Abstract
The alkylation of 1,1,2-trimethyl-1H-benzo[e]indole with 2-chloroacetamide, followed by work-up of the reaction mixture with a base and the subsequent treatment of a crude product with acetic acid gives 10a,11,11-trimethyl-10a,11-dihydro-8H-benzo[e]imidazo[1,2-a]indol-9(10H)-one. The structure assignments were based on data from 1H, 13C, and 15N-NMR spectroscopy. The optical properties of the obtained compound were studied by UV–Vis and fluorescence spectroscopy.
1. Introduction
1,1,2-Trimethyl-1H-benzo[e]indole is an important precursor for the synthesis of building blocks that have wide application in the preparation of a number of near-infrared fluorescence emitting dyes. Alkylation of 1,1,2-trimethyl-1H-benzo[e]indole with various alkylating agents—such as iodomethane [], iodoethane [], 4-iodobutane [], 1-iodo-3-phenylpropane [], benzyl bromides [,], or 3-iodopropanoic acid [] — affords the corresponding N-quaternary 1,1,2-trimethyl-1H-benzo[e]indolium salts. The reactions are usually performed in aprotic solvents such as acetonitrile or toluene with conventional or microwave heating []. Upon treatment with various electrophilic counterparts, these salts are converted into dyes, which have found wide application for the development of the near-infrared fluorescent probes [,,,,,,]. Furthermore, organic squaraine dyes based on the N-quaternized 1,1,2-trimethyl-1H-benzo[e]indolium salts have been used for the preparation of dye-sensitized solar cells []. It was recently shown by us that the microwave-assisted reaction of 1,1,2-trimethyl-1H-benzo[e]indole with acrylamide or acrylic acid gave functionalized benzo[e]indoline derivatives possessing intense fluorescence and significant Stokes shifts []. The aim of the present work is the synthesis of 10a,11,11-trimethyl-10a,11-dihydro-8H-benzo[e]imidazo[1,2-a]indol-9(10H)-one as a novel fluorescent building block, through the reaction of 1,1,2-trimethyl-1H-benzo[e]indole with 2-chloroacetamide. The structurally similar 9,9a-dihydro-1H-imidazo[1,2-a]indol-2(3H)-one derivatives have found application in the preparation of optical molecular switches [].
2. Results and Discussion
The reaction of 1,1,2-trimethyl-1H-benzo[e]indole 1 with 2-chloroacetamide was carried out in o-xylene at 140 °C (Scheme 1) and gave a solid, which was separated by filtration (Scheme 1). The obtained solid material, which constituted a complex product mixture, was dissolved in water, the solution was basified with solid sodium carbonate and the separated resinous substance was extracted with diethyl ether. After the solvent removal from the organic phase, the residue was dissolved in ethanol, acetic acid was added to the solution, and the mixture was refluxed for several minutes in order to perform ring-closure of the imidazolidine ring. The neutralization of the solution, followed by the extraction and purification of the crude product by column chromatography on silica gel gave the target compound 2 in 30% isolated yield.
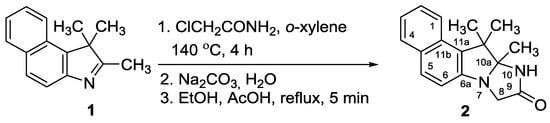
Scheme 1.
Synthesis of 10a,11,11-trimethyl-10a,11-dihydro-8H-benzo[e]imidazo[1.2-a] indol-9(10H)-one.
