Methyl N-[1-(Benzoylamino)-2-methoxy-2-oxoethyl]-tryptophanate
Abstract
:1. Introduction
2. Results
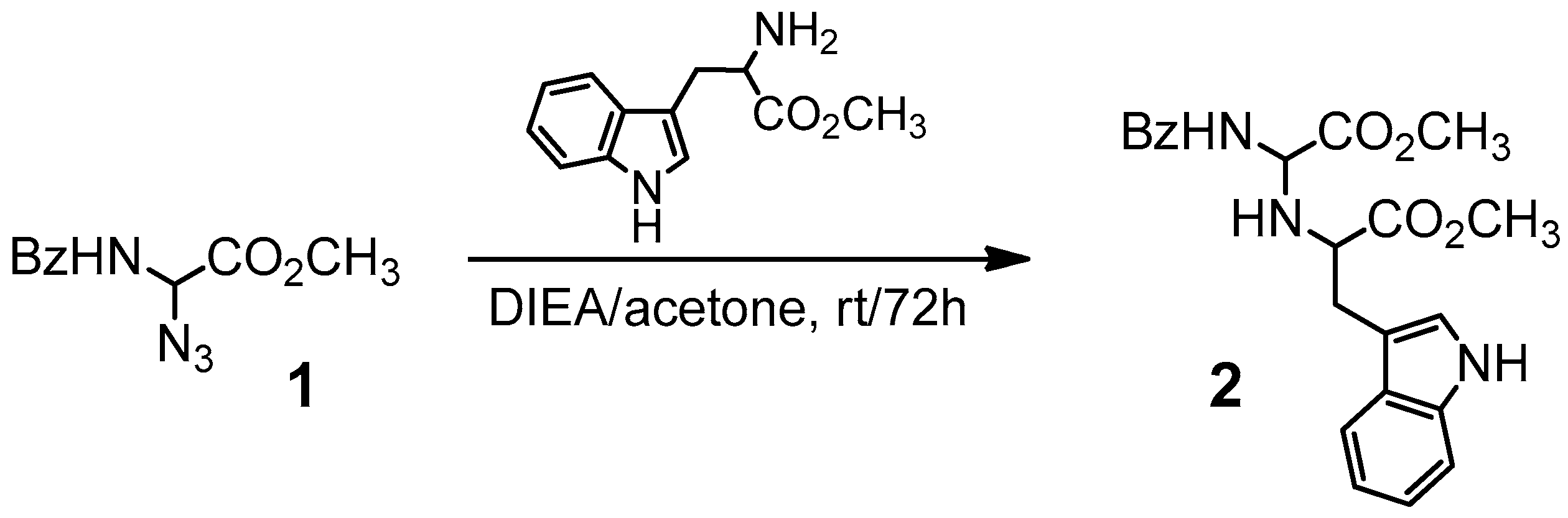
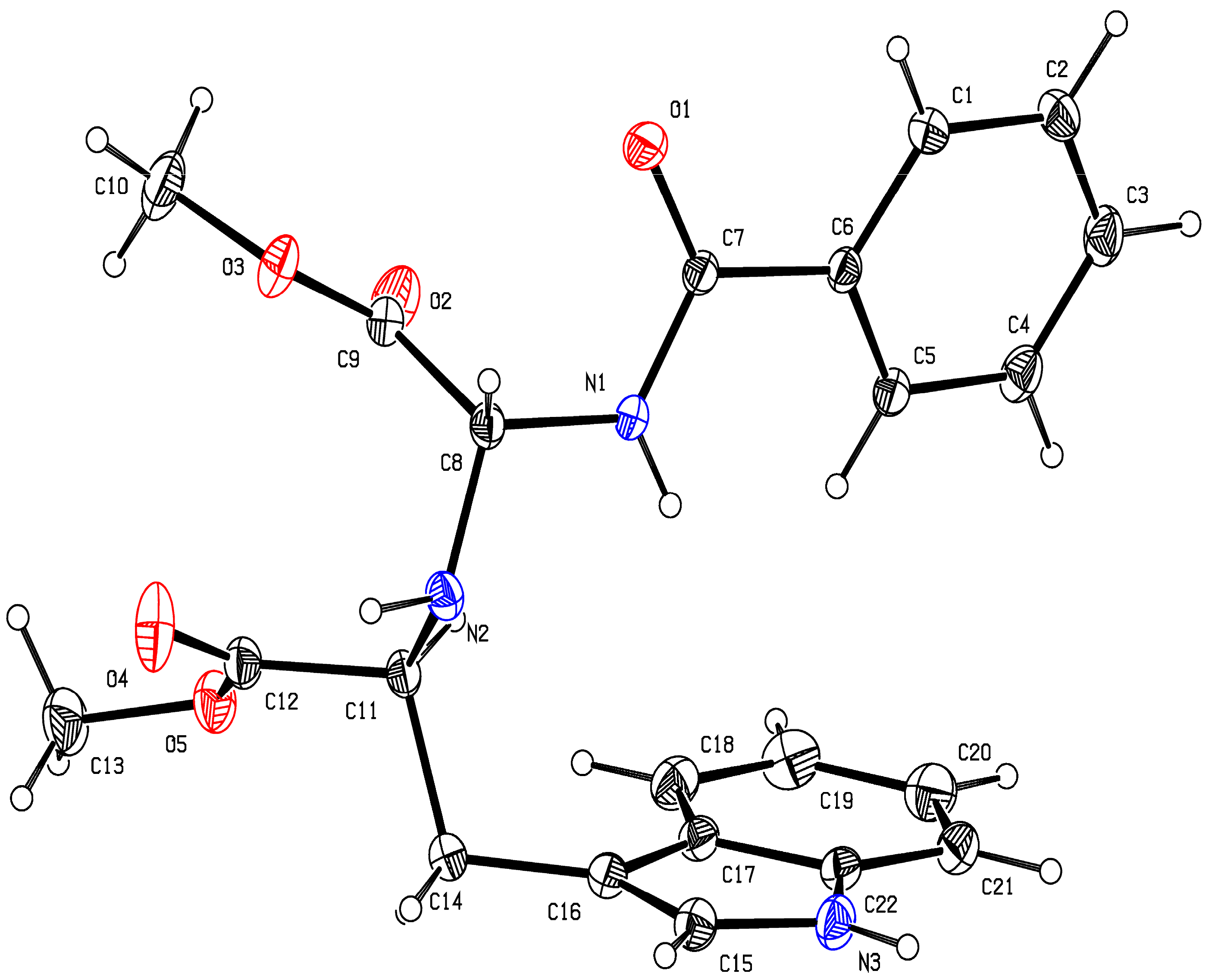
2.1. Chemistry
2.2. Biological Activity
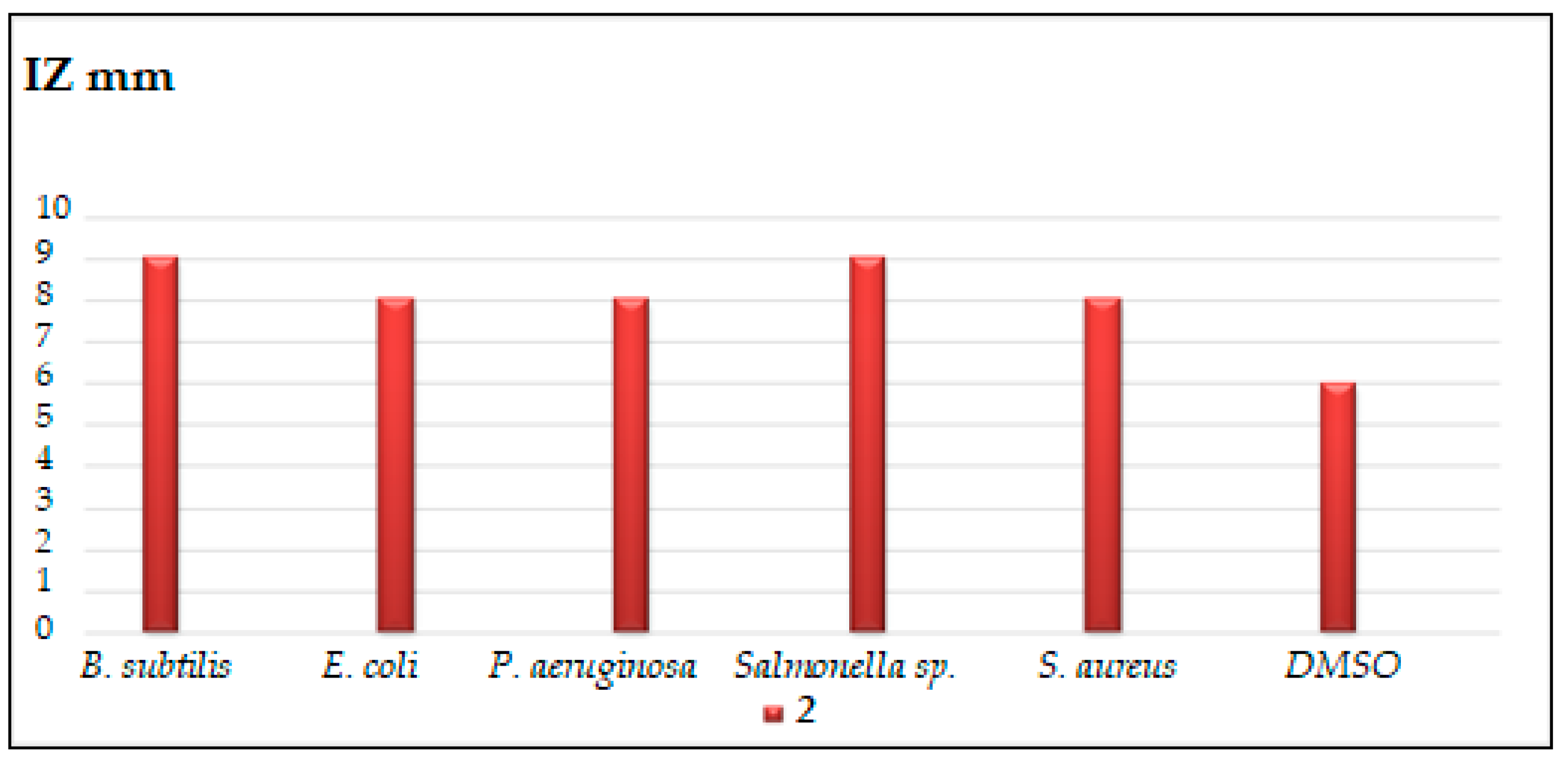
2.2.1. Disc Diffusion Method
2.2.2. Resazurin Microtiter Plate Assay
3. Materials and Methods
3.1. Chemistry
3.2. Biological Activity
3.2.1. Disc Diffusion Method
3.2.2. Resazurin Microtiter Plate Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henry, G.D. De novo synthesis of substituted pyridines. Tetrahedron 2004, 60, 6043–6061. [Google Scholar] [CrossRef]
- Bagley, M.C.; Chapaneri, K.D.; Dale, W.; Xiong, X.; Bower, J. One-pot multistep Bohlmann−Rahtz heteroannulation reactions: Synthesis of dimethyl sulfomycinamate. J. Org. Chem. 2005, 70, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, T.L. Synthesis of aromatic heterocycles. J. Chem. Soc. Perkin Trans. 