Concerted Solvent Processes for Common Sulfonyl Chloride Precursors used in the Synthesis of Sulfonamide-based Drugs
Abstract
:1. Introduction

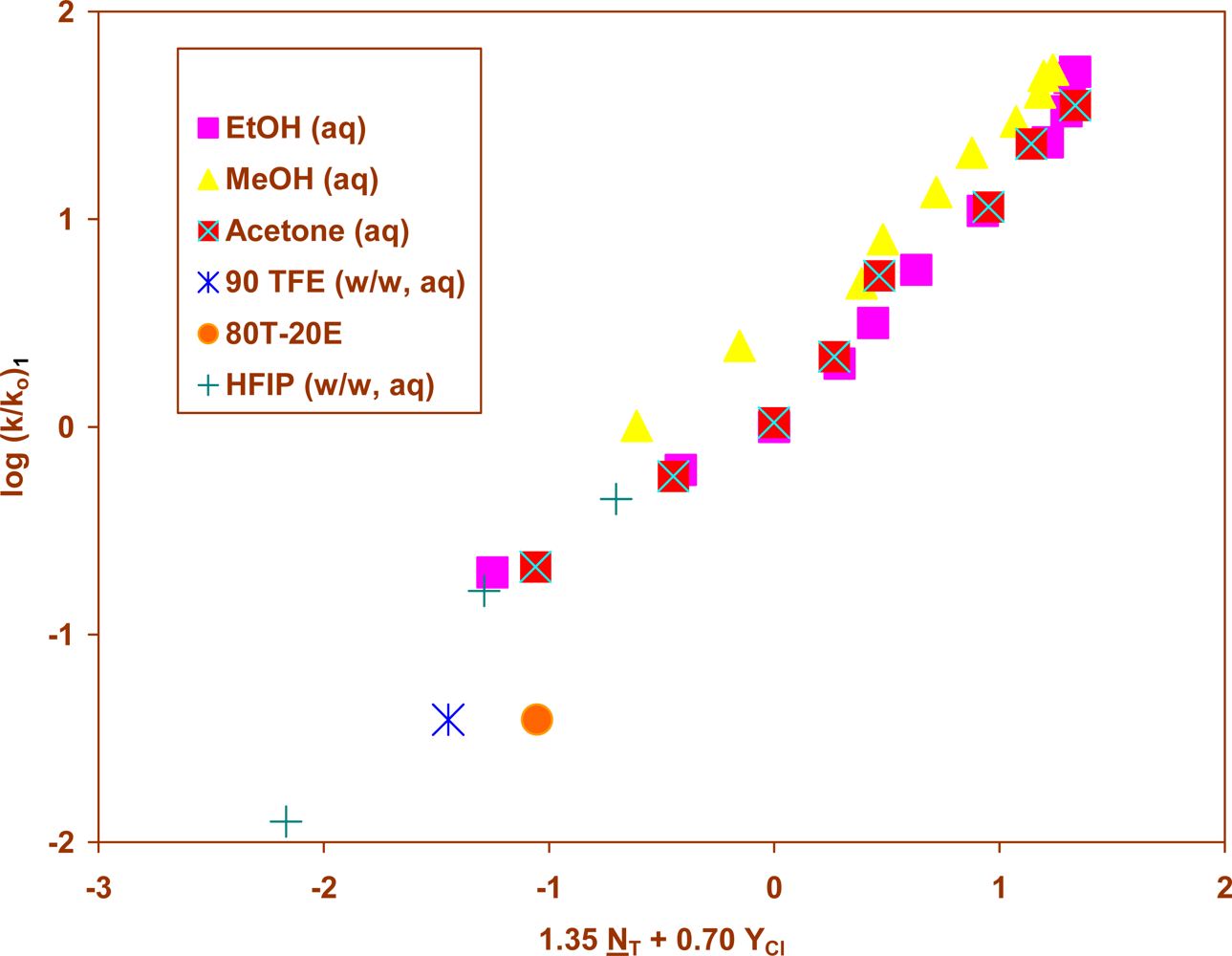
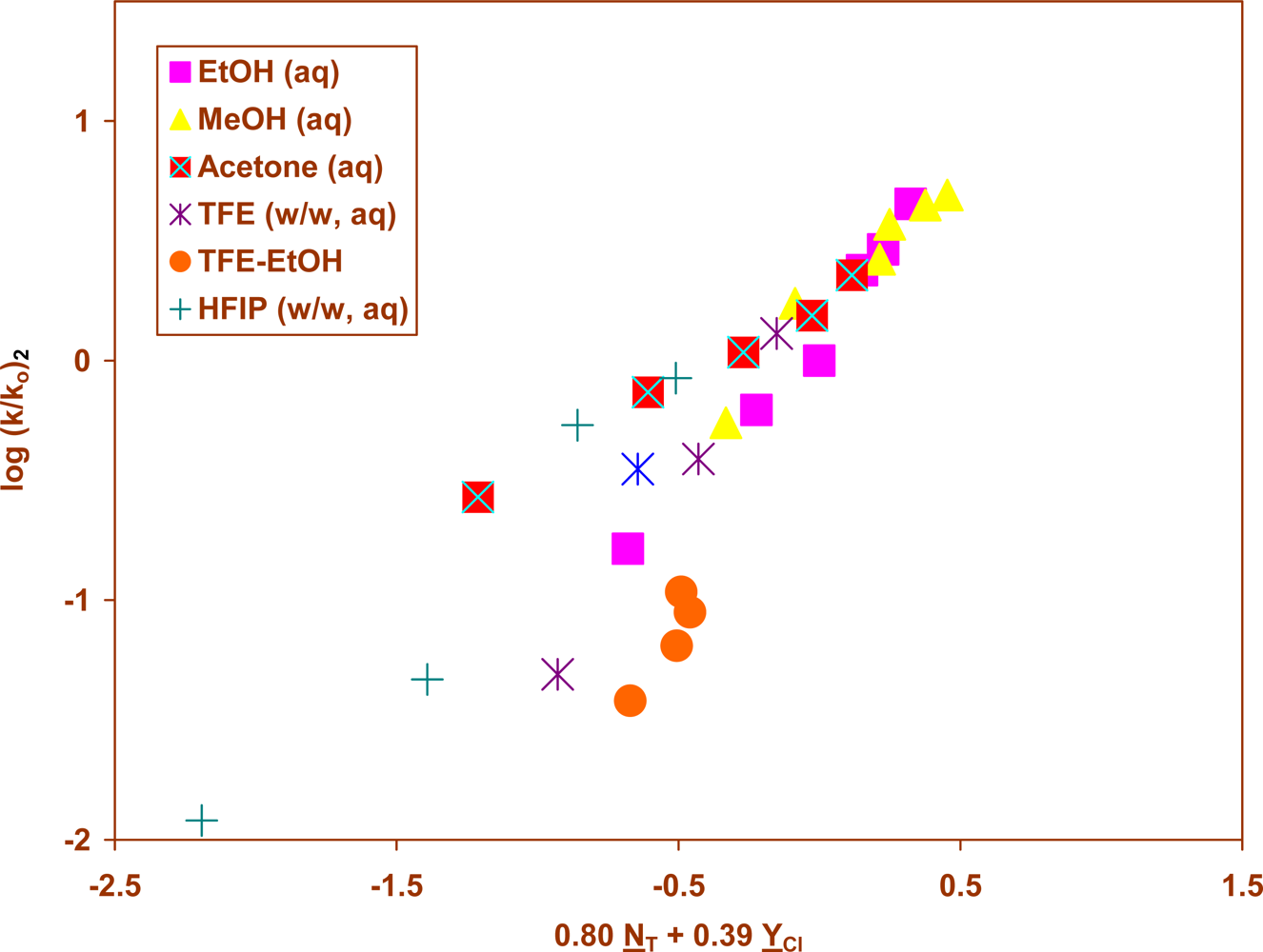
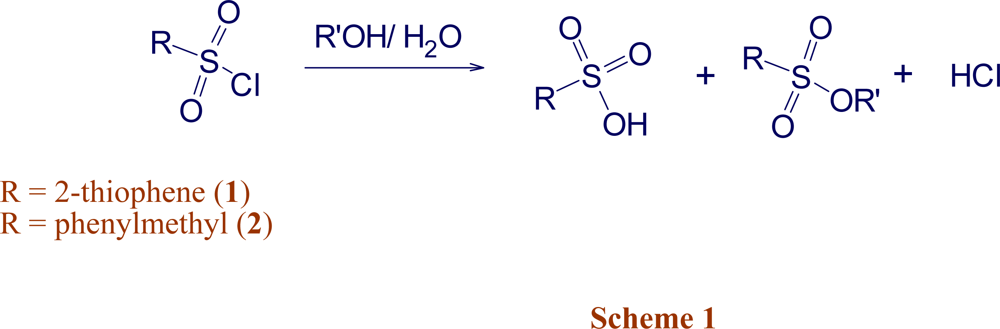
2. Results and Discussion
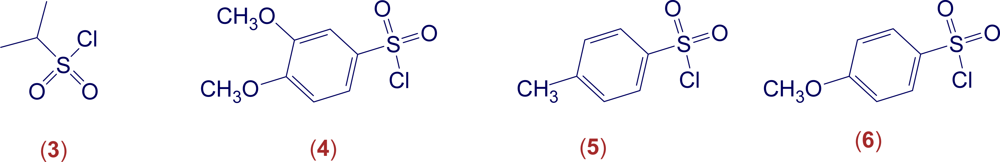
3. Conclusions
4. Experimental Section
Acknowledgments
References and Notes
- Gordon, IM; Maskill, H; Ruasse, M-F. Sufonyl Transfer Reactions. Chem. Soc. Rev. 1989, 18, 123–151. [Google Scholar]
- King, JF; Gill, MS; Klassen, DF. Mechanisms of Reactions of Sulfonyl Compounds with Nucleophiles in Protic Solvents. Pure and Appl. Chem. 1996, 68, 825–830. [Google Scholar]
- King, JF; Lam, JYL; Dave, V. tert-Butyl Cation Formation in the Hydrolysis of 2-Methyl-2-propanesulfonyl Chloride, the Simplest Tertiary Alkanesulfonyl Chloride. J. Org. Chem. 1995, 60, 2831–2834. [Google Scholar]
- King, JF; Gill, MS. Reversible and Irreversible ElcB Mechanisms in the Hydrolysis of 2,2,2,-Trifluorethanesulfonyl Chloride: Carbanion Intermediates in Aqueous Acid. J. Org. Chem. 1998, 63, 808–811. [Google Scholar]
- Seifert, R; Zbirovsky, M; Sauer, M. Hydrolysis of Methane-, Chloromethane-, and Dichloromethanesulfonyl Chlorides. Collection Czechoslv. Chem Commun. 1973, 38, 2477–2483. [Google Scholar]
- King, JF. The Return of Sulfenes. Acc. Chem. Res. 1975, 8, 10–17. [Google Scholar]
- Bendale, P; Olepu, S; Sivyadevara, PK; Bulbube, V; Rivas, K; Nallan, L; Smart, B; Yokoyama, K; Ankala, S; Pendyala, PR; Floyd, D; Lombardo, LJ; Williams, DK; Buckner, FS; Chakrabarti, D; Verlinde, CLMJ; Van Voorhis, WC; Gelb, MH. Second Generation Tetrahydroquinoline-based Protein Farnesyltransferese Inhibitors as Antimatarials. J. Med. Chem. 2007, 50, 4585–4605. [Google Scholar]
