5,9,11-Trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one from Calophyllum pseudomole
Abstract
:1. Introduction
2. Result and Discussion
3. Experiment Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cytotoxic Assay
3.5. Antiplasmodial Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. 5,9,11-Trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)pyrano[2,3-a]xanthen-12(2H)-one from the stem bark of Calophyllum pseudomole. Molbank 2016, M906. [Google Scholar] [CrossRef]
- Tjahjandarie, T.S.; Saputri, R.D.; Tanjung, M. 5,9,11-Trihydroxy-2,2-dimethyl-3-(2-methylbut-3-en-2-yl) pyrano[2,3-a]xanthen-12(2H)-one from the stem bark of Calophyllum tetrapterum Miq. Molbank 2017, M936. [Google Scholar] [CrossRef]
- Wei, D.J.; Mei, W.L.; Zhong, H.M.; Zeng, Y.B.; Wu, X.D.; Dai, H.F. A new prenylated xanthone from the branches of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011, 13, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Daud, S.B.; Ee, G.C.L.; Malek, E.A.; Teh, S.S.; See, I. A new coumarin from Calophyllum hosei. Nat. Prod. Res. 2014, 28, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.P.; Kulkarni, S.R.; Phalgune, U.D.; Puranik, V.G. New dipyranocoumarin from the leaves of Calophyllum apetalum Willd. Nat. Prod. Res. 2013, 27, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Ha, L.D.; Hansen, P.E.; Duus, F.; Pham, H.D.; Nguyen, L.D. A new chromanone acid from the bark of Calophyllum dryobalanoides. Phytochem. Lett. 2012, 5, 287–291. [Google Scholar] [CrossRef]
