Corrrelation of the Specific Rates of Solvolysis of Ethyl Fluoroformate Using the Extended Grunwald-Winstein Equation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Experimental
Acknowledgments
References
- Grunwald, E; Winstein, S; Jones, HW. The Correlation of Solvolyses Rates and the Classification of Solvolysis Reactions into Mechanistic Categories. J. Am. Chem. Soc 1951, 73, 2700–2707. [Google Scholar]
- Schadt, FL; Bentley, TW; Schleyer, PvR. The SN2-SN1 Spectrum 2. Quantitative Treatments of Nucleophilic Solvent Assistance. A Scale of Solvent Nucleophilicities. J. Am. Chem. Soc 1976, 98, 7667–7674. [Google Scholar]
- Kevill, DN. Development and Uses of Scales of Solvent Nucleophilicity. In Advances in Quantitative Structure-Property Relationships; Charton, M, Ed.; JAI Press: Greenwich, CT, 1996; Volume 1, pp. 81–115. [Google Scholar]
- Bentley, TW; Llewellyn, G. Yx Scales of Solvent Ionizing Power. Prog. Phys. Org. Chem 1990, 17, 121. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Correlation of the Rates of Solvolysis of Benzoyl Chloride and Derivatives Using Extended Forms of the Gunwald-Winstein Equation. J. Phys. Org. Chem 2002, 15, 881–888. [Google Scholar]
- D’Souza, MJ; Stant-Boggs, ME; White, R; Kevill, DN. Correlation of the Rates of Solvolysis of 2-Thiophenecarbonyl Chloride. J. Chem. Res., Synop 2003, 775–777. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Correlation of the Rates of Solvolysis of Benzoyl Fluoride and a Consideration of Leaving-Group Effects. J. Org. Chem 2004, 69, 7044–7050. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Correlation of the Rates of Solvolysis of Phenyl Chloroformate. J Chem Soc, Perkin Trans 1997, 2, 1721–1724. [Google Scholar]
- Kevill, DN; Bond, MW; D’Souza, MJ. Dual Pathways in the Solvolyses of Phenyl Chlorothioformate. J. Org. Chem 1997, 62, 7869–7871. [Google Scholar]
