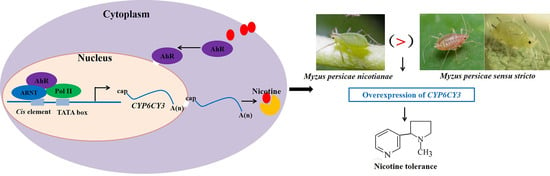
Transcription Factors AhR/ARNT Regulate the Expression of CYP6CY3 and CYP6CY4 Switch Conferring Nicotine Adaptation
Abstract
1. Introduction
2. Results
2.1. Knockdown of CYP6CY3 and CYP6CY4 Together Significantly Increases Nicotine Toxicity in M. Persicae Nicotianae
2.2. Characterization of the Element in the CYP6CY3 Promoter Sequence
2.3. Identification of Core Cis Element Binding Proteins
2.4. Regulation Effect of AhR and ARNT on CYP6CY3
2.5. Transcription Factor Modulation Impacts CYP6CY4 Expression
3. Discussion
4. Materials and Methods
4.1. Insects and Cell Culture
4.2. Quantitative RT-PCR and Data Analysis
4.3. Cloning of CYP6CY3 5′ Flanking Sequences and Sequence Analyses
4.4. Construction of CYP6CY3 Promoter-pGL3 Constructs
4.5. Transient Transfection and Dual Luciferase Assay
4.6. Isolation of Cis Element Binding Proteins, Protein Identification and Bioinformatics Analysis
4.7. Co-transfection of AhR, ARNT or CncC with CYP6CY3 Promoter-pGL3
4.8. Rearing on an Artificial Diet and dsRNA Feeding
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| P450 | cytochrome P450 monooxygenase |
| EcRE | ecdysone response element |
| ARE | antioxidant response element |
| XRE | xenobiotic response element |
| XRE-xan | xenobiotic response element to xanthotoxin |
| AhR | aryl hydrocarbon receptor |
| ARNT | aryl hydrocarbon receptor nuclear translocator |
| XRE-Fla | xenobiotic response element to flavone |
| bHLH | basic helix-loop-helix |
| CncC Camp | cap ‘n’ collar/basic region leucine zipper (Cnc-bZIP) transcription factor; cyclic AMP-dependent transcription factor |
| PAS (PER-ARNT-SIM) | period (Per)-ARNT-single-minded (Sim) |
| Q-rich | Glutamine-rich |
| UTR | Untranslated Regions |
| TSS | transcription start site |
| CE/HPLC-MS | capillary high-performance liquid chromatography-mass spectral analysis |
| NCBI | The National Center for Biotechnology Information |
| SRA | Sequence Read Archive |
| Hsp90 | heat shock protein 90 |
| ECFP | Enhanced cyan fluorescent protein |
| dsRNA | double-stranded RNA |
| RNAi | RNA interference |
| qRT-PCR | quantitative RT-PCR |
References
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Blackman, R.L. Morphological discrimination of a tobacco-feeding form from Myzus persicae (Sulzer) (Hemiptera: Aphididae), and a key to New world Myzus (Nectarosiphon) species. Bull. Entomol. Res. 1987, 77, 713–730. [Google Scholar] [CrossRef]
- Margaritopoulos, J.T.; Malarky, G.; Tsitsipis, J.A.; Blackman, R.L. Microsatellite DNA and behavioural studies provide evidence of host-mediated speciation in Myzus persicae (Hemiptera: Aphididae). Biol. J. Linn. Soc. Lond. 2007, 91, 687–702. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Wang, S.F.; Reidy-Crofts, J.; Edwards, O.R. Phloem alkaloid tolerance allows feeding on resistant Lupinus angustifolius by the aphid Myzus persicae. J. Chem. Ecol. 2006, 32, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Berenbaum, M.R.; Schuler, M.A. Molecular cloning and expression of CYP6B8: A xanthotoxin-inducible cytochrome P450 cDNA from Helicoverpa zea. Insect Biochem. Mol. Biol. 2000, 30, 75–84. [Google Scholar] [CrossRef]
- Li, W.; Berenbaum, M.R.; Schuler, M.A. Molecular analysis of multiple CYP6B genes from polyphagous papilio species. Insect Biochem. Mol. Biol. 2001, 31, 999–1011. [Google Scholar] [CrossRef]
