Shape-Controlled Syntheses of Magnetite Microparticles and Their Magnetorheology
Abstract
1. Introduction
2. Results
2.1. Syntheses of Spherical and Octahedral Fe3O4 Microparticles
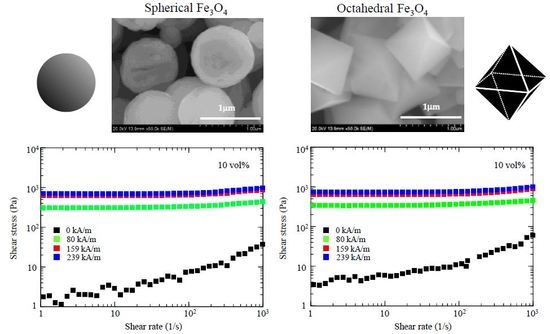
2.2. Magnetorheology of The Shape-Controlled Fe3O4 Microparticles
3. Discussion
3.1. Synthesis Mechanism
3.1.1. Phase Transformation
3.1.2. Shape Control
3.2. Shape Effect on Magnetorheology
4. Materials and Methods
4.1. Shape-Controlled Synthesis of Fe3O4 Microparticles
4.2. Preparation of Fe3O4 Microparticle-Based Suspensions
4.3. Characterization of Fe3O4 Microparticles and Evaluation of MR Fluids
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carlson, J.D.; Jolly, M.R. MR fluid, foam and elastomer devices. Mechatronics 2000, 10, 555–569. [Google Scholar] [CrossRef]
- Murakami, T.; Sakai, M.; Nakano, M. Study on the development of passive MR damper with displacement-dependent damping characteristics. J. Fluid Sci. Technol. 2010, 5, 985–992. [Google Scholar] [CrossRef][Green Version]
- Kikuchi, T.; Noma, J.; Akaiwa, S.; Uechima, Y. Response time of magnetorheological fluid-based haptic devices. J. Intell. Materal Syst. Struct. 2016, 27, 859–865. [Google Scholar] [CrossRef]
- Kikuchi, T.; Tanaka, T.; Anzai, K.; Hosaka, M.; Niino, K. Evaluation of line-tracing controller of intelligently controllable walker. Adv. Robot. 2013, 27, 493–502. [Google Scholar] [CrossRef]
- Park, B.J.; Fang, F.F.; Choi, H.J. Magnetorheology: Materials and application. Soft Matter 2010, 16, 5246–5252. [Google Scholar] [CrossRef]
- Ashtiani, M.; Hashemabadi, S.H.; Ghaffari, A. A review on the magnetorheological fluid preparation and stabilization. J. Magn. Magn. Mater. 2015, 374, 716–730. [Google Scholar] [CrossRef]
- Lemaire, E.; Meunier, A.; Bossis, G.; Liu, J.; Felt, D.; Bashtovoi, P.; Matoussevitch, N. Influence of the particle size on the rheology of magnetorheological fluids. J. Rheol. 1995, 39, 1011–1020. [Google Scholar] [CrossRef]
- Lopez-Lopez, M.T.; Kuzhir, P.; Meunier, A.; Bossis, G. Synthesis and magnetorheology of suspensions of submicron-sized cobalt particles with tunable particle size. J. Phys. Condens. Matter 2010, 22, 324106. [Google Scholar] [CrossRef]
- Pei, L.; Pang, H.; Chen, K.; Xuan, S.; Gong, X. Simulation of the optimal and wall thickness of hollow Fe3O4 microspheres in magnetorheological fluids. Soft Mater. 2018, 15, 5080–5091. [Google Scholar] [CrossRef]
- Bae, D.H.; Han, W.J.; Gao, C.Y.; Dong, Y.Z.; Choi, H.J. Preparation and magnetorheological response of triangular-shaped single-crystalline magnetite particle-based magnetic fluid. IEEE Tans. Mag. 2018, 54, 2001504. [Google Scholar]
- Han, S.; Choi, J.; Seo, Y.P.; Park, J.I.; Choi, H.J. High-performance magnetorheological suspensions of pickering-emulsion-polymerized polystyrene/Fe3O4 particles with enhanced stability. Langmuir 2018, 43, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, S.; Wu, W. Shape controlled inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Fu, Z.; Ghosen, J.; Zeng, M.; Stebbins, J.; Pasad, P.N.; Swihart, M.T. Standardizing size-and shape-controlled synthesis of monodisperse magnetite (Fe3O4) nanocrystals by identifying and exploiting effects of organic impurities. ACS Nano. 2017, 11, 6370–6381. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Chan, H.; Gelman, E.; Repnin, N.; Karl, P.; Kajn, R. Self-assembly of magnetite nanocubes into helical superstructures. Science 2014, 345, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Lanerisheth, Z.; Upadhyay, R.V. Influence of particle shape on the magnetic and steady shear magnetorheological properties of nanoparticles based MR fluids. Smart Mater. Struct. 2017, 26, 054008. [Google Scholar] [CrossRef]
- Jung, H.S.; Choi, H.J. Hydrothermal fabrication of octahedral-shaped Fe3O4 nanoparticles and their magnetorheological response. J. Appl. Phys. 2015, 117, 17E708. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Blin, B.; Beaudoin, B.; Figlarz, M. Homogeneous and heterogeneous nucleations in the polyol process for the preparation of micron and submicron size metal particles. Solid Sate Ion. 1989, 32, 198–205. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory, 2nd ed.; WILEY-VCH: Weinheim, Germany, 2000; pp. 27–32. [Google Scholar]
