ERK Activity Imaging During Migration of Living Cells In Vitro and In Vivo
Abstract
:1. Introduction
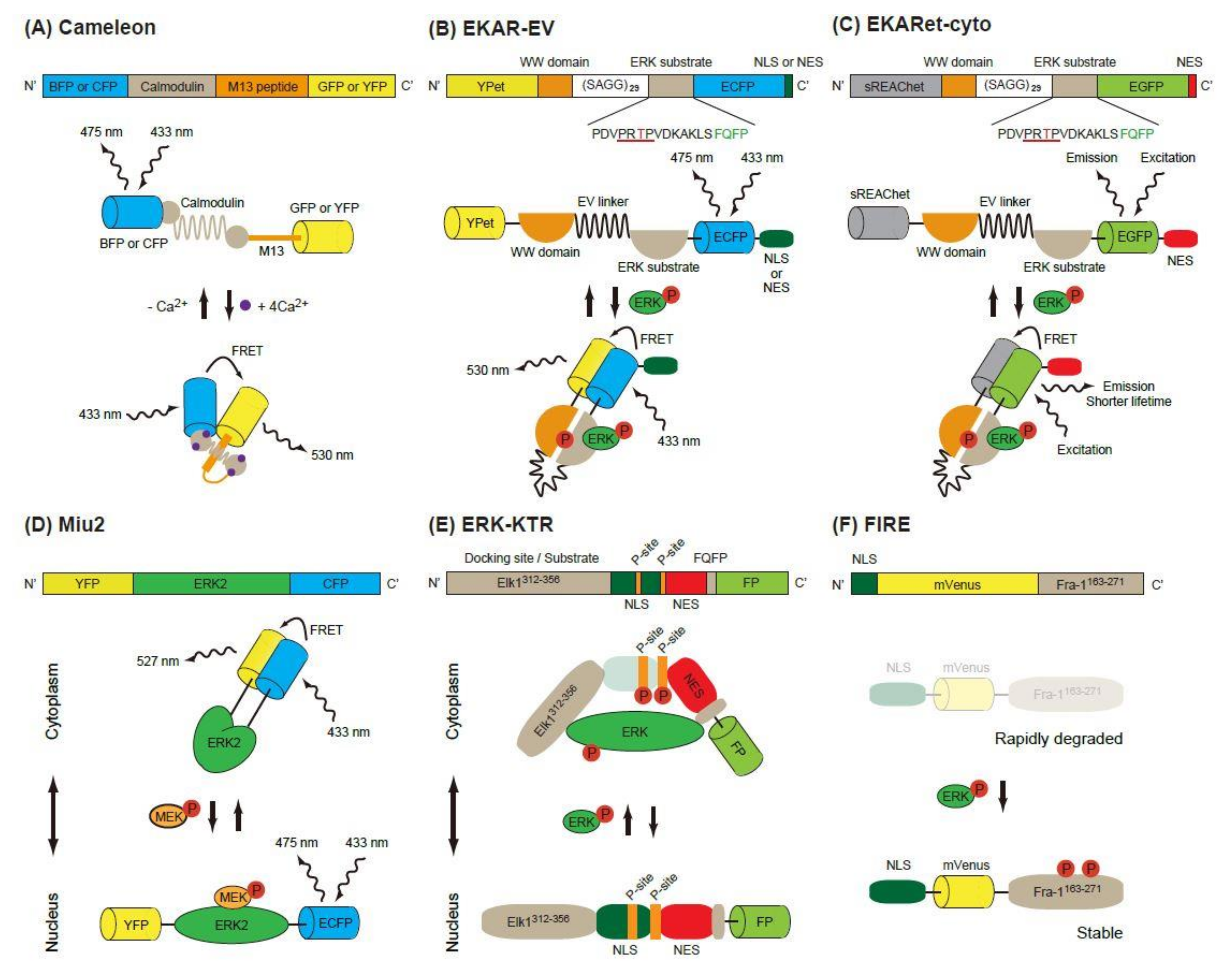
2. ERK Biosensors
2.1. FRET-Based Biosensors
2.1.1. EKAR-Based FRET Biosensors
2.1.2. Miu2 FRET Biosensor
2.2. Kinase Translocation Reporters
2.3. FIRE
3. ERK Activities In Vitro
3.1. Pulse Activation of ERK for Proliferation
3.2. ERK in Cell Migration In Vitro
3.2.1. Pulse Activation of ERK for Migration
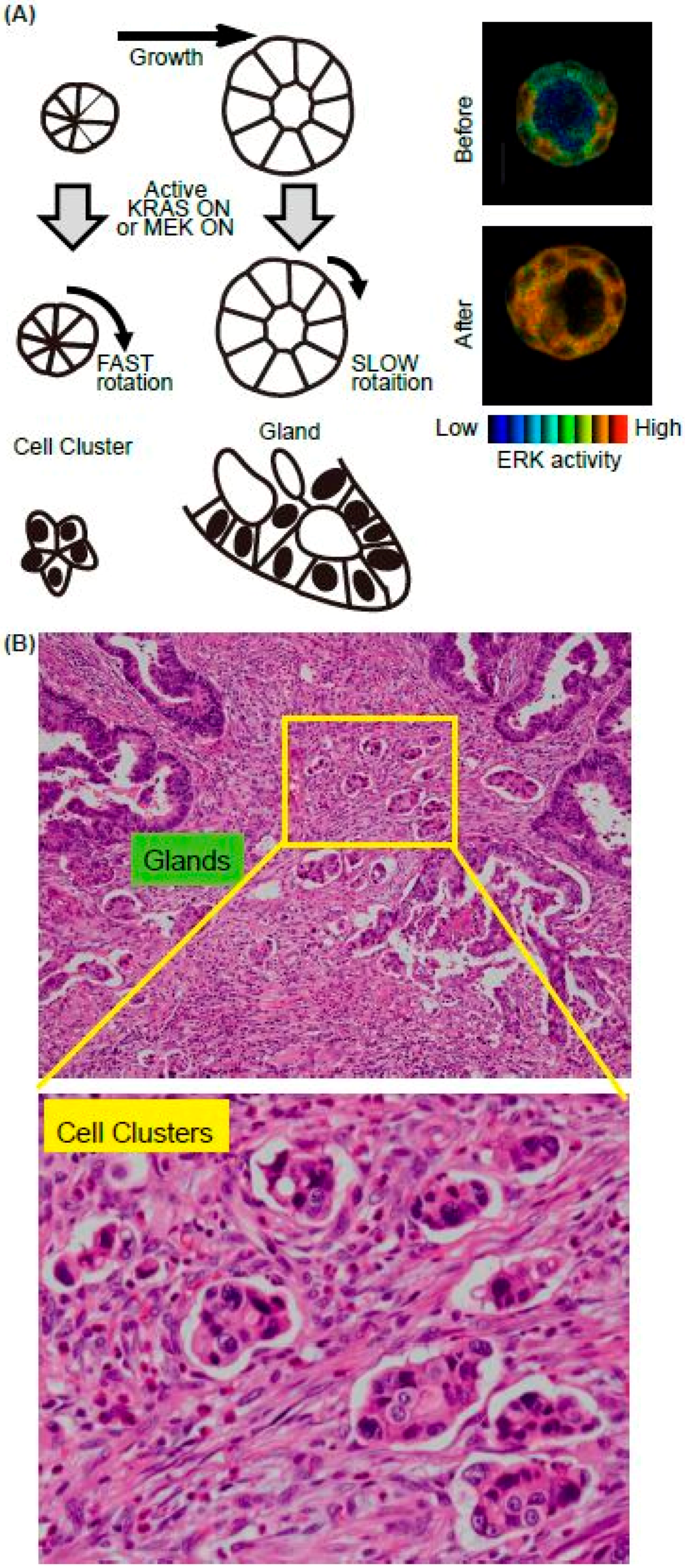
3.2.2. ERK in a Cell Cluster/Cyst Rotation in 3-D Culture
4. ERK Activities in Cell Migration In Vivo
4.1. Neutrophil Migration upon LPS Treatment
4.2. Myeloid-Derived Suppressor Cells (MDSCs)
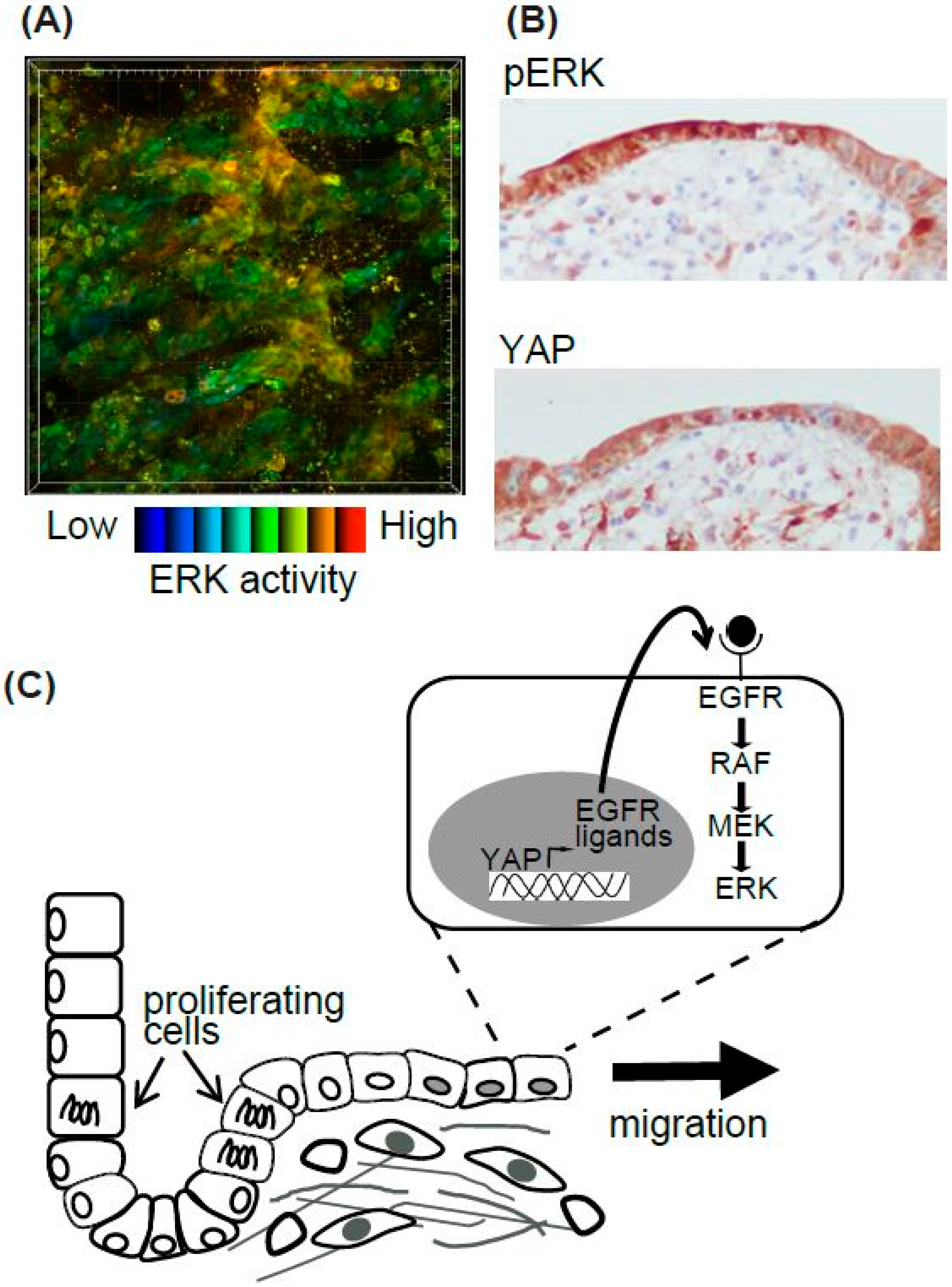
4.3. Epithelial Cell Migration In Vivo
4.3.1. Intestinal Epithelial Cells After Ischemic Injury
4.3.2. Epidermis and Basal Cell Migration of the Urothelium During Wound Healing
5. Perspective
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ADAMs | a disintegrin and metalloproteases |
| AKT | AKT8 virus oncogene cellular homolog |
| AP1 | activator protein 1 |
| BCL-2 | B cell lymphoma 2 |
| BFP | blue fluorescent protein |
| BLT1 | a leukotriene B4 receptor |
| CFP | cyan fluorescent protein |
| CRC | colorectal cancer |
| ddFPs | dimerization-dependent fluorescent proteins |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EGFR | EGF receptor |
| Egr1 | Early growth response gene 1 |
| EP4 | eicosapentaenoic receptor 4 |
| ERK | extracellular signal-regulated kinase |
| ERT2 | Tamoxifen inducible Estrogen receptor |
| ETS | E26 transformation-specific |
| FIRE | Fra-1-based integrative reporter of ERK |
| FLIM | fluorescence lifetime imaging microscopy |
| FRET | Förster (or fluorescence) resonance energy transfer |
| GFP | green fluorescent protein |
| GFs | growth factors |
| GEFs | guanine nucleotide exchange factors |
| HDAC | Histone Deacetylase |
| JNK | c-jun N-terminal kinase |
| KTRs | kinase translocation reporters |
| LPS | lipopolysaccharide |
| LTB4 | leukotriene B4 |
| MDCK | Madin-Darby canine kidney |
| MDSCs | myeloid-derived suppressor cells |
| MEK | MAPK/Erk kinase |
| Miu2 | MAPK indicator unit ERK2 |
| NES | nuclear export signal |
| NET | neutrophil extracellular trap |
| NLS | nuclear localization signal |
| OPN | osteopontin |
| PDCs | poorly differentiated clusters |
| PDO | patient-derived tumor organoid |