- Park, KH; Kyong, JB; Kevill, DN. Nucleophilic Substution Reactions of p-Nitrobenzyl Chloroformate. Bull. Korean Chem. Soc 2000, 21, 1267–1270. [Google Scholar]
- Kevill, DN; Kim, JC; Kyong, JB. Correlation of the Rates of Solvolysis of Methyl Chloroformate with Solvent Properties. J Chem Res, Synop 1999, 150–151. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Correlation of the Rates of Solvolysis of Phenyl Chlorothioformate and Phenyl Chlorodithioformate. Can. J. Chem 1999, 77, 1118–3291. [Google Scholar]
- Kyong, JB; Park, BC; Kim, CB; Kevill, DN. Rate and Product Studies with Benzyl and p-Nitrobenzyl Chloroformates Under Sovolytic Conditions. J. Org. Chem 2000, 65, 8051–8058. [Google Scholar]
- Kyong, JB; Kim, YG; Kim, DK; Kevill, DN. Dual Pathways in the Solvolyses of Isopropyl Chloroformate. Bull. Korean Chem. Soc 2000, 21, 662–664. [Google Scholar]
- Kyong, JB; Yoo, JS; Kevill, DN. Solvolysis-Decomposition of 2-Adamantyl Chloroformate: Evidence for Two Reaction Pathway. J. Org. Chem 2003, 68, 3425–3432. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Application of the NT Solvent Nucleophilicity Scale to Attack at Sulfur: Solvolyses of Benzenesulfonyl Chlorides. Collect Czech Chem Commun 1999, 64, 1790–1796. [Google Scholar]
- Villas-Boas, SG; Delicado, DG; Akesson, M; Nielson, J. Simultaneous Analysis of Amino and Nonamino Organic Acids as Methyl Chloroformate Derivatives Using Gas Chromatography-Mass Spectrometry. Anal. Biochem 2003, 322, 134–138. [Google Scholar]
- Biermann, U; Metzger, JO. Alkylation of Alkenes: Ethylaluminum Sesquichloride-Mediated Hydro-Alkyl Addition with Alkyl Chloroformates and Di-tert-butylpyrocarbonate. J. Am. Chem. Soc 2004, 126, 10319–10330. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Concerning the Two Reaction Channels for the Solvolyses of Ethyl Chloroformate and Ethyl Chlorothioformate. J. Org. Chem 1998, 63, 2120–2124. [Google Scholar]