- Ding, Y; Smith, KL; Varaprasad, CVNS; Chang, E; Alexander, J; Yao, N. Synthesis of Thiazolone-based Sulfonamides as Inhibitors of HCV NS5B Polymerase. Bioorganic and Medicinal Chemistry Letters 2007, 17, 841–845. [Google Scholar]
- Winstein, S; Grunwald, E; Jones, HW. The Correlations of Solvolysis Rates and the Classification of Solvolyses Reactions into Mechanistic Categories. J. Am. Chem. Soc. 1951, 73, 2700–2707. [Google Scholar]
- Schadt, FL; Bentley, TW; Schleyer, PvR. The SN2-SN1 Spectrum. 2. Quantitative Treatments of Nucleophilic Solvent Assistance. A Scale of Solvent Nucleophilicities. J. Am. Chem. Soc. 1976, 98, 7667–7674. [Google Scholar]
- Kevill, DN. Development and Uses of Solvent Nucleophilicity. In Advances in Quantitative Structure-Property Relationships; Volume 1, Charton, M, Ed.; JAI Press: Greenwich, CT, 1996; pp. 81–115. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Sixty Years of the Grunwald-Winstein Equation: Development and Recent Applications. J. Chem. Res. 2008, 61–66. [Google Scholar]
- Choi, J-C; Oh, J; Kang, DH; Koo, IS; Lee, I. Solvolysis of 2-Thiophenesulfonyl Chloride. J. Korean Chem. Soc. 1993, 37, 695–701. [Google Scholar]
- King, JF; Durst, T. Sulfenes in the Base-Induced Solvolysis of Alkanesulfonyl Chlorides. J. Am. Chem. Soc. 1965, 87, 5684–5692. [Google Scholar]
- Lee, I; Kim, WK. Nucleophilic Displacement at a Sulfur Center (X). Solvolysis of Phenylmethanesulfonyl Chloride. J. Korean Chem. Soc. 1978, 22, 111–116. [Google Scholar]