| PDX | patient-derived tumor xenograft |
| PGE2 | Prostaglandin E2 |
| PI-3K | Phosphatidyl Inositol 3 kinase |
| PKA | protein kinase A |
| RALGEF | RAL guanine nucleotide exchange factor |
| REACh | a dark YFP-based resonance energy-accepting chromoprotein |
| RAS | Rat sarcoma |
| RAF | Rapidly Accelerated Fibrosarcoma |
| RFP | red fluorescent protein |
| RTKs | receptor tyrosine kinases |
| sREACh | super REACh |
| SRF | serum response factor |
| TG | transgenic |
| YAP | yes-associated protein |
References
- Caunt, C.J.; Sale, M.J.; Smith, P.D.; Cook, S.J. MEK1 and MEK2 inhibitors and cancer therapy: The long and winding road. Nat. Rev. Cancer 2015, 15, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Meloche, S.; Pouyssegur, J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007, 26, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Balmanno, K.; Cook, S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Zhang, W.; Ballif, B.A.; Elliott, H.L.; Danuser, G.; Blenis, J. ERK-MAPK drives lamellipodia protrusion by activating the WAVE2 regulatory complex. Mol. Cell 2011, 41, 661–671. [Google Scholar] [CrossRef]
- Tanimura, S.; Hashizume, J.; Arichika, N.; Watanabe, K.; Ohyama, K.; Takeda, K.; Kohno, M. ERK signaling promotes cell motility by inducing the localization of myosin 1E to lamellipodial tips. J. Cell Biol. 2016, 214, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Gromnitza, S.; Lepa, C.; Weide, T.; Schwab, A.; Pavensadt, H.; George, B. Tropomyosin-related kinase C (TrkC) enhances podocyte migration by ERK-mediated WAVE2 activation. FASEB J. 2018, 32, 1665–1676. [Google Scholar] [CrossRef]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef]
- Hirata, E.; Kiyokawa, E. Future perspective of single-molecule FRET biosensors and intravital FRET microscopy. Biophys. J. 2016, 111, 1103–1111. [Google Scholar] [CrossRef]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef]
- Mochizuki, N.; Yamashita, S.; Kurokawa, K.; Ohba, Y.; Nagai, T.; Miyawaki, A.; Matsuda, M. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 2001, 411, 1065–1068. [Google Scholar] [CrossRef]