- Kevill, DN; D’Souza, MJ. Correlation of the Rates of Solvolysis of n-Octyl Fluoroformate and a Comparison with n-Octyl Chloroformate Solvolysis. J Chem Soc, Perkin Trans 2002, 2, 240–243. [Google Scholar]
- Queen, A; Nour, TA. Kinetics and Mechanism of the Acetate Catalyzed Hydrolysis of 4-Methoxyphenyl Chloroformate and 4-Methoxyphenyl Fluoroformate in Aqueous Dioxane. J Chem Soc, Perkin Trans 1976, 2, 935–939. [Google Scholar]
- Orlov, SI; Chimishkyan, AL; Grabarnik, MS. Kinetic Relationships Governing the Ethanolysis of Halogenoformates. J. Org. Chem. USSR (Engl Transl) 1983, 19, 1981–1987. [Google Scholar]
- Kevill, DN. The Chemistry of the Functional Groups: The Chemistry of Acyl Halides; Patai, S, Ed.; Wiley: New York, 1972; Chapter 12. [Google Scholar]
- Harris, JM; Shafer, SG; Moffatt, JR; Becker, AR. Prediction of SN2 Transition State Variation by the Use of More O’Ferrall Plots. J. Am. Chem. Soc 1979, 101, 3295–3300. [Google Scholar]
- Bentley, TW; Bowen, CT; Parker, W; Watt, CIF. Evidence against Appreciable Internal Ion Pair Return in the Solvolyses of Tertiary Aliphatic Halides. Measurement of α-Methyl/Hydrogen Rate ratios in Hexafluoropropan-2-ol-Water. J Chem Soc, Perkin Trans 1980, 2, 1244–1252. [Google Scholar]
- Swain, CG; Scott, CB. Rates of Solvolysis of Some Alkyl Fluorides and Chlorides. J. Am. Chem. Soc 1953, 75, 246–248. [Google Scholar]
- Song, BD; Jencks, WP. Mechaism of Solvolysis of Substituted Benzoyl Halides. J. Am. Chem. Soc 1989, 111, 8470–8479. [Google Scholar]
- Wiberg, KB; Rablen, PR. Substituent Effects. 7. Phenyl Derivatives. When is a Fluorine a π-Donor? J. Org. Chem 1998, 63, 3722–3730. [Google Scholar]
- Kevill, DN; Kyong, JB; Weitl, FL. Solvolysis-Decomposition of 1-Adamantyl Chloroformate: Evidence for Ion Pair Return in 1-Adamantyl Chloride Solvolysis. J. Org. Chem 1990, 55, 4304–4311. [Google Scholar]