- Bentley, TW; Llewellyn, G. Yx Scales of Solvent Ionizing Power. Prog. Phys. Org. Chem. 1990, 17, 121–158. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Additional YCl Values and Correlation of the Specific Rates of Solvolysis of tert-Butyl Chloride in Terms of NT and YCl Scales. J. Chem. Res. Synop. 1993, 174–175. [Google Scholar]
- Lomas, JS; D’Souza, MJ; Kevill, DN. Extremely Large Acceleration of the Solvolysis of 1-Adamantyl Chloride Upon Incorporation of a Spiro Adamantane Substituent: Solvolysis of 1-Chlorospiro[adamantane-2, 2′-adamantane]. J. Am. Chem. Soc. 1995, 117, 5891–5892. [Google Scholar]
- Arconia, A; Ballistreri, FP; Tomaselli, GA. Reactions of 2-Thiophenesulphonyl Chloride with Anion and Neutral Nucleophiles. Solvent Effects on Nucleophilic Reactivity Correlations. Tetrahedon 1978, 34, 2545–2556. [Google Scholar]
- Kevill, DN; Park, B-C; Park, K-H; D’Souza, MJ; Yaakoubd, L; Mlynarksi, SL; Kyong, JB. Rate and Product Studies in the Solvolyses of N,N-Dimethylsulfamoyl and 2-Propanesulfonyl Chlorides. Org. Biomol. Chem. 2006, 4, 1580–1586. [Google Scholar]
- Koo, IS; Yang, K; Shin, HB; An, SK; Lee, JP; Lee, I. Stoichiometric Solvation Effects. Solvolysis of Isopropylsulfonyl Chloride. Bull. Korean Chem. Soc. 2004, 25, 699–703. [Google Scholar]
- Koo, IS; Kwon, E; Choi, H; Yang, K; Park, JK; Lee, JP; Lee, I; Bentley, TW. Limitations of the Transition State Variation Model. Part 8 Dual Reaction Channels for Solvolyses of 3, 4-Dimethoxybenzenesulfonyl Chloride. Bull. Korean Chem. Soc. 2007, 28, 2377–2381. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Application of the NT Solvent Nucleophilicity Scale to Attack at Sulfur: Solvolyses of Benzenesulfonyl Chlorides. Collect. Czech. Chem. Commun. 1999, 64, 1790–1796. [Google Scholar]
