Sterol Characteristics in Silkworm Brain and Various Tissues Characterized by Precise Sterol Profiling Using LC-MS/MS
Abstract
1. Introduction
2. Results
2.1. Changes in Sterol Composition in the Midgut and Hemolymph as the Suppliers of Sterols to Other Tissues
2.2. Sterol Profiles in Various Tissues
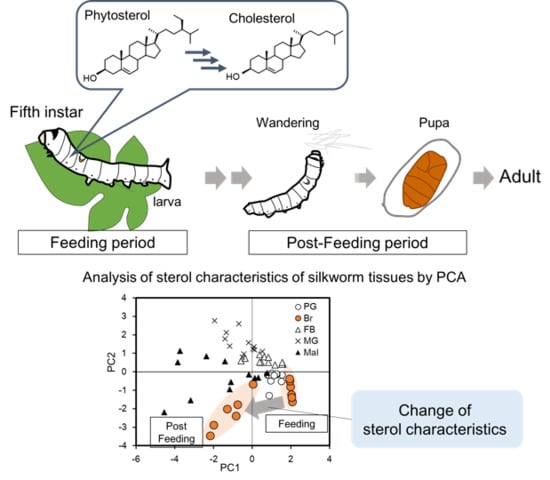
2.3. Visualization of Tissue Characteristics of Sterol Profiles Using Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Silkworm
4.3. Extraction of Sterols from Silkworm Tissues and Hemolymph
4.4. Quantitation of Sterols in Silkworm Tissue or Hemolymph Using LC-MS/MS
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Chl | Cholesterol |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| PCA | principal component analysis |
| PGs | prothoracic glands |
| MRM | multiple reaction monitoring |
| Lp | Lipophorin |
| 7dC | 7-dehydrocholesterol |
| 20E | 20-hydroxyecdysone |
| SCRB | scavenger receptor class B type 1 |
| LpR | lipophorin receptor |
References
- Haines, T.H. Do Sterols Reduce Proton and Sodium Leaks through Lipid Bilayers? Prog. Lipid Res. 2001, 40, 299–324. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Rybczynski, R.; Warren, J.T. Control and Biochemical Nature of the Ecdysteroidogenic Pathway. Annu. Rev. Entomol. 2002, 47, 883–916. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.A.; Young, K.E.; Beachy, P.A. Cholesterol Modification of Hedgehog Signaling Proteins in Animal Development. Science 1996, 274, 255–259. [Google Scholar] [CrossRef]
- Kuwabara, P.E.; Labouesse, M. The Sterol-Sensing Domain: Multiple Families, a Unique Role? Trends Genet. 2002, 18, 193–201. [Google Scholar] [CrossRef]
- Waelsch, H.; Sperry, W.M.; Stoyanoff, V.A. A Study of the Synthesis and Deposition of Lipids and Other Tissues with Deuterium as an Indicator. J. Biol. Chem. 1940, 135, 291–296. [Google Scholar]
- Björkhem, I.; Meaney, S.; Fogelman, A.M. Brain Cholesterol: Long Secret Life behind a Barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef]
- Dietschy, J.M. Central Nervous System: Cholesterol Turnover, Brain Development and Neurodegeneration. Biol. Chem. 2009, 390, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Magot, T.; Chevallier, F. Measurement of the Rate of Cholesterol Synthesis in Various Organs of the Rat in Vivo. Ann. Biol. Anim. Biochim. Biophys. 1979, 19, 1757–1770. [Google Scholar] [CrossRef]
- Daniels, T.F.; Killinger, K.M.; Michal, J.J.; Wright, R.W.; Jiang, Z. Lipoproteins, Cholesterol Homeostasis and Cardiac Health. Int. J. Biol. Sci. 2009, 5, 474–488. [Google Scholar] [CrossRef]
- Saini, H.K.; Arneja, A.S.; Dhalla, N.S. Role of Cholesterol in Cardiovascular Dysfunction. Can. J. Cardiol. 2004, 20, 333–346. [Google Scholar]
- Wollmer, M.A. Cholesterol-Related Genes in Alzheimer’s Disease. Biochim. Biophys. Acta 2010, 1801, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.; Clark, A.J.; Fraenkel, G.; Langdon, R.G. Impaired Steroid Biogenesis in Insect Larvae. Biochim. Biophys. Acta 1956, 21, 176. [Google Scholar] [CrossRef]