- Lim, C.K.; Subramaniam, H.; Say, Y.H.; Jong, V.Y.M.; Khaledi, H.; Chee, C.F. A new chromanone acid from the stem bark of Calophyllum teysmannii. Nat. Prod. Res. 2015, 29, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.C.; Covington, C.D.; Fuller, R.W.; Bokesch, H.R.; Young, S.; Cardellina, J.H.; Kadushin, M.R.; Soejarto, D.D.; Stevens, P.F.; Cragg, G.M. Pyranocoumarins from tropical species of the genus Calophyllum: Chemotaxonomic study of extracts in the national cancer institute collection. J. Nat. Prod. 1998, 61, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.H.; Basualdo, M.C.; Abe, F.; Estrada, M.J.; Soler, C.; Chilpa, R.R. HIV-1 inhibitory compounds from Calophyllum brasiliense leaves. Biol. Pharm. Bull. 2004, 27, 1471–1475. [Google Scholar] [CrossRef]
- Xiao, Q.; Zeng, Y-B.; Mei, W.L.; Zhao, Y-X.; Deng, Y-Y.; Dai, H-F. Cytotoxic prenylated xanthones from Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2008, 10, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Mah, S.H.; Ee, G.C.L.; Teh, S.S.; Sukari, M.A. Calophyllum inophyllum and Calophyllum soulattri source of anti-proliferative xanthones and their structure-activity relationships. Nat. Prod. Res. 2013, 27, 98–101. [Google Scholar]
- Hay, A.; Helesbeux, J.; Duval, O.; Labaied, M.; Grellier, P.; Richomme, P. Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci. 2004, 75, 3077–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. Antioxidant activity of two isomeric benzoxepinderivatives from the stem bark of Bauhinia acuelata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]