2001, 2491–2515. [Google Scholar] [CrossRef]
- Leite, A.L.; Lima, R.S.D.; Moreira, R.M.; Cardoso, M.V.; Brito, A.C.G.; Santos, L.M.F.; Hernandes, M.Z.; Kiperstok, A.C.; Limac, R.S.; Soaresc, M.B.P. Synthesis, docking, and in vitro activity of thiosemicarbazones, aminoacyl-thiosemicarbazides and acyl-thiazolidones against Trypanosoma cruzi. Bioorg. Med. Chem. 2006, 14, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, M.J. Acyclic and cyclic aminophosphonic acids: Asymmetric syntheses mediated by chiral sulfinyl auxiliary. J. Organomet. Chem. 2005, 690, 2488–2496. [Google Scholar] [CrossRef]
- Joly, G.D.; Jacobsen, E.N. Thiourea-catalyzed enantioselective hydrophosphonylation of imines: Practical access to enantiomerically enriched α-amino phosphonic acids. J. Am. Chem. Soc. 2004, 126, 4102–4103. [Google Scholar] [CrossRef] [PubMed]
- Prakasha, K.C.; Raghavendra, G.M.; Harisha, R.; Channe, G.D. Design, synthesis and antimicrobial screening of amino acids conjugated 2-amino-4-arylthiazole derivatives. Int. J. Pharm. Pharm. Sci. 2011, 3, 120–125. [Google Scholar]
- Mabrouk, E.H.; Elachqar, A.; Alami, A.; El Hallaoui, A. Synthesis of new racemic α,α-diaminocarboxylic ester derivatives. Molecules 2010, 13, 9354–9363. [Google Scholar] [CrossRef]
- Boukallaba, K.; Elachqar, A.; El Hallaoui, A.; Alami, A.; El Hajji, S.; Labriti, B.; Martinez, J.; Rolland, V. Synthesis of new α-heterocyclic α-aminophosphonates. Phosphorus Sulfur Silicon 2006, 181, 819–823. [Google Scholar] [CrossRef]