- Clayton, R.B. The Utilization of Sterols by Insects. J. Lipid Res. 1964, 15, 3–19. [Google Scholar]
- Ito, T. Sterol Requirements of the Silkworm, Bombyx Mori. Nature 1961, 191, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Ikekawa, N.; Suzuki, M.; Kobayashi, M.; Tsuda, K. Studies on the Sterol of Bombyx Mori L. Ⅳ. Conversion of the Sterol in the Silkworm. Chem. Pharm. Bull. 1966, 14, 834–836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naito, K.; Hamamura, Y. Studies on the Micro Constituent in Mulberry Leaves Part VII. Isolation of Rutin and Quercetin from Mulberry Leaves. Agric. Chem. Soc. Japan 1961, 35, 848–850. [Google Scholar] [CrossRef]
- Igarashi, F.; Hikiba, J.; Ogihara, M.H.; Nakaoka, T.; Suzuki, M.; Kataoka, H. A Highly Specific and Sensitive Quantification Analysis of the Sterols in Silkworm Larvae by High Performance Liquid Chromatography-Atmospheric Pressure Chemical Ionization-Tandem Mass Spectrometry. Anal. Biochem. 2011, 419, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Nagasawa, H. Sterol Composition in Larvae of the Silkworm, Bombyx Mori. Biosci. Biotechnol. Biochem. 2011, 75, 1003–1005. [Google Scholar] [CrossRef]
- Svoboda, J.A. Variability of Metabolism and Function of Sterols in Insects. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 49–57. [Google Scholar] [CrossRef]
- Ciufo, L.F.; Murray, P.A.; Thompson, A.; Rigden, D.J.; Rees, H.H. Characterisation of a Desmosterol Reductase Involved in Phytosterol Dealkylation in the Silkworm, Bombyx Mori. PLoS ONE 2011, 6, e21316. [Google Scholar] [CrossRef]
- Van der Horst, D.J.; Roosendaal, S.D.; Rodenburg, K.W. Circulatory Lipid Transport: Lipoprotein Assembly and Function from an Evolutionary Perspective. Mol. Cell. Biochem. 2009, 326, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Chino, H.; Gilbert, L.I. Diglyceride Release from Insect Fat Body: A Possible Means of Lipid Transport. Science 1964, 143, 359–361. [Google Scholar] [CrossRef]
- Chino, H.; Downer, R.G.H.; Wyatt, G.R.; Gilbert, L.I. Lipophorins, a Major Class of Lipoproteins of Insect Haemolymph. Insect Biochem. 1981, 11, 491. [Google Scholar] [CrossRef]
- Chino, H.; Downer, R.G.H. Insect Hemolymph Lipophorin: A Mechanism of Lipid Transport in Insects. Adv. Biophys. 1982, 15, 67–92. [Google Scholar] [CrossRef]
- Van Hoof, D.; Rodenburg, K.W.; Van der Horst, D.J. Lipophorin Receptor-Mediated Lipoprotein Endocytosis in Insect Fat Body Cells. J. Lipid Res. 2003, 44, 1431–1440. [Google Scholar] [CrossRef]
- Miura, K.; Shimizu, I. Identification and Properties of Lipophorin of the Silkworm, Bombyx Mori. Biochem. Physiol. 1988, 89, 95–103. [Google Scholar] [CrossRef]
- Behmer, S.T. Overturning Dogma: Tolerance of Insects to Mixed-Sterol Diets Is Not Universal. Curr. Opin. Insect Sci. 2017, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.E.; Woodruff, E.A.; Liang, P.; Patten, M.; Broadie, K. Neuronal Loss of Drosophila NPC1a Causes Cholesterol Aggregation and Age-Progressive Neurodegeneration. J. Neurosci. 2008, 28, 6569–6582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Kamiyama, T.; Niwa, R.; King-Jones, K. The Drosophila CCR4-NOT Complex Is Required for Cholesterol Homeostasis and Steroid Hormone Synthesis. Dev. Biol. 2018, 443, 10–18. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vaquero, D.; Parra-Peralbo, E.; Mejía-Morales, J.E.; Culi, J. Drosophila Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake. PLoS Genet. 2015. [Google Scholar] [CrossRef]