- Tanjung, M.; Hakim, E.H.; Syah, Y.M. Prenylated dihydrostilbenes from Macaranga rubiginosa. Chem. Nat. Compd. 2017, 53, 215–218. [Google Scholar] [CrossRef]
- Tanjung, M.; Tjahjandarie, T.S.; Sentosa, M.H. Antioxidant and cytotoxic agent from the rhizomes of Kaempferia pandurata. Asian Pac. J. Trop. Dis. 2013, 3, 401–404. [Google Scholar] [CrossRef]
- Tanjung, M.; Hakim, E.H.; Elfahmi; Latip, J.; Syah, Y.M. Dihydroflavonol and flavonol derivatives from Macaranga recurvata. Nat. Prod. Commun. 2012, 10, 1309–1310. [Google Scholar]
- Tanjung, M.; Saputri, R.D.; Wahjoedi, R.A.; Tjahjandarie, T.S. 4-Methoxy-3-(3-methylbut-2-en-1-yl)-7-(3-methylbut-2-en-1-yl)oxy)quinolin-2(1H)-one from Melicope moluccana T.G. Hartley. Molbank 2017, M939. [Google Scholar] [CrossRef]
- Tjahjandarie, T.S.; Pudjiastuti, P.; Saputri, R.D.; Tanjung, M. Antimalaria and antioxidant activity of phenolic compounds isolated from Erythrina crysta-galli L. J. Chem. Pharm. Res. 2014, 6, 786–790. [Google Scholar]
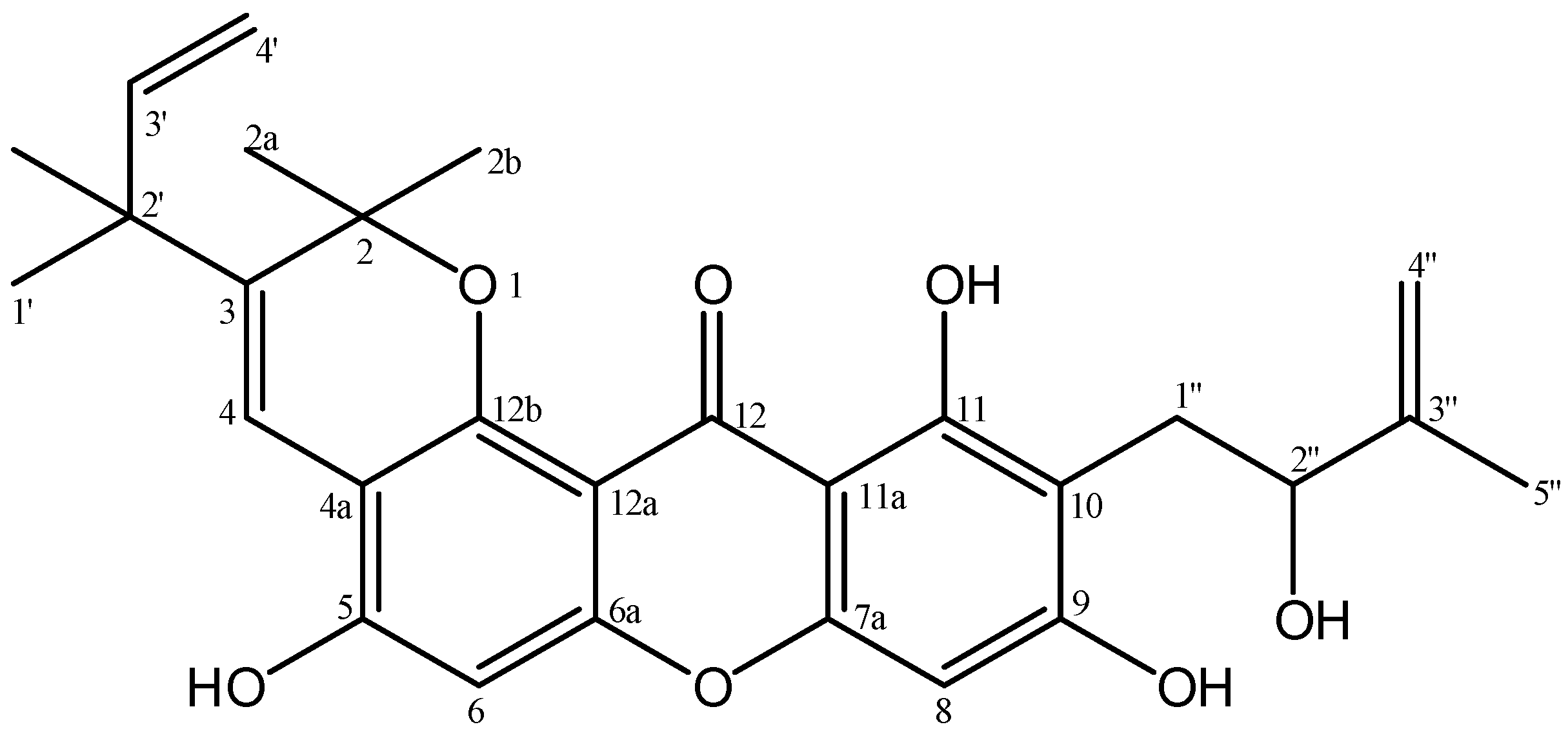
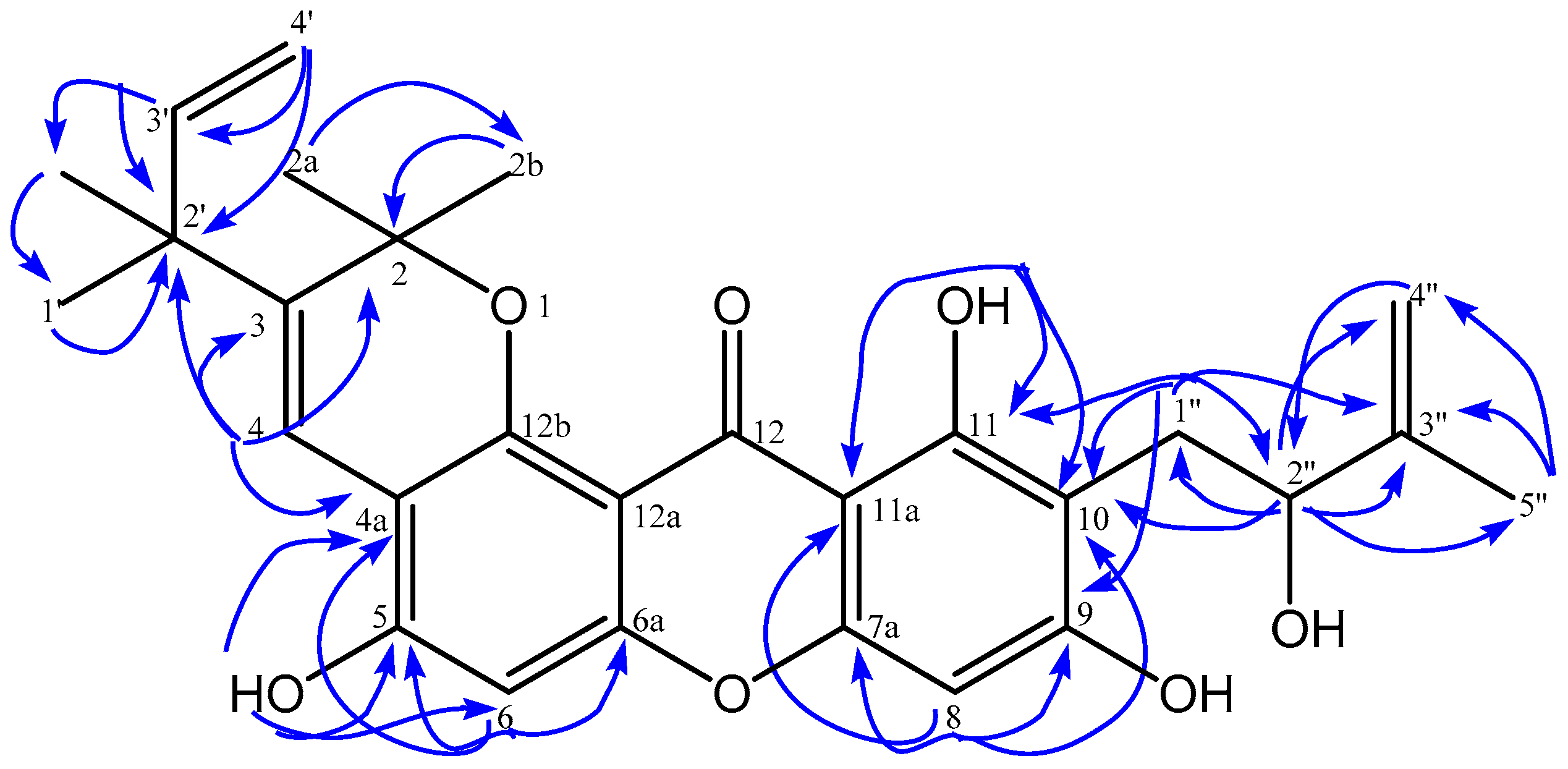
| No.C | δH (Mult, J in Hz) | δC | HMBC |
|---|---|---|---|
| 2 | - | 80.6 | - |
| 2a | 1.54 (s, 3H) | 27.4 | C-2; C-2b; C-12b |
| 2b | 1.53 (s, 3H) | 27.4 | C-2; C-2a; C-12b |
| 3 | - | 135.7 | - |
| 4 | 8.13 (s, 1H) | 118.0 | C-2; C-3; C-4a;C-2′ |
| 4a | - | 108.5 | - |
| 5 | - | 150.6 | - |
| 6 | 6.81 (s, 1H) | 101.8 | C-3; C-4a; C-5; C-6a |
| 6a | - | 153.4 | - |
| 7a | - | 155.8 | - |
| 8 | 6.39 (s, 1H) | 94.5 | C-7a; C-9; C-10; C-11a |