- Kelen, M.; Tepe, B. Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresour. Technol. 2008, 99, 4096–4104. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Cruz, C.; Duarte, A.P.; Domingues, F. An alkenylresorcinol derivative from Hakea sericea Fruits and their antimicrobial activity. Nat. Prod. Commun. 2013, 8, 1459–1462. [Google Scholar] [PubMed]
- Sarker, S.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, B.P.; McGrath, J.W.; Quinn, J.P. Development and application of a resazurin-based biomass activity test for activated sludge plant management. Water Res. 2007, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.G.; Fritz, R.; Del Valle, C.; Roura, S.I. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. Soc. Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
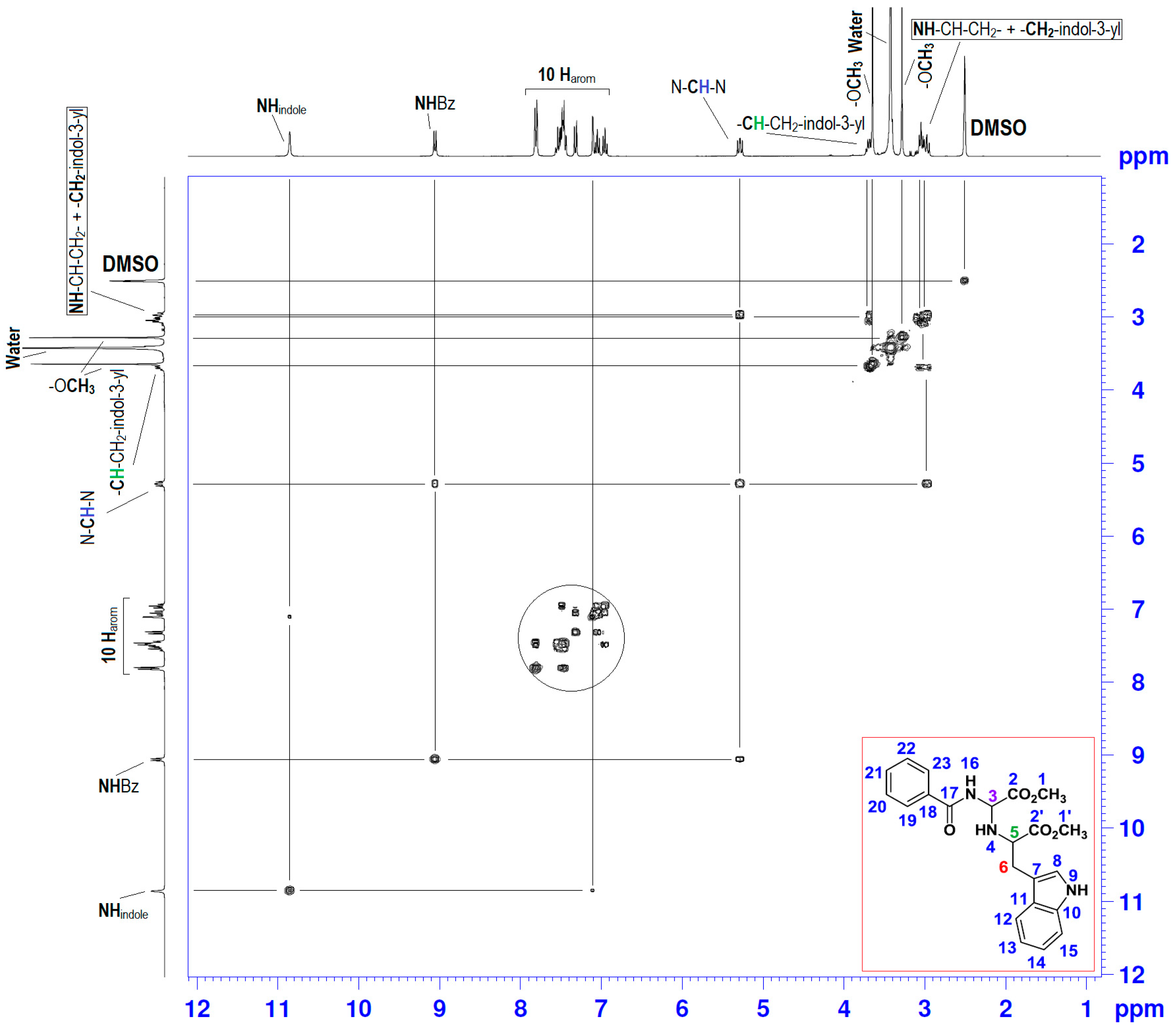
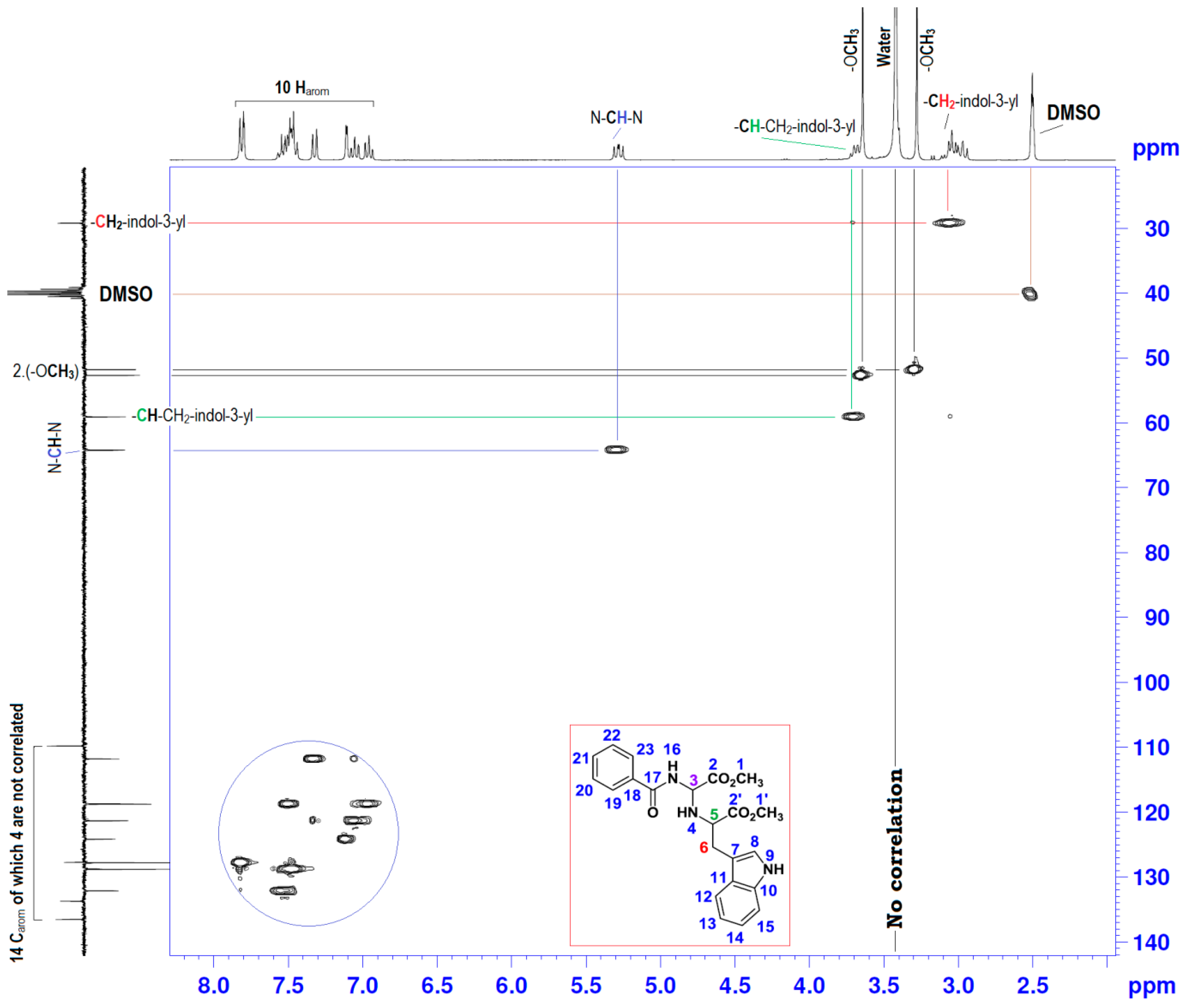
| Position | δH | δC | Correlation H-H | Correlation C-H |
