1. Introduction
The syntheses of biologically active sulfonamides usually involve nucleophilic attack of an amine on a sulfonyl chloride, such as benzenesulfonyl chloride (1) or p-nitrobenzenesulfonyl chloride (2). Several thousand sulfonamides have been evaluated in terms of biological activity and this synthesis area remains a very active one. For example, within the last two years (2007-2008) over fifty patent applications have been filed relating to the utility of sulfonamides synthesized from trans-β-styrene-sulfonyl chloride [(E)-2-phenylethenesulfonyl chloride, 3].
Despite the widespread application of nucleophilic substitution reactions of sulfonyl chlorides, there has been considerable uncertainty regarding the detailed mechanism of the process [
1]. Such knowledge would be useful in the selection of optimum reaction conditions. Solvolytic reactions afford a good prototype for these substitutions [
2].
The original Grunwald-Winstein equation was developed in 1948 [
3] so as to give a linear free energy relationship treatment of the specific rates (first-order rate coefficients) for solvolysis of initially neutral substrates reacting by an ionization (S
N1 + E1) mechanism. The equation can be
expressed as in equation 1 where
k and
ko are the specific rates of solvolysis in a given solvent and in the standard solvent (arbitrary chosen as 80% ethanol), respectively,
m represents the sensitivity to changes in the solvent ionizing power
Y (arbitrary set at unity for
tert-butyl chloride solvolyses in the early studies), and
c is a constant (residual) term. It has been demonstrated [
3] that different
Y scales are needed for different leaving groups and that the use of adamantyl derivatives minimizes nucleophilic solvation effects at the α-carbon, leading to a purer ionization process than for
t-butyl chloride.
For bimolecular (S
N2 and/or E2) reactions, in which the solvent also acts as a nucleophile or base, the correlation can be extended [
4] to include a term governed by the sensitivity (
l) to changes in solvent nucleophilicity (
N), as shown in equation 2.
Initially, methyl
p-toluenesulfonate was used as the standard substrate to arrive at a solvent nucleophilicity scale termed
NOTs [
5]. This required making an estimate of the sensitivity of the solvolysis of methyl
p-toluenesulfonate towards changes in solvent ionizing power (the
m value of equation 2), which cannot be obtained directly. More recently, to minimize leaving group effects upon specific rate variation as the solvent is varied, a scale, labeled
NT, has been developed [
6] based upon the solvolyses of the
S-methyldibenzothiophenium ion. In these solvolyses, the leaving group is a neutral molecule and the appreciable solvent-leaving group interaction, when the leaving group is anionic, is avoided. The development of solvent nucleophilicity scales has been reviewed [
2,
7].
It is generally accepted that, for most sulfonyl chlorides, the mechanism of solvolysis is bimolecular in character [
1,
2,
8–
22]. There have, however, been claims of S
N1 (ionization) reactions for a few substrates. The solvolyses of
N,
N-dimethylsulfamoyl chloride were considered by Hall [
8] to be unimolecular, and this view was supported by Robertson [
23].
Convincing evidence has been presented that the solvolysis are S
N2 in character [
21,
24,
25]. Similarily, a claim that reactions of alkanesulfonyl chlorides solvolyze, at least in part, by S
N1 reactions in polar solvents [
26] is no longer tenable [
1,
2]. Also, a claim that 2,4,6-trimethylbenzene-sulfonyl chloride solvolyzes by the S
N1 mechanism [
16,
27,
28] has been shown to be incorrect [
1,
2,
17,
18]. At the present time, there does not seem to be any convincing evidence for S
N1 reaction in the solvolysis of a sulfonyl chloride.
Although the solvolyses are established as bimolecular, other more detailed aspects remain to be considered. There has been considerable discussion as to whether the mechanism involves a concerted S
N2 pathway or an addition-elimination (S
AN) pathway and, irrespective of which of these pathways operates, whether attack by one solvent molecule is assisted by a general base catalysis from the involvement of a second solvent molecule. The S
AN mechanism has largely been proposed for reactions with added nucleophiles under what otherwise would be solvolytic conditions [
1] and the mechanism has only occasionally been suggested for solvolytic reactions. In proposing a duality of reaction channels for solvolyses of electron-rich benzenesulfonyl chlorides, it was suggested that one channel involves S
N2 and the other S
AN or a base catalyzed S
N2 reaction [
18]. Subsequent publication by the authors, favor the base catalyzed S
N2 reaction as the second channel [
29,
30], without totally ruling out the possibility of the addition-elimination pathway [
31].