- Maryu, G.; Miura, H.; Uda, Y.; Komatsubara, T.; Matsuda, M.; Aoki, K. Live-cell imaging with genetically encoded protein kinase activity reporters. Cell Struct. Funct. 2018, 41, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.D.; Ehrhardt, A.G.; Cellurale, C.; Zhong, H.; Yasuda, R.; Davis, R.J.; Svoboda, K. A genetically encoded fluorescent sensor of ERK activity. Proc. Natl. Acad. Sci. USA 2008, 105, 19264–19269. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Aoki, K.; Yamada, M.; Yukinaga, H.; Fujita, Y.; Kamioka, Y.; Matsuda, M. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol. Biol. Cell 2011, 22, 4647–4656. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, J.; Enterina, J.R.; Shen, Y.; Zhang, I.; Tewson, P.H.; Mo, G.C.H.; Zhang, J.; Quinn, A.M.; Hughes, T.E.; et al. Ratiometric biosensors based on dimerization-dependent fluorescent protein exchange. Nat. Method 2015, 12, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Zhang, Y.; Roth, R.H.; Zhang, J.F.; Mo, A.; Tenner, B.; Huganir, R.L.; Zhang, J. Single-fluorophore biosensors for sensitive and multiplexed detection of signalling activities. Nat. Cell Biol. 2018, 20, 1215–1225. [Google Scholar] [CrossRef]
- Tang, S.; Yasuda, R. Imaging ERK and PKA activation in single dendritic spines during structural plasticity. Neuron 2017, 93, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Murakoshi, H.; Lee, S.J.; Yasuda, R. Highly sensitive and quantitative FRET-FLIM imaging in single dendritic spines using improved non-radiative YFP. Brain Cell Biol. 2008, 36, 31–42. [Google Scholar] [CrossRef]
- Yellen, G.; Mongeon, R. Quantitative two-photon imaging of fluorescent biosensors. Curr. Opin. Chem. Biol. 2015, 27, 24–30. [Google Scholar] [CrossRef]
- Fujioka, A.; Terai, K.; Itoh, R.E.; Aoki, K.; Nakamura, T.; Kuroda, S.; Nishida, E.; Matsuda, M. Dynamics of the Ras/ERK MAPK cascade as monitored by fluorescent probes. J. Biol. Chem. 2006, 281, 8917–8926. [Google Scholar] [CrossRef]
- Regot, S.; Hughey, J.J.; Bajar, B.T.; Carrasco, S.; Covert, M.W. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 2014, 157, 1724–1734. [Google Scholar] [CrossRef]
- Maryu, G.; Matsuda, M.; Aoki, K. Multiplexed fluorescence imaging of ERK and Akt activities and cell-cycle progression. Cell Struct. Funct. 2016, 41, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef]