- Li, X.; Berenbaum, M.R.; Schuler, M.A. Plant allelochemicals differentially regulate Helicoverpa zea cytochrome P450 genes. Insect Mol. Biol. 2002, 11, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 236–295. [Google Scholar]
- Schuler, M.A. P450s in plant-insect interactions. Biochim. Biophys. Acta. 2011, 1814, 36–45. [Google Scholar] [CrossRef]
- Schuler, M.A. Insect P450s: Mounted for battle in their war against toxins. Mol. Ecol. 2012, 21, 4157–4159. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef]
- Mao, W.; Berhow, M.A.; Zangerl, A.R.; McGovern, J.; Berenbaum, M.R. Cytochrome P450-mediated metabolism of xanthotoxin by papilio multicaudatus. J. Chem. Ecol. 2006, 32, 523–536. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef]
- Snyder, M.J.; Glendinning, J.I. Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. J. Comp. Physiol. A 1996, 179, 255–261. [Google Scholar] [CrossRef]
- Bass, C.; Zimmer, C.T.; Riveron, J.M.; Wilding, C.S.; Wondji, C.S.; Kaussmann, M.; Field, L.M.; Williamson, M.S.; Nauen, R. Gene amplification and microsatellite polymorphism underlie a recent insect host shift. Proc. Natl. Acad. Sci. USA 2013, 110, 19460–19465. [Google Scholar] [CrossRef]
- Ramsey, J.S.; Elzinga, D.A.; Sarkar, P.; Xin, Y.R.; Ghanim, M.; Jander, G. Adaptation to nicotine feeding in Myzus persicae. J. Chem. Ecol. 2014, 40, 869–877. [Google Scholar] [CrossRef]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef]
- Brown, R.P.; McDonnell, C.M.; Berenbaum, M.R.; Schuler, M.A. Regulation of an insect cytochrome P450 monooxygenase gene (CYP6B1) by aryl hydrocarbon and xanthotoxin response cascades. Gene 2005, 358, 39–52. [Google Scholar] [CrossRef]
- McDonnell, C.M.; Brown, R.P.; Berenbaum, M.R.; Schuler, M.A. Conserved regulatory elements in the promoters of two allelochemical-inducible cytochrome P450 genes differentially regulate transcription. Insect Biochem. Mol. Biol. 2004, 34, 1129–1139. [Google Scholar] [CrossRef]
- Petersen, R.A.; Niamsup, H.; Berenbaum, M.R.; Schuler, M.A. Transcriptional response elements in the promoter of CYP6B1, an insect P450 gene regulated by plant chemicals. Biochim. Biophys. Acta. 2003, 1619, 269–282. [Google Scholar] [CrossRef]
- Petersen Brown, R.; Berenbaum, M.R.; Schuler, M.A. Transcription of a lepidopteran cytochrome P450 promoter is modulated by multiple elements in its 5′ UTR and repressed by 20-hydroxyecdysone. Insect Mol. Biol. 2004, 13, 337–347. [Google Scholar] [CrossRef]
- Peng, T.; Pan, Y.; Gao, X.; Xi, J.; Zhang, L.; Ma, K.; Wu, Q.; Zhang, J.; Shang, Q. Reduced abundance of the CYP6CY3-targeting let-7 and miR-100 miRNAs accounts for host adaptation of Myzus persicae nicotianae. Insect Biochem. Mol. Biol. 2016, 75, 89–97. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Coumailleau, P.; Poellinger, L.; Gustafsson, J.A.; Whitelaw, M.L. Definition of a minimal domain of the dioxin receptor that is associated with Hsp90 and maintains wild type ligand binding affinity and specificity. J. Biol. Chem. 1995, 270, 25291–25300. [Google Scholar] [CrossRef]
- Goryo, K.; Suzuki, A.; Del Carpio, C.A.; Siizaki, K.; Kuriyama, E.; Mikami, Y.; Kinoshita, K.; Yasumoto, K.; Rannug, A.; Miyamoto, A.; et al. Identification of amino acid residues in the Ah receptor involved in ligand binding. Biochem. Biophys. Res. Commun. 2007, 354, 396–402. [Google Scholar] [CrossRef]