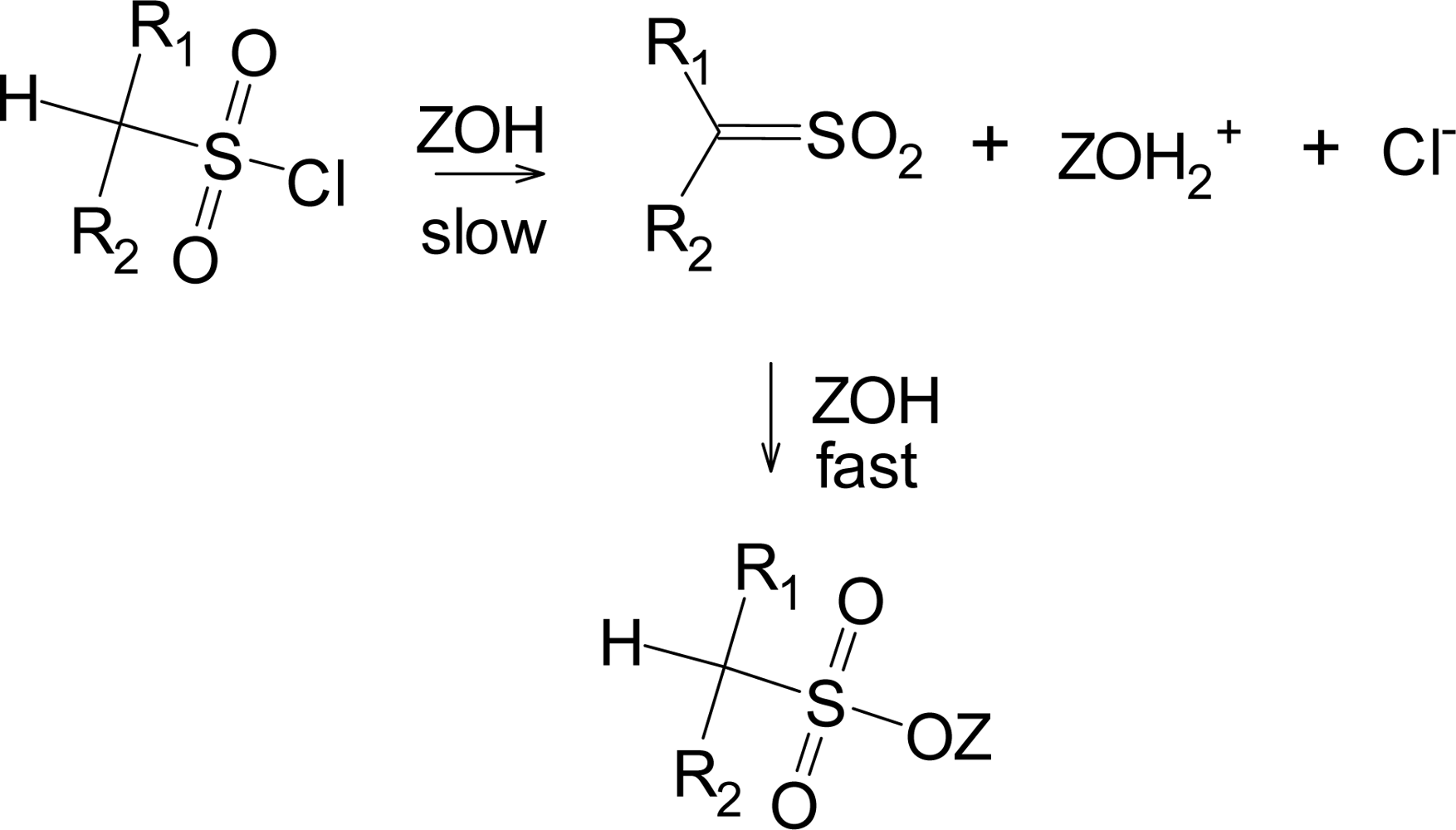
Although the addition-elimination pathway appears to be disfavored for sulfonyl chlorides, the elimination-addition reaction (
Scheme 1) is well established for suitable substrates, primarily those which have a hydrogen plus electron-withdrawing groups on the α-carbon [
11,
32]. The sulfene intermediate formed in the slow step then readily adds solvent (ZOH) to give (in the absence of isotopic labeling) a product identical to the direct substitution product.