- Albeck, J.; Mills, G.; Brugge, J. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol. Cell 2013, 49, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Sparta, B.; Pargett, M.; Minguet, M.; Distor, K.; Bell, G.; Albeck, J.G. Receptor-Level Mechanisms Are Required for EGF-Stimulated ERK Activity Pulses. J. Biol. Chem. 2015, 290, 24784–24792. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Kumagai, Y.; Sakurai, A.; Komatsu, N.; Fujita, Y.; Shionyu, C.; Matsuda, M. Stochastic ERK activation induced by noise and cell-to-cell propagation regulates cell density-dependent proliferation. Mol. Cell 2013, 52, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Kondo, Y.; Naoki, H.; Hiratsuka, T.; Itoh, R.E.; Matsuda, M. Propagating wave of ERK activation orients collective cell migration. Dev. Cell 2017, 43, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Mouw, J.K.; Weaver, V.M. Forcing form and function: Biomechanical regulation of tumor evolution. Trend Cell Biol. 2011, 21, 47–56. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- O’Brien, L.E.; Zegers, M.M.; Mostov, K.E. Opinion: Building epithelial architecture: Insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 2002, 3, 531–537. [Google Scholar] [CrossRef]
- Yagi, S.; Matsuda, M.; Kiyokawa, E. Suppression of Rac1 activity at the apical membrane of MDCK cells is essential for cyst structure maintenance. EMBO Rep. 2012, 13, 237. [Google Scholar] [CrossRef]
- Hirata, E.; Ichikawa, T.; Horike, S.I.; Kiyokawa, E. Active K-RAS induces the coherent rotation of epithelial cells: A model for collective cell invasion in vitro. Cancer Sci. 2018, 109, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Matsuda, M.; Kiyokawa, E. Activated Ras protein accelerates cell cycle progression to perturb Madin-Darby Canine Kidney cystogenesis. J. Biol. Chem. 2012, 287, 31703–31711. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying collective cancer cell invasion. Nat. Cell Biol. 2012, 14, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Haeger, A.; Wolf, K.; Zegers, M.M.; Friedl, P. Collective cell migration: Guidance principles and hierarchies. Trend Cell Biol. 2015, 25, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Murphy, J.; Jass, J.R.; Mochizuki, H.; Talbot, I.C. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002, 40, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kajiwara, Y.; Shimazaki, H.; Shinto, E.; Hashiguchi, Y.; Nakanishi, K.; Maekawa, K.; Katsurada, Y.; Nakamura, T.; Mochizuki, H.; et al. New criteria for histologic grading of colorectal cancer. Am. J. Surg. Pathol. 2012, 36, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.C.; Winter, D.C.; Heeney, A.; Gibbons, D.; Lugli, A.; Puppa, G.; Sheahan, K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br. J. Cancer 2016, 115, 831–840. [Google Scholar] [CrossRef]