The structure assignment of 2 was based on spectral data. The molecular formula of compound 2 was found to be C17H18N2O by HRMS. The IR spectrum shows a broad band at 3157 cm−1 for NH and a sharp band at 1698 cm−1 for the C=O group. The broad-band decoupled 13C-NMR spectrum of compound 2, showed resonances for all 17 carbon atoms. The DEPT-90 and 135 spectra indicated the presence of 3 methyl, 1 methylene, and 6 methine carbon atoms. Comparison of the DEPT spectrum with the broad-band decoupled 13C-NMR spectrum, revealed the presence of seven quaternary carbons. The multiplicity edited 1H-13C HSQC spectrum indicated that the methylene protons H-8, which are diastereotopic due to chirality of the molecule and seen as an AB-system with δ = 3.92 and 3.96 ppm in the 1H-NMR spectrum, have one bond connectivities with the carbon C-8 at 55.3 ppm. The data from 1H-13C HMBC spectrum revealed long range correlations of those methylene protons with the quaternary carbons C-6a (at 148.0 ppm), C-10a (at 92.1 ppm), and C-9 (174.1 ppm), respectively. The aforementioned protonated carbon C-8 showed correlation with carbonyl carbon C-9 in the 1,1-ADEQUATE spectrum, which revealed also correlations of C-6 with C-6a, and of 10a-CH3 with C-10a, respectively. The 1H-15N HSQC experiment (optimized for 1JNH = 90 Hz) indicated that the amide N-H proton at 7.83 ppm has one-bond connectivity with the N-10, which resonates at −243.3 ppm. The resonance for N-10 was also confirmed via the 1H-15N HMBC experiment, which additionally revealed the data for N-7 (−288.7 ppm). Both nitrogen atoms showed appropriate strong couplings to the H-8 and 10a-CH3 protons, and in the case of N-7 it had a weak coupling with the aromatic proton 6-H.
The optical properties of compound 2 were investigated by UV–Vis spectroscopy and fluorimetric measurement. The electronic absorption spectra of compound in THF did not have absorption bands in the visible part of the electronic spectra (Figure 1a). The fluorescence spectra of 2, measured in THF, displayed emission maximum at 418 nm with a Stokes shift of 74 nm (Figure 1b). The fluorescence quantum yield (Φf) of the solution was estimated by the integrating sphere method and gave the Φf value of 37.6%.
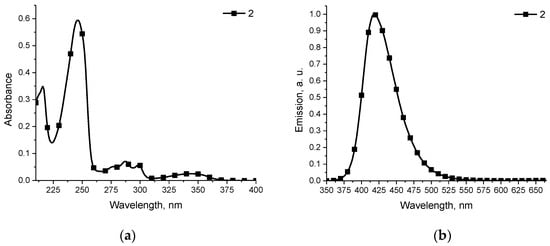
Figure 1.
(a) Absorption spectrum of 2 in THF (0.1 mM, 298 K); (b) Fluorescence emission spectrum of 2 in THF (λex = 310 nm).
3. Materials and Methods
3.1. Materials
All reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification.
3.2. Instrumentation
For thin layer chromatography (TLC), Merck pre-coated TLC plates (Silica gel 60 F254, Merck KgaA, Darmstadt, Germany) were employed. The purification of the products was performed using flash chromatography on a glass column with silica gel (high purity grade 9385, pore size 60 A, 230–400 mesh particle size). The melting points were determined on a Melt-Temp (Capillary Melting point Apparatus, Barnstead International, Dubuque, IOWA, USA) and are uncorrected. The 1H, 13C and 15N-NMR spectra were recorded in CDCl3 solutions at 25 °C on a Bruker Avance III 700 (Bruker BioSpin AG, Fallanden, Switzerland), (700 MHz for 1H, 176 MHz for 13C, 71 MHz for 15N) spectrometer equipped with a 5 mm TCI 1H-13C/15N/D z-gradient cryoprobe. The chemical shifts, expressed in ppm, were relative to tetramethylsilane (TMS). The 15N-NMR spectra were referenced to neat, external nitromethane (coaxial capillary). The full and unambiguous assignments of the 1H, 13C, and 15N-NMR resonances were achieved using standard Bruker software (TopSpin 3.2.7) and a combination of standard NMR spectroscopic techniques, such as DEPT, COSY, TOCSY, NOESY, gs-HSQC, gs-HMBC, H2BC, and 1,1-ADEQUATE. The infrared spectra were recorded on a Bruker TENSOR 27 spectrometer (Bruker Optik GmbH, Ettlingen, Germany), using potassium bromide pellets. The UV–Vis spectra were recorded using 0.1 mM solutions of the compounds in THF on a Shimadzu 2600 UV-Vis spectrometer (Shimadzu EUROPA GmbH, Duisburg, Germany). The fluorescence spectra were recorded on a FL920 fluorescence spectrometer from Edinburgh Instruments (Edinburg Instruments Ltd, Livingston, UK). The PL quantum yields were measured from dilute solutions by an absolute method using Edinburgh Instruments (Edinburg Instruments Ltd, Livingston, UK)integrating sphere excited with a Xe lamp. Optical densities of the sample solutions were ensured to be below 0.1 to avoid reabsorption effects. All optical measurements were performed at rt under ambient conditions. HRMS spectra were recorded with a Bruker micrOTOF - QIII spectrometer (Bruker Daltonik GmbH, Bremen, Germany).