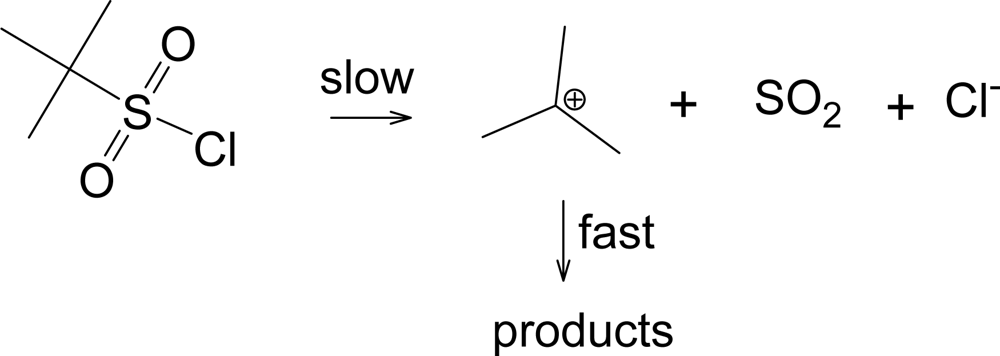
Another pathway has been observed when the R group of RSO
2Cl forms a relatively stable carbocation, such as a
tertiary-butyl group [
33]. This pathway shown in
Scheme 2, leads to the products typically formed from a tertiary carbocation, including
tert-butyl chloride. This solvolysis-decomposition reaction is closely related to that observed [
34] with similarily constituted tert-alkyl chloroformates (ROCOCl), but with loss of SO
2 rather than CO
2.
The three substrates of the present study would not be expected to proceed by the ionization, elimination-addition, or solvolysis-decomposition routes and one can concentrate on whether the rate-determining nucleophilic attack by the solvent involves a concerted or stepwise substitution pathway, and on whether general base catalysis is assisting the process.
2. Results and Discussion
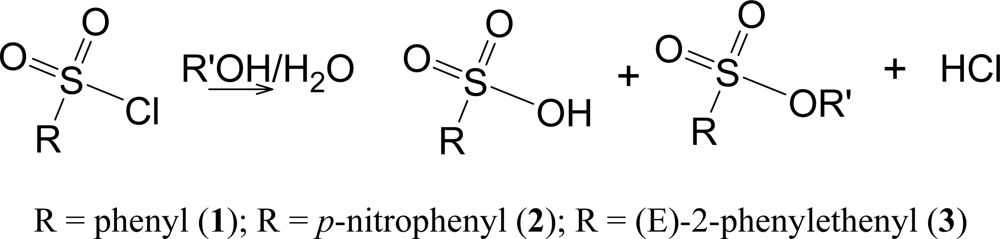
The solvolyses lead to the shown products (
Scheme 3) at rates which are convenient for measurement by the titration of the developed acid. Both the sulfonic acid and the hydrochloric acid titrate as strong acids.