- Ueno, H.; Mochizuki, H.; Hashiguchi, Y.; Ishiguro, M.; Kajiwara, Y.; Sato, T.; Shimazaki, H.; Hase, K.; Talbot, I.C. Histological grading of colorectal cancer: A simple and objective method. Ann. Surg. 2008, 247, 811–818. [Google Scholar] [CrossRef]
- Ueno, H.; Hashiguchi, Y.; Kajiwara, Y.; Shinto, E.; Shimazaki, H.; Kurihara, H.; Mochizuki, H.; Hase, K. Proposed objective criteria for grade 3 in early invasive colorectal cancer. Am. J. Clin. Pathol. 2010, 134, 312–322. [Google Scholar] [CrossRef]
- Pearson, G.W.; Hunter, T. Real-time imaging reveals that noninvasive mammary epithelial acini can contain motile cells. J. Cell Biol. 2007, 179, 1555–1567. [Google Scholar] [CrossRef]
- Tanner, K.; Mori, H.; Mroue, R.; Bruni-Cardoso, A.; Bissell, M.J. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc. Natl. Acad. Sci. USA 2012, 109, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lacoche, S.; Huang, L.; Xue, B.; Muthuswamy, S.K. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc. Natl. Acad. Sci. USA 2013, 110, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Saitou, T.; Kawakami, R. In vivo optical imaging of cancer cell function and tumor microenvironment. Cancer Sci. 2018, 109, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, Y.; Sumiyama, K.; Mizuno, R.; Sakai, Y.; Hirata, E.; Kiyokawa, E.; Matsuda, M. Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors. Cell Struct. Funct. 2012, 37, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Komatsu, N.; Hirata, E.; Kamioka, Y.; Matsuda, M. Stable expression of FRET biosensors: A new light in cancer research. Cancer Sci. 2012, 103, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Okuchi, Y.; Imajo, M.; Mizuno, R.; Kamioka, Y.; Miyoshi, H.; Taketo, M.M.; Nagayama, S.; Sakai, Y.; Matsuda, M. Identification of aging-associated gene expression signatures that precede intestinal tumorigenesis. PLoS ONE 2016, 11, e0162300. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Nakahara, I.; Yamaguchi, T.; Kamioka, Y.; Sumiyama, K.; Matsuda, M.; Nakanishi, S.; Funabiki, K. Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc. Natl. Acad. Sci. USA 2015, 112, 6718–6723. [Google Scholar] [CrossRef]