- Forbes, RM; Maskill, H. Sulfonyl Transfer Reactions: Solvolysis of Arenesulfonyl Chlorides in Aqueous Trifluoroethanol. J. Chem. Soc., Chem. Commun. 1991, 854–856. [Google Scholar]
- Koo, IS; Bentley, TW; Llewellyn, G; Yang, K. Limitations of the Transition-state Variation Method. Part 3. Solvolyses of Electron-rich Benzenesulfonyl Chlorides. In J. Chem. Soc. Perkin Trans.; Volume 2, 1991; pp. 1175–1179. [Google Scholar]
- Bentley, TW; Jones, RO. Substituent Effects on the Formation of Sulfonyl Cations from Sulfonyl Chlorides: Comparisons of Solvolytic Kinetic Data with Calculated Gas Phase Energies. J. Phys. Org. Chem. 2007, 20, 1093–1101. [Google Scholar]
- King, JF; Lam, JYL; Skonieczny, S. Mechanisms of Hydrolysis and Related Nucleophilic Displacement Reactions of Alkanesulfonyl Chlorides: pH Dependence and the Mechanism of Hydration of Sulfenes. J. Am. Chem. Soc. 1992, 114, 1743–1749. [Google Scholar]
- Ingold, CK. Structure and Mechanism in Organic Chemistry, 2nd Ed ed; Cornell University Press: Ithaca, NY, 1969; p. 437. [Google Scholar]
- Kevill, DN; Miller, B. Application of the NT Solvent Nucleophilicity Scale to Attack at Phosphorus: Solvolyses of N, N, N′, N′-Tetramethyldiaminophosphorochloridate. J. Org. Chem. 2002, 67, 7399–7406. [Google Scholar]
- Kevill, DN; Ryu, ZH; Niedermeyer, MA; Koyoshi, F; D’Souza, MJ. Rate and Product Studies in the Solvolyses of Methanesulfonic Anhydride and a Comparison with Methanesulfonyl Chloride Solvolysis. J. Phys. Org. Chem. 2007, 20, 431–438. [Google Scholar]
- Kevill, DN; Anderson, SW. An Improved Scale for Solvent Nucleophilicity Based on the Solvolyses of the S-Methyldibenzothiophenium Ion. J. Org. Chem. 1991, 56, 1845–1850. [Google Scholar]