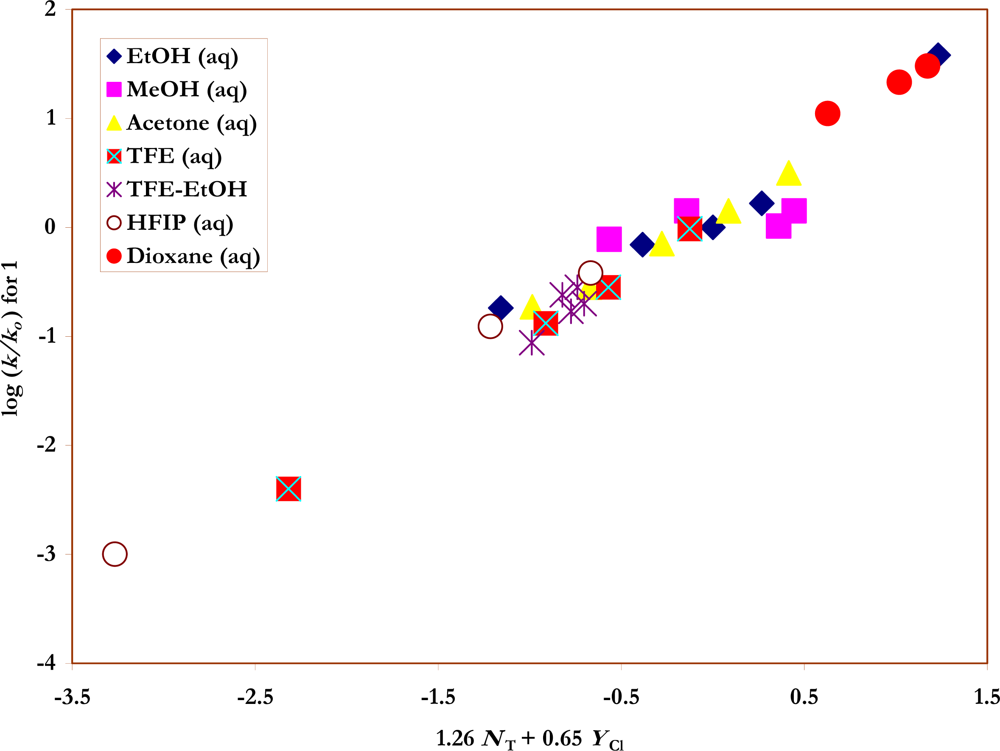
The specific rates already available for
1 and
2 in the literature were complemented by the determination of additional values. For
1 values were already available in aqueous acetone [
35], aqueous dioxane [
28], water [
28,
36], and 97% 2,2,2-trifluoroethanol (TFE) [
22]. Values were also available for ethanol and mixtures of ethanol with water or TFE [
37] but a repeat of these determinations led to specific rates some 20 - 35% lower; the lower values were used in the correlations. Additional values were obtained in aqueous methanol, 80% acetone, 80 – 50% TFE, TFE-ethanol, and aqueous 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP). All of the specific rate values at 35.0 °C for solvolyses of
1, as used in the correlation, are shown in
Table 1.
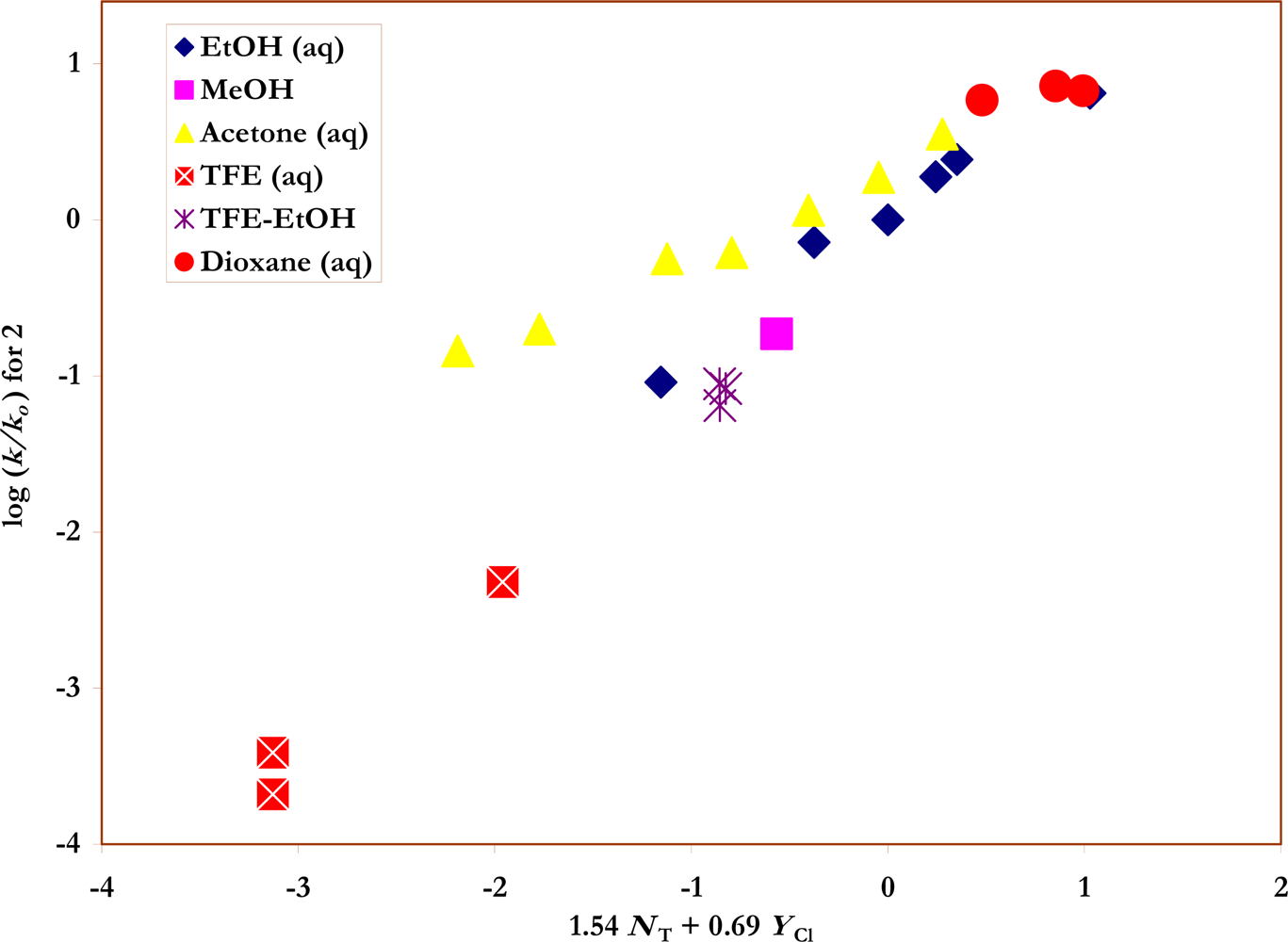
Also shown in
Table 1 are the specific rates of solvolysis for
2 used in the correlations. Values were available for ethanol [
37], water [
28] and their mixtures [
37], for several aqueous acetone [
35] and aqueous dioxane [
28] compositions, and for TFE-ethanol mixtures [
37]. Additional determinations were made in methanol, 80% ethanol, 90% and 80% acetone, and in 100%, 97% and 90% TFE. The
NT [
6,
7] and
YCl [
3,
38,
39] values needed for the application of equation 2 to the specific rate values for
1 and
2 (
Figures 2 and
3) are also listed in the Table.
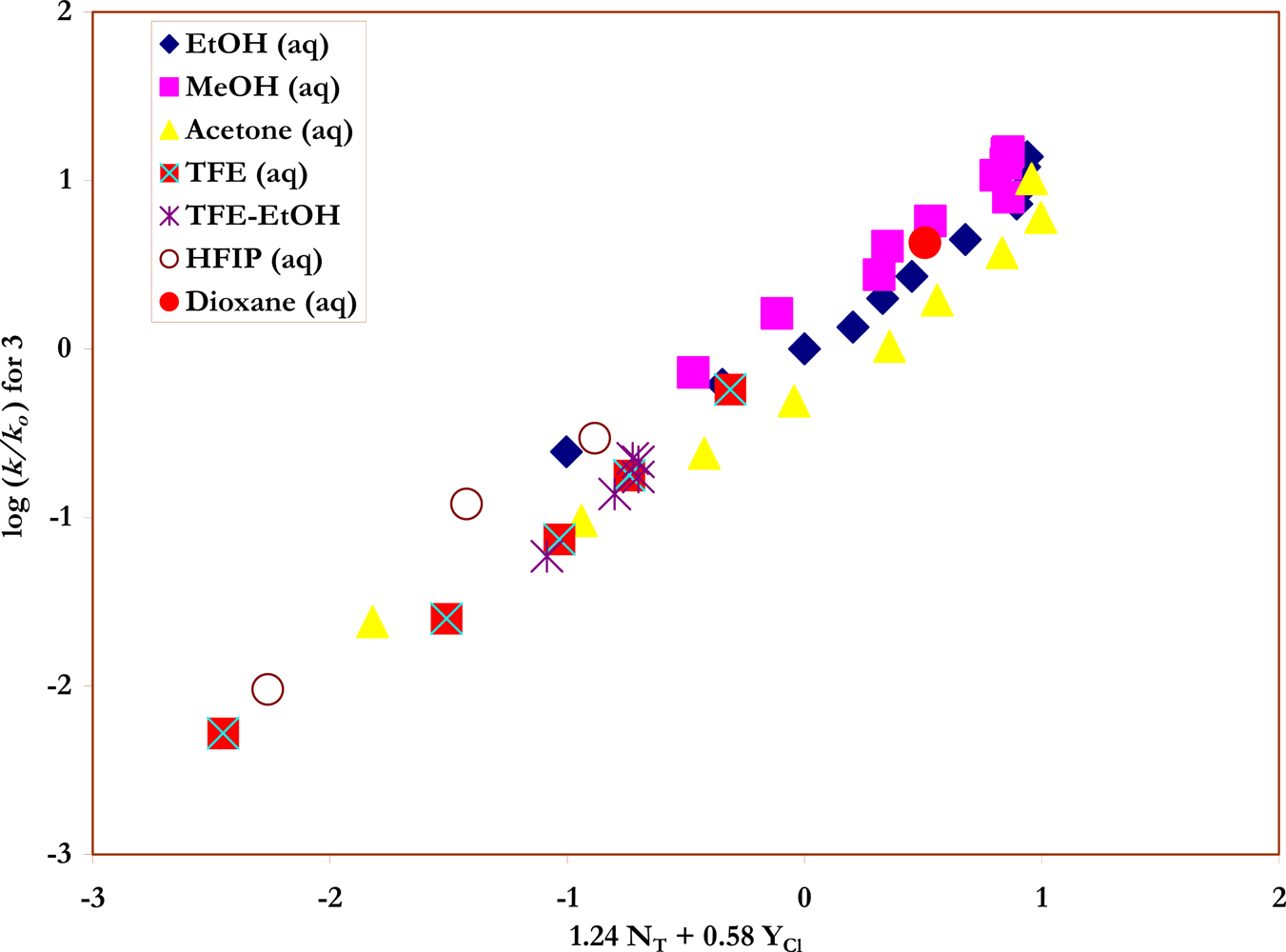
Specific rates of solvolysis of
trans-β-styrenesulfonyl chloride (
3) have not been previously determined and a study was carried out, at 45.0 °C, in terms of increases in conductivity observed as the strong acids are produced (