- Enya, S.; Ameku, T.; Igarashi, F.; Iga, M.; Kataoka, H.; Shinoda, T.; Niwa, R. A Halloween Gene Noppera-Bo Encodes a Glutathione S-Transferase Essential for Ecdysteroid Biosynthesis via Regulating the Behaviour of Cholesterol in Drosophila. Sci. Rep. 2015, 4, 6586. [Google Scholar] [CrossRef] [PubMed]
- Weers, P.M.; van der Horst, D.J.; van Marrewijk, W.J.; van den Eijnden, M.; van Doorn, J.M.; Beenakkers, A.M. Biosynthesis and Secretion of Insect Lipoprotein. J. Lipid Res. 1992, 33, 485–491. [Google Scholar] [PubMed]
- Dantuma, N.P.; Potters, M.; De Winther, M.P.; Tensen, C.P.; Kooiman, F.P.; Bogerd, J.; Van der Horst, D.J. An Insect Homolog of the Vertebrate Very Low Density Lipoprotein Receptor Mediates Endocytosis of Lipophorins. J. Lipid Res. 1999, 40, 973–978. [Google Scholar]
- Van Hoof, D.; Rodenburg, K.W.; Van Der Horst, D.J. Receptor-Mediated Endocytosis and Intracellular Trafficking of Lipoproteins and Transferrin in Insect Cells. Insect Biochem. Mol. Biol. 2005, 35, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Gopalapillai, R.; Kadono-Okuda, K.; Tsuchida, K.; Yamamoto, K.; Nohata, J.; Ajimura, M.; Mita, K. Lipophorin Receptor of Bombyx Mori: CDNA Cloning, Genomic Structure, Alternative Splicing, and Isolation of a New Isoform. J. Lipid Res. 2006, 47, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, F.; Ogihara, M.H.; Iga, M.; Kataoka, H. Cholesterol Internalization and Metabolism in Insect Prothoracic Gland, a Steroidogenic Organ, via Lipoproteins. Steroids 2018, 134, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lavrynenko, O.; Rodenfels, J.; Carvalho, M.; Dye, N.A.; Lafont, R.; Eaton, S.; Shevchenko, A. The Ecdysteroidome of Drosophila: Influence of Diet and Development. Development 2015, 142, 3758–3768. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Wang, Y.; Green, M.; Lin, D.S. Effects of Diet and Metamorphosis upon the Sterol Composition of the Butterfly Morpho Peleides. J. Lipid Res. 2006, 47, 1444–1448. [Google Scholar] [CrossRef]
- Roth, G.E.; Gierl, M.S.; Vollborn, L.; Meise, M.; Lintermann, R.; Korge, G. The Drosophila Gene Start1: A Putative Cholesterol Transporter and Key Regulator of Ecdysteroid Synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1601–1606. [Google Scholar] [CrossRef]
- Huang, X. A Drosophila Model of the Niemann-Pick Type C Lysosome Storage Disease: Dnpc1a Is Required for Molting and Sterol Homeostasis. Development 2005, 132, 5115–5124. [Google Scholar] [CrossRef]
- Voght, S.P.; Fluegel, M.L.; Andrews, L.A.; Pallanck, L.J. Drosophila NPC1b Promotes an Early Step in Sterol Absorption from the Midgut Epithelium. Cell Metab. 2007, 5, 195–205. [Google Scholar] [CrossRef]