- Vereda, F.; de Vicente, J.; del Puerto Morales, M.; Rull, F.; Hidalgo-Alvarez, R. Synthesis and Characterization of Single-Domain Monocrystalline Magnetite Particles by Oxidative Aging of Fe(OH)2. J. Phys. Chem. C. 2008, 115, 5843–5849. [Google Scholar] [CrossRef]
- Domingo, C.; Rodriguez-Clemente, R.; Blesa, M. Morphological properties of α-FeOOOG, γ-FeOOH and Fe3O4 obtained by oxidation of aqueous Fe(II) solutions. J. Colloid. Inter. Sci. 1994, 165, 244–252. [Google Scholar] [CrossRef]
- Yamanaka, S.; Abe, H.; Naito, M.; Fujimoto, T.; Kuga, Y. Colloidal dispersibility of fatty acid-capped iron nanoparticles and its effect on static and dynamic magnetorheological response. Colloids and Surfaces A: Physicochem. Eng. Aspects. 2012, 415, 239–246. [Google Scholar] [CrossRef]
- Vereda, F.; Segovia-Gutierrez, J.P.; de Vicente, J.; Hidalgo-Alvarez, R. Faceted particles: An approach for the enhancement of the elasticity and the yield-stress of magnetorheological fluids. J. Appl. Phys. 2016, 108, 211904. [Google Scholar] [CrossRef]
- Byrne, J.M.; Telling, N.D.; Coker, V.S.; Pattrick, R.A.D.; van der Laan, G.; Arenholz, E.; Tuna, F.; Lloyd, J.R. Control of nanoparticle size, reactivity and magnetic production during the bioproduction of magnetite by geobacter sulfurreducens. Nanotechnology 2011, 22, 455709. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, M.; Mitsui, D.; Wu, H.L.; Sato, R.; Teranishi, T. Simple reductant concentration-dependent shape control of polyhedral gold nanoparticles and their plasmonic properties. Langmuir 2012, 28, 9021–9026. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, A. Iron(III) hydrolysis and solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [PubMed]
- Baumgartner, J.; Dey, A.; Bomans, P.H.H.; Coadou, C.; Fratz, P.; Sommerdijk, N.A.J.M.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetite single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Wang, Y.-X.J.; Yu, J.C.; Leung, K.C.-F. Tuning the grain size and particle size of superparamagnetic Fe3O4. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matijevic, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid. Inter. Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of monodispersed spherical nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, B.; Vereda, F.; de Vicente, J.; Hidalgo-Alvarez, R. Rough and hollow spherical magnetite microparticles; revealing the morphology, internal structure, and growth mechanism. J. Phys. Chem. C 2013, 117, 5397–5406. [Google Scholar] [CrossRef]
- Sugimoto, T.; Sakata, K.; Muramatsu, A. Formation mechanism of monodispersed pseudocubic α-Fe2O3 particles from condensed ferric hydroxide gel. J. Colloid. Inter. Sci. 1993, 159, 372–382. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, H.; Naka, T.; Sato, K.; Suzuki, Y.; Nakano, M. Shape-Controlled Syntheses of Magnetite Microparticles and Their Magnetorheology. Int. J. Mol. Sci. 2019, 20, 3617. https://doi.org/10.3390/ijms20153617
Abe H, Naka T, Sato K, Suzuki Y, Nakano M. Shape-Controlled Syntheses of Magnetite Microparticles and Their Magnetorheology. International Journal of Molecular Sciences. 2019; 20(15):3617. https://doi.org/10.3390/ijms20153617
Chicago/Turabian StyleAbe, Hiroya, Takashi Naka, Kazuyoshi Sato, Yoshikazu Suzuki, and Masami Nakano. 2019. "Shape-Controlled Syntheses of Magnetite Microparticles and Their Magnetorheology" International Journal of Molecular Sciences 20, no. 15: 3617. https://doi.org/10.3390/ijms20153617
APA StyleAbe, H., Naka, T., Sato, K., Suzuki, Y., & Nakano, M. (2019). Shape-Controlled Syntheses of Magnetite Microparticles and Their Magnetorheology. International Journal of Molecular Sciences, 20(15), 3617. https://doi.org/10.3390/ijms20153617