Scheme 3) in ethanol, methanol, and water, and the full range of aqueous ethanol and methanol compositions. These values are reported in
Table 2. A value obtained in deuterium oxide could be combined with the water value to give a kinetic solvent isotope effect (
kH2O/
kD2O) of 1.46 ± 0.02. Similarly the values in methanol and methanol-
d give a KSIE value (
kMeOH/
kMeOD) of 1.76 ± 0.02. In
Table 3, two additional
NT values and one additional
YCl value for dioxane-water mixtures are abstracted from the literature [
18,
40,
41] and specific rates are tabulated for a series of aqueous acetone and aqueous dioxane compositions.
Table 4 contains the specific rates obtained in TFE-water, HFIP-water, and TFE-ethanol. The ratio of the specific rates in 40% ethanol (
Table 2) and 97% TFE (
Table 4), two solvents of similar ionizing power but very different solvent nucleophilicities, (
k40EtOH/
k97TFE) is 846, indicating that solvent nucleophilicity is an important rate-controlling factor [
3,
22,
42]. The correlation of the specific rates of solvolysis of
3, in all 43 solvents and using the extended Grunwald-Winstein equation, is presented in
Figure 4.
For nine representative solvents, specific rates were also determined at 25.0, 35.0, and 55.0 °C (
Table 5) and the determinations at the four temperatures (45.0 °C values from
Tables 2–
4) were analyzed using the Eyring equation, so as to obtain enthalpies and entropies of activation. The entropies of activation were all appreciably negative (-13 to -34 k cal mol
-1 K
-1), consistent with a bimolecular (or higher molecularity) solvolytic process.
The new values for the specifc rates of solvolysis of
1 and