- Kevill, DN; Kyong, JB. Multiple Pathways in the Solvolysis of 1-Adamantyl Fluoroformate. J. Org. Chem 1992, 57, 258–265. [Google Scholar]
- Seong, MH; Kyong, JB; Kim, DK; Kevill, DN. Application of the Extended Grunwald-Winstein Equation to Solvolyses of n-Propyl Fluoroformate and a Consideration of Leaving Group Effects. Bull. Korean Chem. Soc 2008, 29, 1747–1751. [Google Scholar]
- Kyong, JB; Ryu, SH; Kevill, DN. Rate and Product Studies of the Solvolyses of Benzyl Fluoroformate. Int. J. Mol. Sci 2006, 7, 186–196. [Google Scholar]
- Kyong, JB; Rhu, CJ; Kim, YG; Kevill, DN. Rate and Product Studies with 2-Adamantyl Fluoroformate under Solvolytic Conditions. J. Phys. Org. Chem 2007, 20, 525–531. [Google Scholar]
- Ryu, ZH; Shin, SH; Lee, JP; Lim, GT; Bentley, TW. Kinetic Solvent Isotope Effects and Correlations of Rates of Solvolyses for α-Methylthio and Other Substituted Acetyl Chlorides. J. Chem. Soc., Perkin Trans 2002, 2, 1283–1287. [Google Scholar]
- Oh, YH; Jang, GG; Lim, GT; Ryu, ZH. Marked Difference in Solvation Effects and Mechanism between Solvolyses of Substituted Acetyl Chloride with Alkyl Groups and with Aromatic Rings in Aqueous Fluorinated Alcohol and in 2,2,2-Trifluoroethanol-Ethanol Solvent Systems. Bull. Korean Chem. Soc 2002, 23, 1083–1096. [Google Scholar]
- Yew, KH; Koh, HJ; Lee, HW; Lee, I. Nucleophilic Substitution Reactions of Phenyl Chloroformates. J. Chem. Soc., Perkin. Trans 1995, 2, 2263–2268. [Google Scholar]
- Koo, IS; Yang, K; Kang, K; Lee, I. Transition-State Variation in the Solvolyses of para-Substituted Phenyl Chloroformates in Alcohol-Water Mixtures. Bull. Korean Chem. Soc 1998, 19, 968–973. [Google Scholar]
- Kevill, DN; Miller, B. Application of the NT Solvent Nucleophilicity Scale to Attack at Phosphorus: Solvolyses of N,N,N’,N’-Tetramethyldiamidophosphorochloridate. J. Org. Chem 2002, 67, 7399–7406. [Google Scholar]