- Fluegel, M.L.; Parker, T.J.; Pallanck, L.J. Mutations of a Drosophila NPC1 Gene Confer Sterol and Ecdysone Metabolic Defects. Genetics 2006, 172, 185–196. [Google Scholar] [CrossRef]
- Hikiba, J.; Ogihara, M.H.; Iga, M.; Saito, K.; Fujimoto, Y.; Suzuki, M.; Kataoka, H. Simultaneous Quantification of Individual Intermediate Steroids in Silkworm Ecdysone Biosynthesis by Liquid Chromatography-Tandem Mass Spectrometry with Multiple Reaction Monitoring. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 915–916, 52–56. [Google Scholar] [CrossRef]
- Iga, M.; Kataoka, H. Recent Studies on Insect Hormone Metabolic Pathways Mediated by Cytochrome P450 Enzymes. Biol. Pharm. Bull. 2012, 35, 838–843. [Google Scholar] [CrossRef]
- Carvalho, M.; Schwudke, D.; Sampaio, J.L.; Palm, W.; Riezman, I.; Dey, G.; Gupta, G.D.; Mayor, S.; Riezman, H.; Shevchenko, A.; et al. Survival Strategies of a Sterol Auxotroph. Development 2010, 137, 3675–3685. [Google Scholar] [CrossRef]
- Bidet, M.; Joubert, O.; Lacombe, B.; Ciantar, M.; Nehmé, R.; Mollat, P.; Brétillon, L.; Faure, H.; Bittman, R.; Ruat, M.; et al. The Hedgehog Receptor Patched Is Involved in Cholesterol Transport. PLoS ONE 2011, 6, e23834. [Google Scholar] [CrossRef]
- Chai, P.C.; Liu, Z.; Chia, W.; Cai, Y. Hedgehog Signaling Acts with the Temporal Cascade to Promote Neuroblast Cell Cycle Exit. PLoS Biol. 2013, 11, e1001494. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, K.M.; Choi, C.O.; Song, H.Y.; Lee, C.S.; Kim, B.W.; Kang, P.D.; Jeon, S.H.; Cho, B.P.; Bae, Y.J.; et al. Apoptosis of Neuronal Cells in the Brains of Postembryonic Silkworms Bombyx Mori (Lepidoptera: Bombycidae). Eur. J. Entomol. 2009, 106, 335–345. [Google Scholar] [CrossRef]
- Schubiger, M.; Wade, A.A.; Carney, G.E.; Truman, J.W.; Bender, M. Drosophila EcR-B Ecdysone Receptor Isoforms Are Required for Larval Molting and for Neuron Remodeling during Metamorphosis. Development 1998, 125, 2053–2062. [Google Scholar]
- Fujishita, M.; Ohnishi, E.; Ishizaki, H. The Role of Ecdysteroids in the Determination of Gut-Purge Timing in the Saturniid, Samia Cynthia Ricini. J. Insect Physiol. 1982, 28, 961–967. [Google Scholar] [CrossRef]
- Fujishita, M.; Ishizaki, H. Temporal Organization of Endocrine Events in Relation to the Circadian Clock during Larval-Pupal Development in Samia Cynthia Ricini. J. Insect Physiol. 1982, 28, 77–84. [Google Scholar] [CrossRef]
- Dominick, O.S.; Truman, J.W. The Physiology of Wandering Behaviour in Manduca Sexta. III. Organization of Wandering Behaviour in the Larval Nervous System. J. Exp. Biol. 1986, 121, 115–132. [Google Scholar]
- Miller, J.E.; Levine, R.B. Steroid Hormone Activation of Wandering in the Isolated Nervous System of Manduca Sexta. J. Comp. Physiol. A 2006, 192, 1049–1062. [Google Scholar] [CrossRef]
- Warren, J.T.; Sakurai, S.; Rountree, D.B.; Gilbert, L.I.; Lee, S.S.; Nakanishi, K. Regulation of the Ecdysteroid Titer of Manduca Sexta: Reappraisal of the Role of the Prothoracic Glands. Proc. Natl. Acad. Sci. USA 1988, 85, 958–962. [Google Scholar] [CrossRef]