2, in conjunction with earlier values, allow for correlations using equation 2 for a good selection of solvent types, including the important fluoroalcohol solvents. Similarly, within this study, the specific rates of solvolysis of
3 have been determined in 43 well chosen solvents. The sensitivity values, the residual (intercept) value, and the associated standard errors are reported within
Table 6, together with the multiple correlation coefficient and the
F-test values. With
1 and
3, all of the available values are included in the correlation. For
2, (
Figure 3) it is found that two data points, for 90% and 87% acetone, lie appreciably off the correlation line and two data points (80% and 75% acetone) lie somewhat off the plot. In the table, correlation values are given for all solvents and also with the two and four data points obtained in the acetone-rich solvents omitted. The goodness-of-fit parameters show a progressive improvement for the limited analyses. Since the specific rate in 87% acetone is from an independent source [
35] and the values for 90% and 87% are consistent with each other, the deviations appear to be real and not due to experimental error. The observation that these deviations appear only when the
p-nitro group is present suggests a specific interaction between the group and the acetone-rich solvents. It has previously been observed that the Hammett σ values for a
p-nitro group are somewhat dependent on the solvent [
43,
44]. The benefits of a good mix of solvent type are well illustrated by the previous approximate
l and
m values [
20] for 16 solvents for the solvolyses of
2 of 1.39±0.50 for
l, and 0.65±0.13 for
m improving to values of 1.44±0.11 and 0.57±0.06 for the full 23 solvents now available (
Table 6).