|---|---|---|---|---|
| 1 | 3.28 (s) | 51.78 | 3H1-3H1 | C1-3H1 |
| 2 | - | 166.61 | - | - |
| 1′ | 3.64 (s) | 52.61 | 3H1′-3H1′ | C1-3H1′ |
| 2′ | - | 174.89 | - | - |
| 3 | 5.25–5.31 (dd, J1 = 10.17; J2 = 7.73) | 64.18 | 1H3-1H3; 1H3-1H4; 1H3-1H16 | C3-1H3 |
| 4 | 2.94–3.02 (m) | - | 1H4-1H4; 1H4-1H3; 1H4-1H5 | - |
| 5 | 3.67–3.72 (t, J = 6.90) | 59.06 | 1H5-1H5; 1H5-1H4; 1H5-2H6 | - |
| 6 | 2.94–3.02 (m) | 29.18 | 2H6-2H6; 2H6-1H5 | C6-2H6 |
| 9 | 10.9 (s) | - | 1H9-1H9; 1H9-1H8 | - |
| 16 | 9.1 (s) | - | 1H16-1H16; 1H16-1H3 | - |
| 17 | - | 170.38 | - | - |
| 7, 8; 10–15 and 18–23 | 6.93–7.82 (m) | 109.80–136.48 | 10Har-10Har; 1H8-1H9 | 10Car-10Car |
| Compound 2 | Chloramphenicol | |
|---|---|---|
| E. Coli CIP 53126 | 2.5 | 0.05 |
| S. aureus CIP 483 | 5 | 0.095 |
| S. enterica CIP 8039 | 2.5 | 0.05 |
| B. subtilus CIP 5262 | 2.5 | 0.095 |
| P. aeruginosa CIP 82118 | 2.5 | 0.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karai, O.; El Hamdani, M.; Faraj, H.; Alami, A.; Kabbour, M.R.; El Hallaoui, A.; Bouksaim, M.; Aouine, Y. Methyl N-[1-(Benzoylamino)-2-methoxy-2-oxoethyl]-tryptophanate. Molbank 2017, 2017, M958. https://doi.org/10.3390/M958
Karai O, El Hamdani M, Faraj H, Alami A, Kabbour MR, El Hallaoui A, Bouksaim M, Aouine Y. Methyl N-[1-(Benzoylamino)-2-methoxy-2-oxoethyl]-tryptophanate. Molbank. 2017; 2017(4):M958. https://doi.org/10.3390/M958
Chicago/Turabian StyleKarai, Oumaima, Maha El Hamdani, Hassane Faraj, Anouar Alami, Mohammed Rachid Kabbour, Abdelilah El Hallaoui, Mohammed Bouksaim, and Younas Aouine. 2017. "Methyl N-[1-(Benzoylamino)-2-methoxy-2-oxoethyl]-tryptophanate" Molbank 2017, no. 4: M958. https://doi.org/10.3390/M958