- Kevill, DN; Abduljaber, MH. Correlation of the Ratio of Solvolysis of Cyclopropylcarbinyl and Cyclobutyl Bromides Using the Extended Grunwald-Winstein Equation. J. Org. Chem. 2000, 65, 2548–2554. [Google Scholar]
- Frost, AA; Pearson, RG. Kinetics and Mechanism – a Study of Homogeneous Chemical Reactions, 2nd Ed. ed; Wiley: New York, 1961; pp. 49–50. [Google Scholar]
| Solvent (%)a | 106k, s−1 | NTb | YClc | |
|---|---|---|---|---|
| 25.0°C | 45.0°C | |||
| 80% EtOH | 78.7±3.2 | 0.00 | 0.00 | |
| 90% TFE | 0.397±0.020 | 3.88±0.11 | −2.55 | 2.85 |
| 80% TFE | 21.6±0.7 | −2.22 | 2.90 | |
| 50% TFE | 87.2±8.5 | −1.73 | 3.16 | |
| 80T-20Ed | 0.986±0.051 | −1.76 | 1.89 | |
| 90% HFIP | 0.129±0.006 | 2.36±0.17 | −3.84 | 4.31 |
| 70% HFIP | 1.67±0.10 | 16.0±0.8 | −2.94 | 3.83 |
| 50% HFIP | 4.63±0.18 | 40.6±2.0 | −2.49 | 3.80 |
| Solvent (%)a | 106k, s−1 | NTb | YClc | k(2)/k(3)d |
|---|---|---|---|---|
| 100% EtOH | 14.0±0.9 | 0.37 | −2.52 | 8.3 |
| 90% EtOH | 53.3±2.2 | 0.16 | −0.94 | 6.3 |
| 80% EtOH | 85.6±0.5 | 0.00 | 0.00 | 6.3 |
| 100% MeOH | 47.2±2.8 | 0.17 | −1.17 | 6.5 |
| 90% TFE | 4.17±0.28 | −2.55 | 2.85 | 6.1 |
| 80% TFE | 30.2±1.4 | −2.22 | 2.90 | 10.1 |
| 70% TFE | 34.1±2.9 | −1.98 | 2.96 | 6.7 |
| 50% TFE | 111±9 | −1.73 | 3.16 | 8.5 |
| 80T-20Ee | 3.24±0.17 | −1.76 | 1.89 | 11.7 |
| 60T-40Ee | 5.54±0.37 | −0.94 | 0.63 | 5.7 |
| 40T-60Ee | 7.69±0.27 | −0.34 | −0.48 | 4.8 |
| 20T-80Ee | 9.27±0.26 | 0.08 | −1.42 | 5.1 |
| 97% HFIP | 1.02±0.09 | −5.26 | 5.17 | (48.6)f |
| 90% HFIP | 4.03±0.29 | −3.84 | 4.31 | 42.3 |
| 70% HFIP | 46.1±2.3 | −2.94 | 3.83 | 21.3 |
| 50% HFIP | 72.4±2.0 | −2.49 | 3.80 | 9.6 |
| Substrate | T°C | na | ℓb | mb | cb | Rc | Fd | ℓ/m |