- Kyong, JB; Won, H; Kevill, DN. Application of the Extended Grunwald-Winstein Equation to Solvolyses of n-Propyl Chloroformate. Int. J. Mol. Sci 2005, 6, 87–96. [Google Scholar]
- Lee, SH; Rhu, CJ; Kyong, JB; Kim, DK; Kevill, DN. Correlation of the Rates of Solvolysis of Isopropyl Fluoroformate Using the Extended Grunwald-Winstein Equation. Bull. Korean Chem. Soc 2007, 28, 657–661. [Google Scholar]
- Charles, SW; Jones, GIL; Owen, NL; West, LA. Infrared Spectra and Rotational Isomerism of Ethyl Fluoroformate and Ethyl Propiolate. J. Molecular Structure 1975, 26, 249–257. [Google Scholar]
| Solvent(%)b | 104 k, s−1 | NTc | YCld | kF /kCle |
|---|---|---|---|---|
| 100 MeOH f | 0.769±0.040 | 0.17 | −1.17 | 0.93 |
| 90 MeOH | 8.09±0.47 | −0.01 | −0.18 | 4.82 |
| 80 MeOH | 18.3±0.8 | −0.06 | 0.67 | 7.43 |
| 60 MeOH | 44.2±3.1 | −0.54 | 2.07 | 11.1 |
| 100 EtOH | 0.128±0.010 | 0.37 | −2.52 | 0.57 |
| 90 EtOH | 3.15±0.09 | 0.16 | −0.94 | 5.77 |
| 80 EtOH | 6.39±0.06 | 0.00 | 0.00 | 8.74 |
| 70 EtOH | 11.0±0.7 | −0.20 | 0.78 | 11.1 |
| 60 EtOH | 17.0±0.4 | −0.38 | 1.38 | 14.0 |
| 90Me2CO | 0.0536±0.0023 | −0.35 | −2.22 | 1.80 |
| 80 Me2CO | 0.376±0.022 | −0.37 | −0.83 | 3.90 |
| 60 Me2CO | 3.47±0.22 | −0.52 | 0.95 | 9.44 |
| 90TFEg | 0.0794±0.0016 | −2.55 | 2.85 | 13.3 |
| 70 TFEg | 1.18±0.04 | −1.98 | 2.96 | 19.3 |
| 50 TFEg | 5.92±0.56 | −1.73 | 3.16 | 28.1 |
| 80T-20Eh | 0.0313±0.0010 | −1.76 | 1.89 | 5.03 |
| 60T-40Eh | 0.103±0.008 | −0.94 | 0.63 | 3.47 |
| 40T-60Eh | 0.236±0.023 | −0.34 | −0.48 | 2.85 |
| 20T-80Eh | 0.235±0.019 | 0.08 | −1.42 | 1.65 |
| 70HFIPg | 2.38±0.19 | −2.94 | 3.83 | 53.6 |
| 50HFIPg | 4.55±0.35 | −2.49 | 3.80 | 33.2 |
| Solventc (%) | Temp.(°C) | 104 kF (sec−1) | EtOCOF
| ΔS≠297.4°Cd | 104 kCl (sec−1) | EtOCOCl
| ΔS≠297.4°Cd |