3.3. Synthesis
To a solution of 1,1,2-trimethyl-1H-benzo[e]indole (1) (1.0 g, 4.8 mmol) in o-xylene (5 mL), 2-chloroacetamide (0.51 g, 5.5 mmol) was added and the reaction mixture was heated at 140 °C for 4 h to afford a solid, which was separated by filtration. The solid was dissolved in ethanol (5 mL), the solution was diluted with water (15 mL) and treated with solid sodium carbonate till alkaline pH. The separated resinous substance was extracted with diethyl ether (3 × 15 mL), the organic layers were combined, and the organic solvent was removed under reduced pressure. The residue was dissolved in ethanol (5 mL), glacial acetic acid (2 mL) was added to the solution and the mixture was refluxed for 10 min. Upon cooling to rt, the reaction mixture was diluted with water (30 mL), neutralized with solid sodium carbonate and extracted with diethyl ether (3 × 15 mL). The residue was purified by flash chromatography on silica gel (dichloromethane/methanol, 20:1→ dichloromethane/methanol, 9:1, v/v) to yield the title compound 2.
Yield: 380 mg, (30%), brownish crystals, m. p. 225–226 °C. IR (KBr) ν(cm−1): 3157 (NH), 3054 (CHarom), 2966 (CHaliph), 1698 (C=O), 1351, 1307, 811, 745. UV-Vis (THF) λmax, nm (ε × 103, dm3·mol−1·cm−1): 216 (34.98), 246 (59.49), 287 (7.08); 299 (5.89), 344 (2.49). 1H-NMR (700 MHz, CDCl3): δH ppm 1.40 (s, 3H, 11-CH3), 1.58 (s, 3H, 10a-CH3), 1.75 (s, 3H, 11-CH3), 3.92 (d, J = 16.7 Hz, 1H, 8-CHaHb), 3.96 (d, J = 16.5 Hz, 1H, 8-CHaCHb), 7.02 (d, J = 8.7 Hz, 1H, 6-H), 7.30 (t, J = 7.4 Hz, 1H, 3-H), 7.44 (t, J = 7.6 Hz, 1H, 2-H), 7.71 (d, J = 8.7 Hz, 1H, 5-H), 7.80 (d, J = 8.2 Hz, 1H, 4-H), 7.83 (s, 1H, NH), 7.97 (d, J = 8.5 Hz, 1H, 1-H). 13C-NMR (176 MHz, CDCl3): δC ppm 20.7 (10a-CH3), 23.5 (11-CH3), 25.3 (11-CH3), 48.4 (C-11), 55.3 (C-8), 92.1 (C-10a), 114.3 (C-6), 122.0 (C-1), 123.2 (C-3), 126.7 (C-2), 129.0 (C-11a), 129.62 (C-4), 129.64 (C-5), 130.2 (C-11b), 131.2 (C-4a), 148.0 (C-6a), 174.1 (C=O). 15N-NMR (71 MHz, CDCl3): δN ppm –288.7 (N-7), –243.3 (N-10). HRMS (ESI TOF): [M + Na+]+ found 289.1312. [C17H18N2O + Na+]+ requires 289.1311.