- Kumar, M.B.; Ramadoss, P.; Reen, R.K.; Vanden Heuvel, J.P.; Perdew, G.H. The Q-rich subdomain of the human Ah receptor transactivation domain is required for dioxin-mediated transcriptional activity. J. Biol. Chem. 2001, 276, 42302–42310. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, C.; Gao, X.; Peng, T.; Bi, R.; Xi, J.; Xin, X.; Zhu, E.; Wu, Y.; Shang, Q. Spirotetramat resistance adaption analysis of Aphis gossypii Glover by transcriptomic survey. Pestic. Biochem. Physiol. 2015, 124, 73–80. [Google Scholar] [CrossRef]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Mao, Y.B.; Tao, X.Y.; Xue, X.Y.; Wang, L.J.; Chen, X.Y. Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res. 2011, 20, 665–673. [Google Scholar] [CrossRef]
- Lenhard, B.; Sandelin, A.; Carninci, P. Metazoan promoters: Emerging characteristics and insights into transcriptional regulation. Nat. Rev. Genet. 2012, 13, 233–245. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Kalay, G. Cis-regulatory elements: Molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 2012, 13, 59–69. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, X.; Ni, X.; Zhang, Y.; Li, X. Functional characterization of cis-acting elements mediating flavone-inducible expression of CYP321A1. Insect Biochem. Mol. Biol. 2010, 40, 898–908. [Google Scholar] [CrossRef]
- Zhang, C.; Wong, A.; Zhang, Y.; Ni, X.; Li, X. Common and unique cis-acting elements mediate xanthotoxin and flavone induction of the generalist P450 CYP321A1. Sci. Rep. 2014, 4, 6490. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription factors, CncC and Maf, regulate expression of CYP6BQ genes responsible for deltamethrin resistance in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 65, 47–56. [Google Scholar] [CrossRef]
- Misra, J.R.; Horner, M.A.; Lam, G.; Thummel, C.S. Transcriptional regulation of xenobiotic detoxification in drosophila. Genes Dev. 2011, 25, 1796–17806. [Google Scholar] [CrossRef]
- Gemayel, R.; Vinces, M.D.; Legendre, M.; Verstrepen, K.J. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu. Rev. Genet. 2010, 44, 445–477. [Google Scholar] [CrossRef]
- Vinces, M.D.; Legendre, M.; Caldara, M.; Hagihara, M.; Verstrepen, K.J. Unstable tandem repeats in promoters confer transcriptional evolvability. Science 2009, 324, 1213–1216. [Google Scholar] [CrossRef]
- Rockman, M.V.; Wray, G.A. Abundant raw material for cis-regulatory evolution in humans. Mol. Biol. Evol. 2002, 19, 1991–2004. [Google Scholar] [CrossRef]
- Huang, T.S.; Lee, C.C.; Chang, A.C.; Lin, S.; Chao, C.C.; Jou, Y.S.; Chu, Y.W.; Wu, C.W.; Whang-Peng, J. Shortening of microsatellite deoxy(CA) repeats involved in GL331-induced down-regulation of matrix metalloproteinase-9 gene expression. Biochem. Biophys. Res. Commun. 2003, 300, 901–907. [Google Scholar] [CrossRef]
- Shimajiri, S.; Arima, N.; Tanimoto, A.; Murata, Y.; Hamada, T.; Wang, K.Y.; Sasaguri, Y. Shortened microsatellite d(CA)21 sequence down-regulates promoter activity of matrix metalloproteinase 9 gene. FEBS Lett. 1999, 455, 70–74. [Google Scholar] [CrossRef]
- Kewley, R.J.; Whitelaw, M.L.; Chapman-Smith, A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004, 36, 189–204. [Google Scholar] [CrossRef]
- Perdew, G.H. Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. 1988, 263, 13802–13805. [Google Scholar]