- Tsuchida, K.; Sakudoh, T. Recent Progress in Molecular Genetic Studies on the Carotenoid Transport System Using Cocoon-Color Mutants of the Silkworm. Arch. Biochem. Biophys. 2015, 572, 151–157. [Google Scholar] [CrossRef]
- Sakudoh, T.; Kuwazaki, S.; Iizuka, T.; Narukawa, J.; Yamamoto, K.; Uchino, K.; Sezutsu, H.; Banno, Y.; Tsuchida, K. CD36 Homolog Divergence Is Responsible for the Selectivity of Carotenoid Species Migration to the Silk Gland of the Silkworm Bombyx Mori. J. Lipid Res. 2013, 54, 482–495. [Google Scholar] [CrossRef]
- Sakudoh, T.; Iizuka, T.; Narukawa, J.; Sezutsu, H.; Kobayashi, I.; Kuwazaki, S.; Banno, Y.; Kitamura, A.; Sugiyama, H.; Takada, N.; et al. A CD36-Related Transmembrane Protein Is Coordinated with an Intracellular Lipid-Binding Protein in Selective Carotenoid Transport for Cocoon Coloration. J. Biol. Chem. 2010, 285, 7739–7751. [Google Scholar] [CrossRef]
- Duan, L.P.; Wang, H.H.; Wang, D.Q.H. Cholesterol Absorption Is Mainly Regulated by the Jejunal and Ileal ATP-Binding Cassette Sterol Efflux Transporters Abcg5 and Abcg8 in Mice. J. Lipid Res. 2004, 45, 1312–1323. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T. The ABC Gene Family in Arthropods: Comparative Genomics and Role Ininsecticide Transport and Resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, S.; Tian, L.; Guo, E.; Luan, Y.; Zhang, J.; Li, S. Genome-Wide Identification and Characterization of ATP-Binding Cassette Transporters in the Silkworm, Bombyx Mori. BMC Genomics 2011. [Google Scholar] [CrossRef]
- Sissener, N.H.; Rosenlund, G.; Stubhaug, I.; Liland, N.S. Tissue Sterol Composition in Atlantic Salmon (Salmo Salar L.) Depends on the Dietary Cholesterol Content and on the Dietary Phytosterol:Cholesterol Ratio, but Not on the Dietary Phytosterol Content. Br. J. Nutr. 2018, 119, 599–609. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, J.W. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 911–917. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshima, M.; Ogihara, M.H.; Kataoka, H. Sterol Characteristics in Silkworm Brain and Various Tissues Characterized by Precise Sterol Profiling Using LC-MS/MS. Int. J. Mol. Sci. 2019, 20, 4840. https://doi.org/10.3390/ijms20194840
Takeshima M, Ogihara MH, Kataoka H. Sterol Characteristics in Silkworm Brain and Various Tissues Characterized by Precise Sterol Profiling Using LC-MS/MS. International Journal of Molecular Sciences. 2019; 20(19):4840. https://doi.org/10.3390/ijms20194840
Chicago/Turabian StyleTakeshima, Mika, Mari H. Ogihara, and Hiroshi Kataoka. 2019. "Sterol Characteristics in Silkworm Brain and Various Tissues Characterized by Precise Sterol Profiling Using LC-MS/MS" International Journal of Molecular Sciences 20, no. 19: 4840. https://doi.org/10.3390/ijms20194840
APA StyleTakeshima, M., Ogihara, M. H., & Kataoka, H. (2019). Sterol Characteristics in Silkworm Brain and Various Tissues Characterized by Precise Sterol Profiling Using LC-MS/MS. International Journal of Molecular Sciences, 20(19), 4840. https://doi.org/10.3390/ijms20194840