- Takeda, H.; Kiyokawa, E. Activation of Erk in ileal epithelial cells engaged in ischemic-injury repair. Sci. Rep. 2017, 7, 16469. [Google Scholar] [CrossRef]
- Okada, T.; Takahashi, S.; Ishida, A.; Ishigame, H. In vivo multiphoton imaging of immune cell dynamics. Pflugers Archiv. Eur. J. Phys. 2016, 468, 1793–1801. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martinez-Guzman, M.; Iniguez-Gutierrez, L.; Garcia-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil extracellular traps and its implications in inflammation: An overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Tan, S.Y.; Weninger, W. Neutrophil migration in inflammation: Intercellular signal relay and crosstalk. Curr. Opin. Immunol. 2017, 44, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Kamioka, Y.; Kabashima, K.; Imajo, M.; Sumiyama, K.; Nakasho, E.; Ito, T.; Hamazaki, Y.; Okuchi, Y.; Sakai, Y.; et al. In vivo imaging reveals PKA regulation of ERK activity during neutrophil recruitment to inflamed intestines. J. Exp. Med. 2014, 211, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, M.H.; Feoktistov, A.S.; Capodici, C.; Solitar, B.; Levy, J.; Oei, T.T.; Philips, M.R. Mitogen-activated protein kinase in neutrophils and enucleate neutrophil cytoplasts: Evidence for regulation of cell-cell adheion. J. Biol. Chem. 1996, 271, 12049–12056. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, Y.; Takakura, K.; Sumiyama, K.; Matsuda, M. Intravital Förster resonance energy transfer imaging reveals osteopontin-mediated polymorphonuclear leukocyte activation by tumor cell emboli. Cancer Sci. 2017, 108, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2010, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Dignass, A.U. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 348–353. [Google Scholar] [CrossRef]
- El Marjou, F.; Janssen, K.P.; Hung-Junn Chang, B.; Li, M.; Hindie, V.; Chan, L.; Louvard, D.; Chambon, P.; Metzger, D.; Robine, S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004, 39, 186–193. [Google Scholar] [CrossRef]
- Marsh, E.; Gonzalez, D.G.; Lathrop, E.A.; Boucher, J.; Greco, V. Positional stability and membrane occupancy define skin fibroblast homeostasis in vivo. Cell 2018, 175, 1620–1633. [Google Scholar] [CrossRef]
- Gregorieff, A.; Liu, Y.; Inanlou, M.R.; Khomchuk, Y.; Wrana, J.L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 2015, 526, 715–718. [Google Scholar] [CrossRef]