|---|---|---|---|---|---|---|---|
| ΔH≠297.4°Cd | ΔH≠297.4°Cd | ||||||
| 100MeOH | 24.2 | 0.769±0.040 | 12.0±0.4 | −36.9±1.4 | 0.824±0.009e | 14.8±0.2 | −27.5±0.8 |
| 35.0 | 1.74±0.16 | 2.16±0.01 | |||||
| 45.0 | 4.59±0.17 | ||||||
| 55.0 | 5.78±0.08 | 9.59±0.23 | |||||
| 100EtOH | 24.2 | 0.128±0.010 | 12.6±0.1 | −38.5±0.5 | 0.226±0.005e | 14.6±0.3 | −30.7±1.1 |
| 35.0 | 0.528±0.028 | ||||||
| 45.0 | 0.544±0.020 | 1.15±0.01 | |||||
| 55.0 | 1.05±0.03 | 2.54±0.08 | |||||
| 80EtOH | 24.2 | 6.39±0.4 | 9.4±0.6 | −41.5±2.0 | 0.731±0.006e | 13.6±0.2 | −31.8±0.5 |
| 35.0 | 10.8±0.3 | 1.75±0.03 | |||||
| 40.0 | 14.3±0.2 | ||||||
| 45.0 | 19.6±0.3 | 3.72±0.05 | |||||
| 55.0 | 7.21±0.24 | ||||||
| 70TFE | 24.2 | 1.18±0.04 | 13.3±0.5 | −31.7±1.5 | 0.0611±0.002e | 18.5±0.2 | −20.4±0.7 |
| 45.0 | 4.47±0.19 | 0.503±0.005 | |||||
| 50.0 | 7.78±0.13 | ||||||
| 55.0 | 1.30± 0.02 | ||||||
| 60.0 | 1.90±0.05 | ||||||
| 70HFIP | 24.2 | 2.38±0.19 | 14.0±0.4 | −27.9±1.5 | |||
| 35.0 | 5.52±0.07 | ||||||
| 40.0 | 8.40±0.07 | ||||||
| Solvent(%)a | ethylc | n-propyld | i-propyle | n-octylf | benzylg | 1-adamantylh | 2-adamantyli |
|---|---|---|---|---|---|---|---|
| 100EtOH | 0.57 | 0.57 | 0.18 | 0.62 | 1.19 | 1.31×10−17 | 0.37 |
| 80EtOH | 8.74 | 5.62 | 2.11 | 8.09 | 11.5 | 1.25×10 −3 | 3.48 |
| 60EtOH | 14.0 | 1.79 | 15.1 | 14.6l | 3.01 | ||
| 100MeOH | 0.93 | 0.75 | 0.39 | 0.95 | 1.78 | 5.91×10−11 | 0.42 |
| 90MeOH | 4.82 | - | 1.76 | - | 7.18 | - | 2.40 |
| 80Me2CO | 3.90 | 4.24j | 0.53 | 2.86 | 5.89 | - | 0.65 |
| 70TFEb | 19.3 | 7.72 | 0.067 | 10.2k | 6.36 | - | 0.011 |
| Substrate | Mech.a | nb | lc | mc | cc | Rd | l/m |
|---|---|---|---|---|---|---|---|
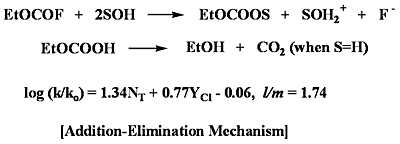
| EtOCOF | A-E | 21 | 1.51±0.20 | 0.85±0.11 | −0.14±0.14 | 0.883 | 1.78 |
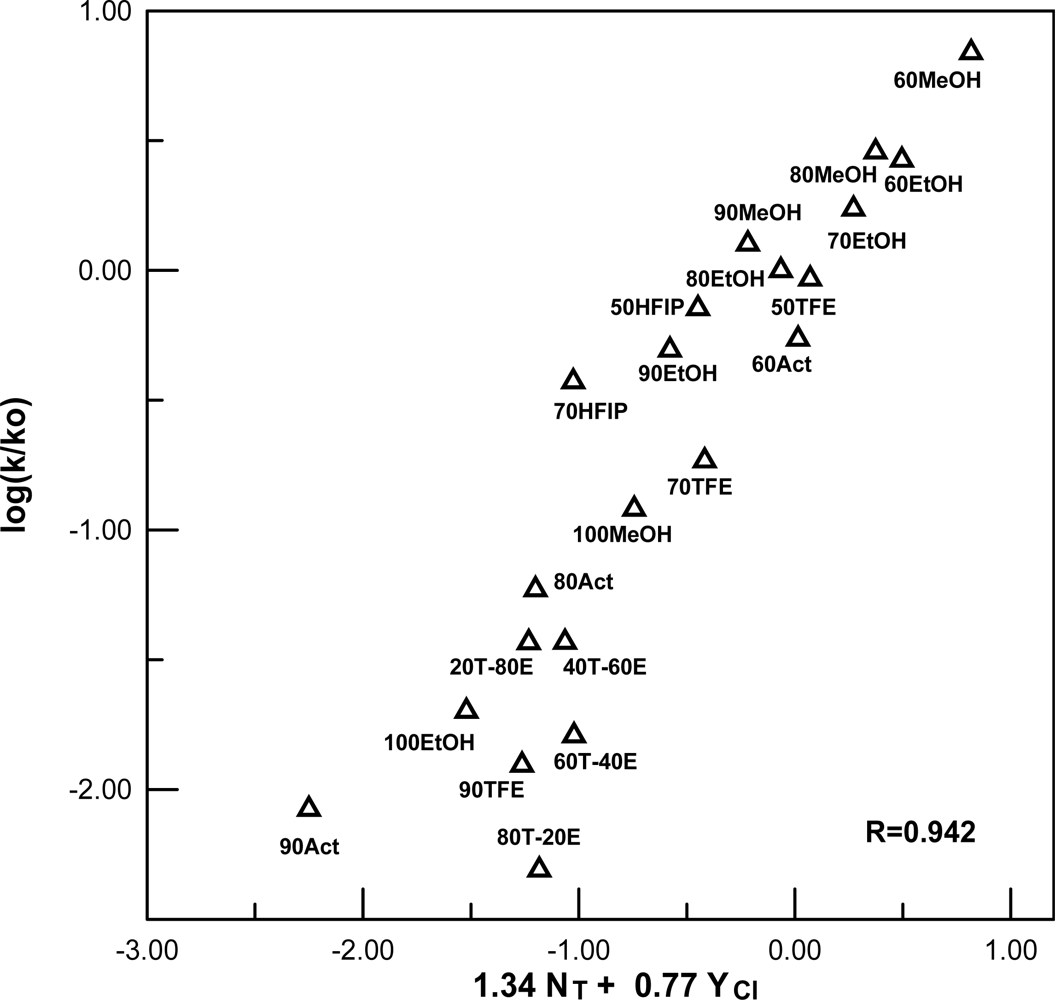
| EtOCOF | A-E | 17e | 1.34±0.14 | 0.77±0.07 | −0.06±0.10 | 0.942 | 1.74 |
