef]
- Newkome, G.R.; Fronczek, F.R.; Gupta, V.K.; Puckett, W.E.; Pantaleo, D.C.; Kiefer, G.E. Acute bonding deviation in square-planar d8 palladium(II) complexes. J. Am. Chem. Soc. 1982, 104, 1782–1783. [Google Scholar] [CrossRef]
- Beer, P.D.; Fletcher, N.C.; Drew, M.G.B.; Wear, T.J. Chloride anion recognition by neutral platinum(II) and palladium(II) 5,5′-bis-amide substituted bipyridyl receptor molecules. Polyhedron 1997, 16, 815–823. [Google Scholar] [CrossRef]
- Crystal Structure 4.2.4: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2000–2016.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, S.; Nomoto, A.; Sakai, Y.; Katao, S.; Kakiuchi, K.; Yano, S.; Ogawa, A. (2-Deoxy-2-{[2-(2-pyridinyl-κN)-4-thiazolecarbonyl-κN3]amino}-α-d-glucopyranose)dichloropalladium(II) Methanol Solvate. Molbank 2017, 2017, M959. https://doi.org/10.3390/M959
Kodama S, Nomoto A, Sakai Y, Katao S, Kakiuchi K, Yano S, Ogawa A. (2-Deoxy-2-{[2-(2-pyridinyl-κN)-4-thiazolecarbonyl-κN3]amino}-α-d-glucopyranose)dichloropalladium(II) Methanol Solvate. Molbank. 2017; 2017(4):M959. https://doi.org/10.3390/M959
Chicago/Turabian StyleKodama, Shintaro, Akihiro Nomoto, Yuta Sakai, Shouhei Katao, Kiyomi Kakiuchi, Shigenobu Yano, and Akiya Ogawa. 2017. "(2-Deoxy-2-{[2-(2-pyridinyl-κN)-4-thiazolecarbonyl-κN3]amino}-α-d-glucopyranose)dichloropalladium(II) Methanol Solvate" Molbank 2017, no. 4: M959. https://doi.org/10.3390/M959