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectrum of 2, Figure S2: 13C-NMR spectrum of 2, Figure S3: 13C and DEPT 13C-NMR spectra of compound 2.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Author Contributions
E.Š.: Experimental synthetic work, NMR interpretation; R.T.: Experimental synthetic work, synthesis planning, NMR interpretation; A.B.: Experimental performance of the NMR, UV–Vis, HRMS spectra and fluorimetric measurements, NMR interpretation, and writing of the manuscript; A.Š.: Synthesis planning, literature research, supervision, and writing of the manuscript.
Conflicts of Interest
The authors declare no conflict interest.
References
- Sun, Y.; Fan, S.; Zhao, D.; Duan, L.; Li, R. A fluorescent turn-on probe based on benzo[e]indolium for cyanide ion in water with high selectivity. J. Fluoresc. 2013, 23, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, H.; Chen, M.; Su, Q.; Feng, W.; Li, F. Mitochondria-targeted near-infrared fluorescent off-on probe for selective detection of cysteine in living cells and in vivo. ACS Appl. Mater. Interfaces 2015, 7, 27968–27975. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Hyun, H.; Tawney, J.G.; Choi, H.S.; Henary, M. Correlating molecular character of NIR imaging agents with tissue-specific uptake. J. Med. Chem. 2015, 58, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yang, X.-F.; Zhong, Y.; Chen, Y.; Li, Z. A mitochondria-targetable near-infrared fluorescent probe for imaging nitroxyl (HNO) in living cells. Dyes Pigm. 2016, 131, 24–32. [Google Scholar] [CrossRef]
- Owens, E.A.; Tawney, J.G.; Henary, M.M. 2-{(E)-2-[(3E)-2-chloro-3-{(2E)-2-[1,1-dimethyl-3-(3-phenylpropyl)-1,3-dihydro-2H-benzo[e]indol-2-ylidene]ethylidene}cyclohex-1-en-1-yl]ethynyl}-1,1-dimethyl-3-(3-phenylpropyl)-1H-benzo[e]indolium iodide. Molbank 2014, M814. [Google Scholar] [CrossRef]
- Sun, C.-L.; Lv, S.-K.; Liu, Y.-P.; Liao, Q.; Zhang, H.-L.; Fu, H.; Yao, J. Benzoindolic squaraine dyes with a large two-photon absorption cross-section. J. Mater. Chem. C 2017, 5, 1224–1250. [Google Scholar] [CrossRef]
- Burke, A.; Schmidt-Mende, L.; Ito, S.; Grätzel, M. A novel blue dye for near-IR ‘dye-sensitised’ solar cell applications. Chem. Commun. 2007, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Winstead, A.J.; Nyambura, G.; Matthews, R.; Toney, D.; Oyaghire, S. Synthesis of quaternary heterocyclic salts. Molecules 2013, 18, 14306–14319. [Google Scholar] [CrossRef] [PubMed]
- Connel, A.; Holliman, P.J.; Davies, M.L.; Gwenin, C.D.; Weiss, S.; Pitak, M.B.; Horton, P.N.; Coles, S.J.; Cooke, G. A study of dye anchoring points in half-squarylium dyes for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Steponavičiūtė, R.; Martynaitis, V.; Bieliauskas, A.; Šačkus, A. Synthesis of new fluorescent building blocks via the microwave-assisted annulation reaction of 1,1,2-trimethyl-1H-benzo[e]indole with acrylic acid and its derivatives. Tetrahedron 2014, 70, 1967–1974. [Google Scholar] [CrossRef]
- Ragaitė, G.; Martynaitis, V.; Kriščiūnienė, V.; Kleizienė, N.; Redeckas, K.; Voiciuk, V.; Vengris, M.; Šačkus, A. Fast and stable light-driven molecular switch based on a 5a,13-methanoindolo[2,1-b][1,3] benzoxazepine ring system. Dyes Pigm. 2015, 113, 546–553. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).