- Beischlag, T.V.; Luis Morales, J.; Hollingshead, B.D.; Perdew, G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250. [Google Scholar] [CrossRef]
- Chan, W.K.; Yao, G.; Gu, Y.Z.; Bradfield, C.A. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J. Biol. Chem. 1999, 274, 12115–12123. [Google Scholar] [CrossRef]
- Chiaro, C.R.; Patel, R.D.; Marcus, C.B.; Perdew, G.H. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol. Pharmacol. 2007, 72, 1369–1379. [Google Scholar] [CrossRef]
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef]
- Nukaya, M.; Moran, S.; Bradfield, C.A. The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc. Natl. Acad. Sci. USA 2009, 106, 4923–4928. [Google Scholar] [CrossRef]
- Sato, W.; Suzuki, H.; Sasaki, T.; Kumagai, T.; Sakaguchi, S.; Mizugaki, M.; Miyairi, S.; Yamazoe, Y.; Nagata, K. Construction of a system that simultaneously evaluates CYP1A1 and CYP1A2 induction in a stable human-derived cell line using a dual reporter plasmid. Drug Metab. Pharmacokinet. 2010, 25, 180–189. [Google Scholar] [CrossRef]
- Schmidt, J.V.; Bradfield, C.A. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996, 12, 55–89. [Google Scholar] [CrossRef]
- Whitlock, J.P., Jr. Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 103–125. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Peng, T.; Pan, Y.; Gao, X.; Xi, J.; Zhang, L.; Yang, C.; Bi, R.; Yang, S.; Xin, X.; Shang, Q. Cytochrome P450 CYP6DA2 regulated by CncC is associated with the gossypol tolerance in Aphis gossypii Glover. Insect Mol. Biol. 2016, 25, 450–459. [Google Scholar] [CrossRef]
- Peng, T.; Pan, Y.; Yang, C.; Gao, X.; Xi, J.; Wu, Y.; Huang, X.; Zhu, E.; Xin, X.; Zhan, C.; et al. Over-expression of CYP6A2 is associated with spirotetramat resistance and cross-resistance in the resistant strain of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2016, 126, 64–69. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, X.; Shang, Q.; Shi, X.; Gao, X. Oral delivery mediated RNA interference of a carboxylesterase gene results in reduced resistance to organophosphorus insecticides in the cotton aphid, Aphis gossypii Glover. PLoS ONE 2014, 9, e102823. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Peng, T.; Xu, P.; Zeng, X.; Tian, F.; Song, J.; Shang, Q. Transcription Factors AhR/ARNT Regulate the Expression of CYP6CY3 and CYP6CY4 Switch Conferring Nicotine Adaptation. Int. J. Mol. Sci. 2019, 20, 4521. https://doi.org/10.3390/ijms20184521
Pan Y, Peng T, Xu P, Zeng X, Tian F, Song J, Shang Q. Transcription Factors AhR/ARNT Regulate the Expression of CYP6CY3 and CYP6CY4 Switch Conferring Nicotine Adaptation. International Journal of Molecular Sciences. 2019; 20(18):4521. https://doi.org/10.3390/ijms20184521
Chicago/Turabian StylePan, Yiou, Tianfei Peng, Pengjun Xu, Xiaochun Zeng, Fayi Tian, Jiabao Song, and Qingli Shang. 2019. "Transcription Factors AhR/ARNT Regulate the Expression of CYP6CY3 and CYP6CY4 Switch Conferring Nicotine Adaptation" International Journal of Molecular Sciences 20, no. 18: 4521. https://doi.org/10.3390/ijms20184521
APA StylePan, Y., Peng, T., Xu, P., Zeng, X., Tian, F., Song, J., & Shang, Q. (2019). Transcription Factors AhR/ARNT Regulate the Expression of CYP6CY3 and CYP6CY4 Switch Conferring Nicotine Adaptation. International Journal of Molecular Sciences, 20(18), 4521. https://doi.org/10.3390/ijms20184521