Also included in
Table 6 are the corresponding sensitivity and goodness-of-fit parameters from previous correlations of sulfonyl chlorides [
45] using the extended Grunwald-Winstein equation. With the exception of low values for both
l and
m for the solvolyses of
α-toluenesulfonyl chloride, the
l values vary from a low of 1.07±0.08 for
p-methoxybenzenesulfonyl chloride to a value of 1.54±0.07 for
2, with the values for
1 (1.26±0.05) and
3 (1.24±0.04) being almost identical and towards the middle of the range. The
l/
m ratios are very close in value for all of the entries in the table, varying from a low of 1.67 for
N,
N-dimethylsulfamoyl chloride to 2.39 to methanesulfonyl chloride. Restricting to arenesulfonyl chlorides, the lowest value is 1.78 for
p-MeOC
6H
4SO
2Cl and the highest is 2.23 for
p-NO
2C
6H
4SO
2Cl. The
l value and the
l/
m value variations for the arenesulfonyl chlorides are consistent with a situation in which all members of the family solvolyze by an S
N2 mechanism but with the detailed pathways involving a looser transition state in the presence of the electron supplying
p-MeO group, relative to
1, and varying to a tighter transition state with the electron-withdrawing
p-NO
2 group as the substituent [
37,
46].
A major consideration over the past two decades involves the extent to which the bimolecular nucleophilic substitution reactions of sulfonyl chlorides may be proceeding with general base catalysis. Evidence comes mainly from two sources: the successful analysis in binary water-alcohol solvents in terms of the four possible specific rates expected to contribute if the pathway is termolecular (first-order in substrate and second-order in “solvent” (alcohol and/or water), as was observed for the solvolyses of
2 [
30], and consideration of the magnitude of KSIEs, using the rationale that higher values would be expected with the involvement of two solvent molecules (as general base catalysis and as nucleophile) compared to the involvement of only one as nucleophile [
18,
19,
29,
31,
47–
49]. Initially, water and deuterium oxide were used as the solvents [
47] but solubility problems can arise with organic substrates and methanol and methanol-
d (MeOD) have been found to afford a useful alternative [
19]. The KSIE values are usually slightly higher in value in the methanol solvents [
19,
31,
49] but the solvolyses of methanesulfonyl chloride provide an exception to this generalization [
48].
The KSIE values (
kMeOH/
kMeOD) for
1-
3, are tabulated in
Table 7, where they are compared with values for other sulfonyl chlorides. The values are all appreciably above unity (1.45 to 2.54), consistent with the nucleophilic attack by solvent indicated by the treatment using equation 2. The monosubstituted arenesulfonyl chlorides have KSIEs which vary from 1.58 for the
p-MeO-derivative to 1.72 for the
p-Me-derivative to 1.79 for the parent benzenesulfonyl chloride (
1) to 2.31 for the
p-NO
2-derivative (
2) [
19]. These increases parallel increases in the
k40EtOH/
k97TFE ratio (a simplified treatment for determining the sensitivity to solvent nucleophilicity, which works well provided there is no intervening change in mechanism), with values of 300, 450, 2900, and 15000, respectively. That care must be taken in interrelating the two types of entry in
Table 7 is indicated, however, by very similar (
k40EtOH/
k97TFE) ratios (2010 and 2790) for MeSO
2Cl [
49,
50] and
i-PrSO
2Cl [
21], but very different (
kMeOH/
kMeOD) ratios of 1.62 and 2.54 at 25 oC and 1.51 and 2.41 at 35 oC, respectively. It has been proposed that the KSIE values in
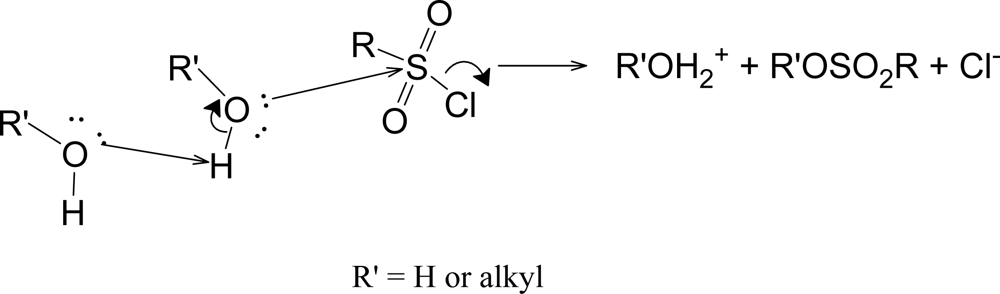