- Leoni, G.; Neumann, P.A.; Sumagin, R.; Denning, T.L.; Nusrat, A. Wound repair: Role of immune-epithelial interactions. Mucosal Immunol. 2015, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Feil, W.; Wenzl, E.; Vattay, P.; Starlinger, M.; Sogukoglu, T.; Schiessel, R. Repair of rabbit duodenal mucosa after acid injury in vivo and in vitro. Gastroenterology 1987, 92, 1973–1986. [Google Scholar] [CrossRef]
- Feil, W.; Lacy, E.R.; Lacy, E.R.; Wong, Y.M.; Burger, D.; Wenzl, E.; Starlinger, M.; Schiessel, R. Rapid epithelial restitution of human and rabbit colonic mucosa. Gastroenterology 1989, 97, 685–701. [Google Scholar] [CrossRef]
- Seno, H.; Miyoshi, H.; Brown, S.L.; Geske, M.J.; Colonna, M.; Stappenbeck, T.S. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Ajima, R.; Luo, C.T.; Yamaguchi, T.P.; Stappenbeck, T.S. Wnt5a potentiates TGF-b signaling to promote colonic crypt regeneration after tissue injury. Science 2012, 338, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.P.; Vongsa, R.A.; Faherty, S.L.; Salzman, N.H.; Dwinell, M.B. Targeted intestinal epithelial deletion of the chemokine receptor CXCR4 reveals important roles for extracellular-regulated kinase-1/2 in restitution. Lab. Investig. 2011, 91, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Hirai, K.; Yamamoto, H.; Tanooka, H.; Sakamoto, H.; Iwamoto, T.; Takahashi, T.; Terada, M.; Ochiya, T. HST-1//FGF-4 plays a critical role in crypt cell survival and facilitates epithelial cell restitution and proliferation. Oncogene 2004, 23, 3681–3688. [Google Scholar] [CrossRef]
- Sano, T.; Kobayashi, T.; Ogawa, O.; Matsuda, M. Gliding basal cell migration of the urothelium during wound healing. Am. J. Pathol. 2018, 188, 2564–2573. [Google Scholar] [CrossRef]
- Tanabe, A.; Hibi, T.; Ipponjima, S.; Matsumoto, K.; Yokoyama, M.; Kurihara, M.; Hashimoto, N.; Nemoto, T. Transmissive liquid-crystal device for correcting primary coma aberration and astigmatism in biospecimen in two-photon excitation laser scanning microscopy. J. Biomed. Opt. 2016, 21, 121503. [Google Scholar] [CrossRef]
- Tanabe, A.; Hibi, T.; Ipponjima, S.; Matsumoto, K.; Yokoyama, M.; Kurihara, M.; Hashimoto, N.; Nemoto, T. Correcting spherical aberrations in a biospecimen using a transmissive liquid crystal device in two-photon excitation laser scanning microscopy. J. Biomed. Opt. 2015, 20, 101204. [Google Scholar] [CrossRef]