| EtOCOClf | A-E | 28 | 1.56±0.09 | 0.55±0.03 | 0.19±0.24 | 0.967 | 2.84 |
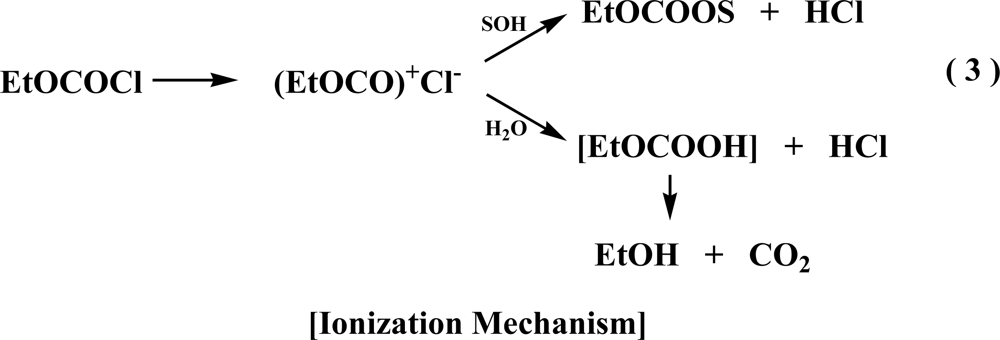
| EtOCOClf | I | 7 | 0.69±0.13 | 0.82±0.16 | −2.40±0.27 | 0.946 | 0.84 |
| n-PrOCOFg | A-E | 19 | 1.80±0.17 | 0.96±0.10 | −0.01±0.11 | 0.940 | 1.88 |
| n-PrOCOClh | A-E | 22 | 1.57±0.12 | 0.56±0.06 | 0.15±0.08 | 0.947 | 2.79 |
| n-PrOCOClh | I | 6 | 0.40±0.12 | 0.64±0.13 | −2.45±0.47 | 0.942 | 0.63 |
| i-PrOCOFi | A-E | 20 | 1.59±0.16 | 0.80±0.06 | −0.12±0.05 | 0.957 | 1.99 |
| i-PrOCOClj | I | 20 | 0.28±0.05 | 0.52±0.03 | −0.12±0.05 | 0.979 | 0.54 |
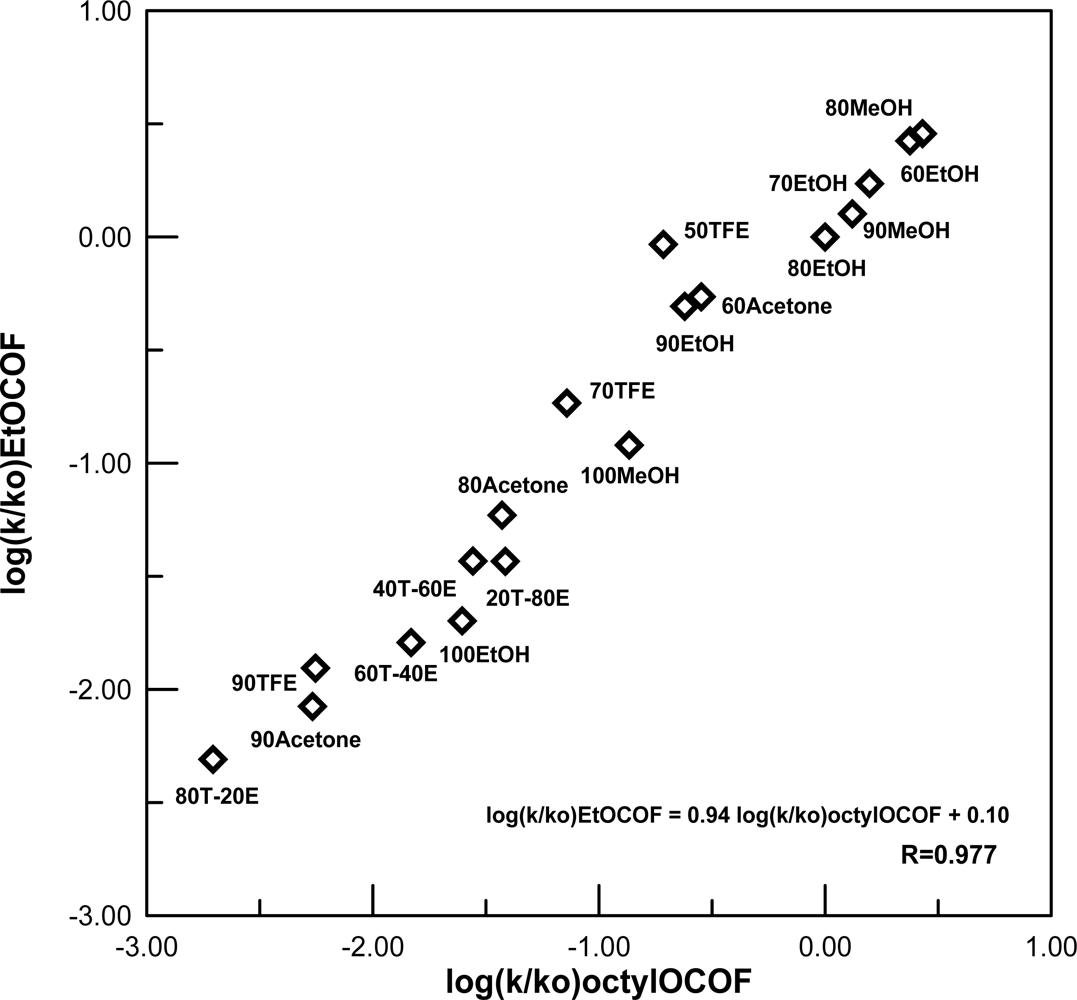
| n-OctOCOFk | A-E | 23 | 1.80±0.13 | 0.79±0.06 | 0.13±0.34 | 0.959 | 2.28 |
| BzOCOFl | A-E | 16 | 1.57±0.20 | 0.76±0.08 | −0.13±0.27 | 0.974 | 2.04 |
| 1-AdOCOFm | A-E | 10 | 2.78±0.21 | 1.01±0.06 | 0.09±0.16 | 0.987 | 2.78 |
| 1-AdOCOFm | I | 16 | ~0 | 0.70±0.01 | −0.02±0.05 | 0.999 | ~0 |
| 2-AdOCOFn | A-E | 17 | 1.92±0.15 | 0.84±0.06 | −0.02±0.06 | 0.968 | 2.28 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Seong, M.H.; Kyong, J.B.; Lee, Y.H.; Kevill, D.N. Corrrelation of the Specific Rates of Solvolysis of Ethyl Fluoroformate Using the Extended Grunwald-Winstein Equation. Int. J. Mol. Sci. 2009, 10, 929-941. https://doi.org/10.3390/ijms10030929
Seong MH, Kyong JB, Lee YH, Kevill DN. Corrrelation of the Specific Rates of Solvolysis of Ethyl Fluoroformate Using the Extended Grunwald-Winstein Equation. International Journal of Molecular Sciences. 2009; 10(3):929-941. https://doi.org/10.3390/ijms10030929
Chicago/Turabian StyleSeong, Mi Hye, Jin Burm Kyong, Young Hoon Lee, and Dennis N. Kevill. 2009. "Corrrelation of the Specific Rates of Solvolysis of Ethyl Fluoroformate Using the Extended Grunwald-Winstein Equation" International Journal of Molecular Sciences 10, no. 3: 929-941. https://doi.org/10.3390/ijms10030929