|---|---|---|---|---|---|---|---|---|
| 4 | 25.0 | 40e | 1.24±0.07 | 0.64±0.03 | 0.14±0.06 | 0.967 | 264 | 1.94 |
| 25.0 | 19f | 1.12 | 0.58 | 0.16 | 1.93 | |||
| 6 | 25.0 | 38g | 1.07±0.08 | 0.60±0.03 | 0.22±0.06 | 0.967 | 254 | 1.78 |
| 5 | 25.0 | 34g | 1.19±0.07 | 0.61±0.02 | 0.20±0.05 | 0.975 | 305 | 1.95 |
| 1 | 25.0 | 34 | 1.35±0.05 | 0.70±0.02 | 0.28±0.05 | 0.983 | 455 | 1.93 |
| 45.0 | 7 | 1.21±0.15 | 0.73±0.13 | −0.07±0.15 | 0.978 | 43 | 1.66 | |
| MeSO2Cl | 45.0 | 39h | 1.17±0.04 | 0.49±0.02 | 0.23±0.05 | 0.981 | 454 | 2.39 |
| 3 | 45.0 | 19i | 1.28±0.05 | 0.64±0.03 | 0.18±0.06 | 0.988 | 333 | 2.00 |
| 2 | 45.0 | 29 | 0.87±0.10 | 0.46±0.06 | 0.09±0.09 | 0.874 | 42 | 1.89 |
| 45.0 | 25j | 0.80±0.06 | 0.39±0.04 | 0.21±0.06 | 0.947 | 95 | 2.05 | |
| (CH3)2NSO2Cl | 25.0 | 32i | 1.20±0.04 | 0.72±0.03 | 0.11±0.04 | 0.985 | 478 | 1.67 |
Share and Cite
D’Souza, M.J.; Yaakoubd, L.; Mlynarski, S.L.; Kevill, D.N. Concerted Solvent Processes for Common Sulfonyl Chloride Precursors used in the Synthesis of Sulfonamide-based Drugs. Int. J. Mol. Sci. 2008, 9, 914-925. https://doi.org/10.3390/ijms9050914
D’Souza MJ, Yaakoubd L, Mlynarski SL, Kevill DN. Concerted Solvent Processes for Common Sulfonyl Chloride Precursors used in the Synthesis of Sulfonamide-based Drugs. International Journal of Molecular Sciences. 2008; 9(5):914-925. https://doi.org/10.3390/ijms9050914
Chicago/Turabian StyleD’Souza, Malcolm J., Lamia Yaakoubd, Stacey L. Mlynarski, and Dennis N. Kevill. 2008. "Concerted Solvent Processes for Common Sulfonyl Chloride Precursors used in the Synthesis of Sulfonamide-based Drugs" International Journal of Molecular Sciences 9, no. 5: 914-925. https://doi.org/10.3390/ijms9050914