Table 7 are sufficiently large to justify the proposal of the substitution reactions being assisted by general base catalysis (
Scheme 4) [
48,
49]. For a series of
para-substituted benzenesulfonyl chlorides, it has been suggested that both assisted and unassisted S
N2 pathways operate, with the presence of an electron-withdrawing substituent strongly favoring the pathway assisted by general-base catalysis [
19]. At the other extreme, it has been proposed that, in TFE [
17] or in highly ionizing aqueous acetic acid [
51], the solvolyses of
p-(dimethylamino)benzenesulfonyl chloride occur through a concerted bimolecular mechanism with bond breaking running ahead of bond formation in the transition state. A problem with the evaluation of the relevance of general-base catalysis effects in the solvolyses of sulfonyl chlorides is that, although the possibility of analyzing in terms of third-order rate coefficients in the solvolyses of
para-nitrobenzenesulfonyl chloride (
2) is suggestive [
30], there are no substrates for which one can say with a high degree of confidence that the bimolecular solvolyses are either assisted or unassisted by general base catalysis. This leads to a situation in which there are no firmly established reference (standard) KSIE values for solvolyses involving nucleophilic attack at the sulfur atom of a sulfonyl chloride.
3. Conclusions
Additional specific rates of solvolysis, mainly in solvents rich in fluoroalcohol, have been determined at 35.0 °C for benzenesulfonyl chloride (
1) and for
p-nitrobenzenesulfonyl chloride (
2). Correlations, using equation 2, previously carried out with 15 or 16 solvents [
20] and handicapped by a shortage of solvents rich in fluoroalcohol, are considerably improved in the present study, with 29 and 23 solvents, respectively, now available. For
2, there is noticeable deviation from the correlation line for solvents rich in acetone and it is suggested that this could be a consequence of specific interactions between the nitro-group of
2 and the solvent.
A comprehensive study of the specific rates of solvolysis of trans-β-styrenesulfonyl chloride (3) in 43 solvents showed that a very good extended Grunwald-Winstein equation treatment of the data (equation 2) can be carried out with all solvents included. Values typical for solvolyses of sulfonyl chlorides (l = 1.24; m = 0.58) were obtained. The KSIEs (at 45.0 °C) of 1.76 in methanol and methanol-d and of 1.46 in H2O and D2O are also typical values for the solvolyses of sulfonyl chlorides.
Indeed, a comparison (
Table 6) of the results from the correlations using equation 2, for all sulfonyl chlorides studied in this way leads, with one exception, to
l values in the range 1.07 – 1.54 and
m values in the range of 0.49 – 0.72. The one exception,
α-toluenesulfonyl chloride, has low values for both
l and
m, which lead to a typical
l/
m ratio of 2.05 (range of value 1.67 – 2.53).
It appears that all of the sulfonyl chlorides so far investigated in terms of equation 2 are solvolyzing by a common mechanism, generally believed to be S
N2. In support of a bimolecular pathway, very recent calculations [
52] indicate that most sulfonyl chlorides solvolyze with relatively little development of cationic character. The trends of values seen for
l,
m, and the
l/
m ratio (
Table 6) plus the trends in the
k40EtOH/
k97TFE ratio (a simplified measure of the sensitivity towards changes in solvent nucleophilicity) of
Table 7 and, to some extent, the trends in KSIE values for solvolyses in methanol and methanol-
d support a mechanism with variations in detailed transition state structure within a conventional S
N2 process and/or variations in the extent of general base catalysis (
Scheme 4) towards a fundamentally S
N2 process.