| 9 | - | 163.2 | - |
| 10 | - | 107.4 | - |
| 11 | - | 161.1 | - |
| 11a | - | 103.4 | - |
| 12 | - | 182.5 | - |
| 12a | - | 121.7 | - |
| 12b | - | 148.8 | - |
| 1′ | 1.43 (s, 3H) | 28.3 | C-2′; C-3′;2′-CH3 |
| 2′ | - | 42.7 | - |
| 2′-CH3 | 1.43 (s, 3H) | 28.2 | C-1′; C-2′; C-3′ |
| 3′ | 5.98 (dd, 10.6; 17.6, 1H) | 146.8 | C-1′; C-2′; 2′-CH3 |
| 4′ | 5.08 (d, 10.6, 1H) 5.13 (d, 17.6, 1H) | 112.0 | C-2′; C-3′ |
| 1″ | 3.16 (d, 15.1, 1H) 2.93 (dd, 7.6; 15.1, 1H) | 28.1 | C-9; C-10;C-11; C-2″; C-3″ |
| 2″ | 4.42 (d, 7.3, 1H) | 77.5 | C-10; C-1″; C-3″; C-4″; C-5″ |
| 3″ | - | 146.7 | - |
| 4″ | 4.97 (s, 1H) 4.86 (s, 1H) | 110.2 | C-2″; C-5″ |
| 5″ | 1.87 (s, 3H) | 18.7 | C-2″; C-3″; C-4″ |
| 5-OH | 6.15 (s, 1H) | - | C-4a; C-5; C-6 |
| 9-OH | 9.15 (s, 1H) | - | - |
| 11-OH | 13.48 (s, 1H) | - | C-10; C-11; C-11a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. 5,9,11-Trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one from Calophyllum pseudomole. Molbank 2017, 2017, M961. https://doi.org/10.3390/M961
Tanjung M, Saputri RD, Tjahjandarie TS. 5,9,11-Trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one from Calophyllum pseudomole. Molbank. 2017; 2017(4):M961. https://doi.org/10.3390/M961
Chicago/Turabian StyleTanjung, Mulyadi, Ratih Dewi Saputri, and Tjitjik Srie Tjahjandarie. 2017. "5,9,11-Trihydroxy-10-(2″-hydroxy-3″-methylbut-3″-en-1-yl)-2,2-dimethyl-3-(2′-methylbut-3′-en-2′-yl)-2H,12H-pyrano[2,3-a]xanthen-12-one from Calophyllum pseudomole" Molbank 2017, no. 4: M961. https://doi.org/10.3390/M961