- Takaoka, S.; Kamioka, Y.; Takakura, K.; Baba, A.; Shime, H.; Seya, T.; Matsuda, M. Live imaging of transforming growth factor-b activated kinase 1 activation in Lewis lung carcinoma 3LL cells implanted into syngeneic mice and treated with polyinosinic:polycytidylic acid. Cancer Sci. 2016, 107, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Infante, J.R.; Janku, F.; Wong, D.J.L.; Sosman, J.A.; Keedy, V.; Patel, M.R.; Shapiro, G.I.; Mier, J.W.; Tolcher, A.W.; et al. First-in-Class ERK1/2 inhibitor Ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: Results of a phase I dose-escalation and expansion study. Cancer Discov. 2018, 8, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Naoki, H.; Nakasyo, E.; Kamioka, Y.; Kiyokawa, E.; Matsuda, M. Heterogeneity in ERK activity as visualized by in vivo FRET imaging of mammary tumor cells developed in MMTV-Neu mice. Oncogene 2015, 34, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Muta, Y.; Fujita, Y.; Sumiyama, K.; Sakurai, A.; Taketo, M.M.; Chiba, T.; Seno, H.; Aoki, K.; Matsuda, M.; Imajo, M. Composite regulation of ERK activity dynamics underlying tumour-specific traits in the intestine. Nat. Commun. 2018, 9, 2174. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Girotti, M.R.; Viros, A.; Hooper, S.; Spencer-Dene, B.; Matsuda, M.; Larkin, J.; Marais, R.; Sahai, E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin b1/FAK signaling. Cancer Cell 2015, 27, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Sahai, E. Tumor Microenvironment and differential responses to therapy. Cold Spring Harb. Perspect. Med. 2017, 7, a026781. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease modeling in stem cell-derived 3D organoid systems. Trend Mol. Med. 2018, 23, 393–410. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirata, E.; Kiyokawa, E. ERK Activity Imaging During Migration of Living Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 679. https://doi.org/10.3390/ijms20030679
Hirata E, Kiyokawa E. ERK Activity Imaging During Migration of Living Cells In Vitro and In Vivo. International Journal of Molecular Sciences. 2019; 20(3):679. https://doi.org/10.3390/ijms20030679
Chicago/Turabian StyleHirata, Eishu, and Etsuko Kiyokawa. 2019. "ERK Activity Imaging During Migration of Living Cells In Vitro and In Vivo" International Journal of Molecular Sciences 20, no. 3: 679. https://doi.org/10.3390/ijms20030679




