Trans-Ferulic Acid-4-β-Glucoside Alleviates Cold-Induced Oxidative Stress and Promotes Cold Tolerance
Abstract
:1. Introduction
2. Results
2.1. Trans-Ferulic Acid-4-β-Glucoside Improves Cold Tolerance in Mice under Cold (4 °C) Condition
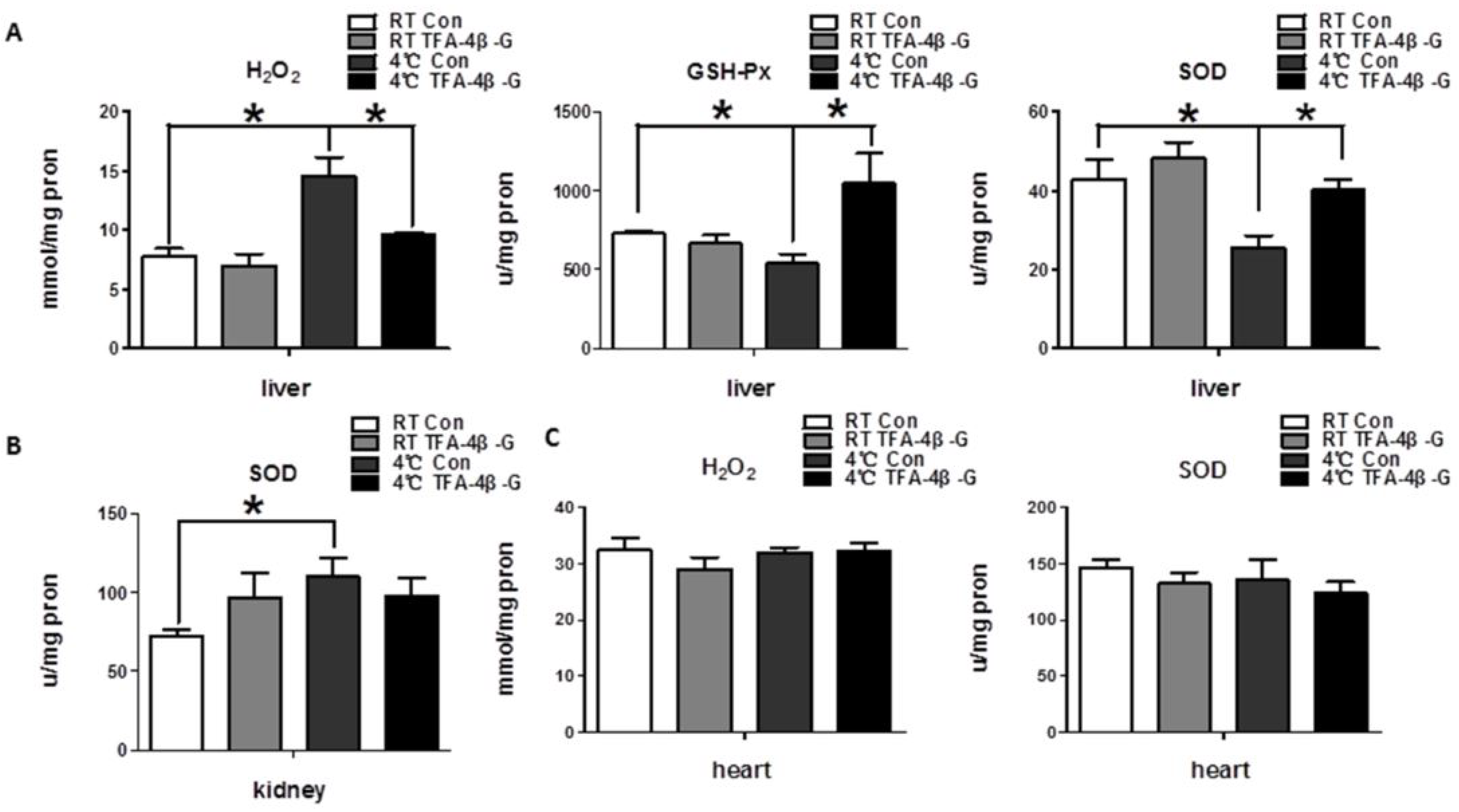
2.2. Trans-Ferulic Acid-4-β-Glucoside Alleviates Cold-Induced Oxidative Stress in Liver
2.3. Trans-Ferulic Acid-4-β-Glucoside Promotes Thermogenesis in Liver upon Cold Exposure
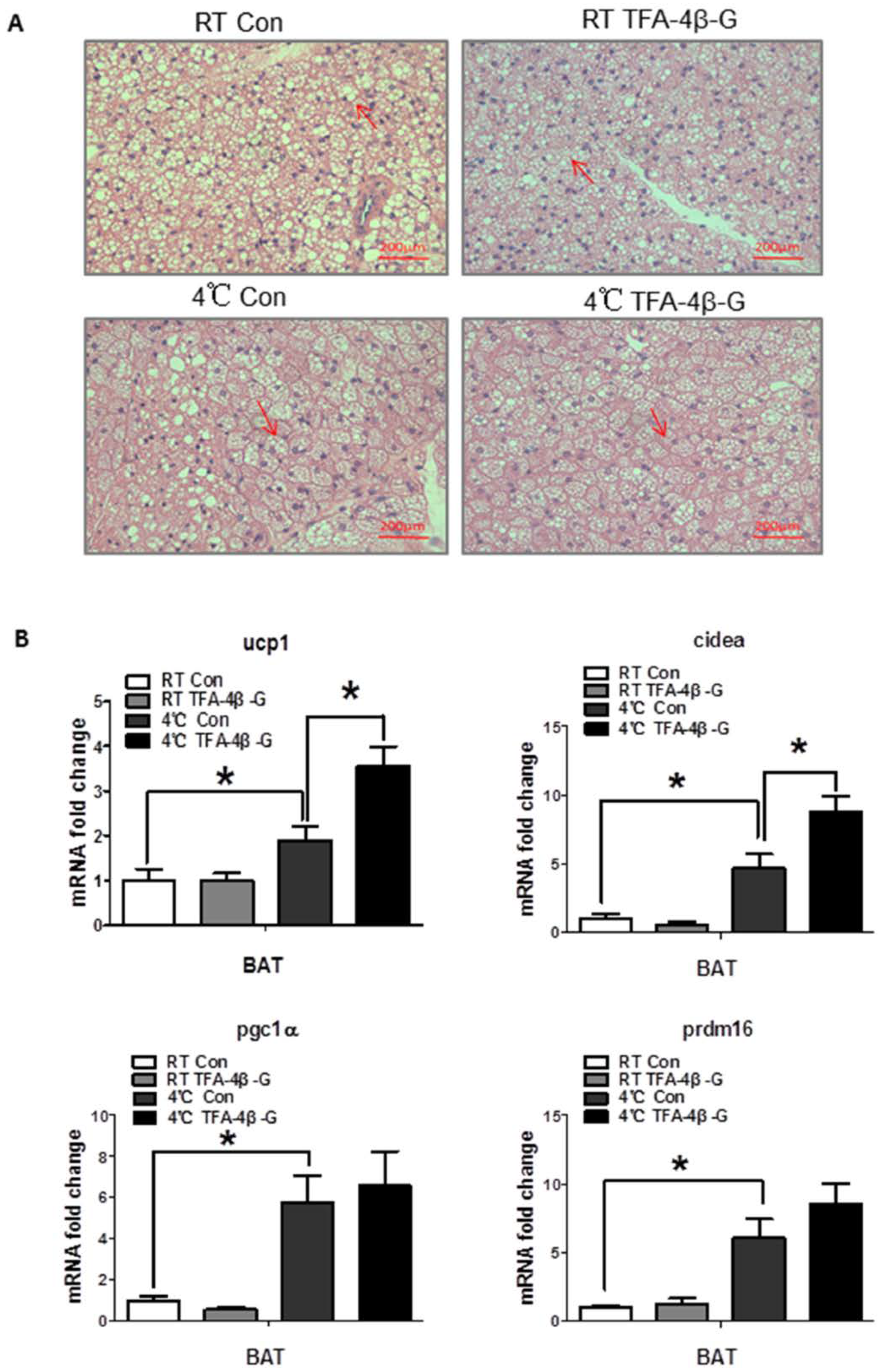
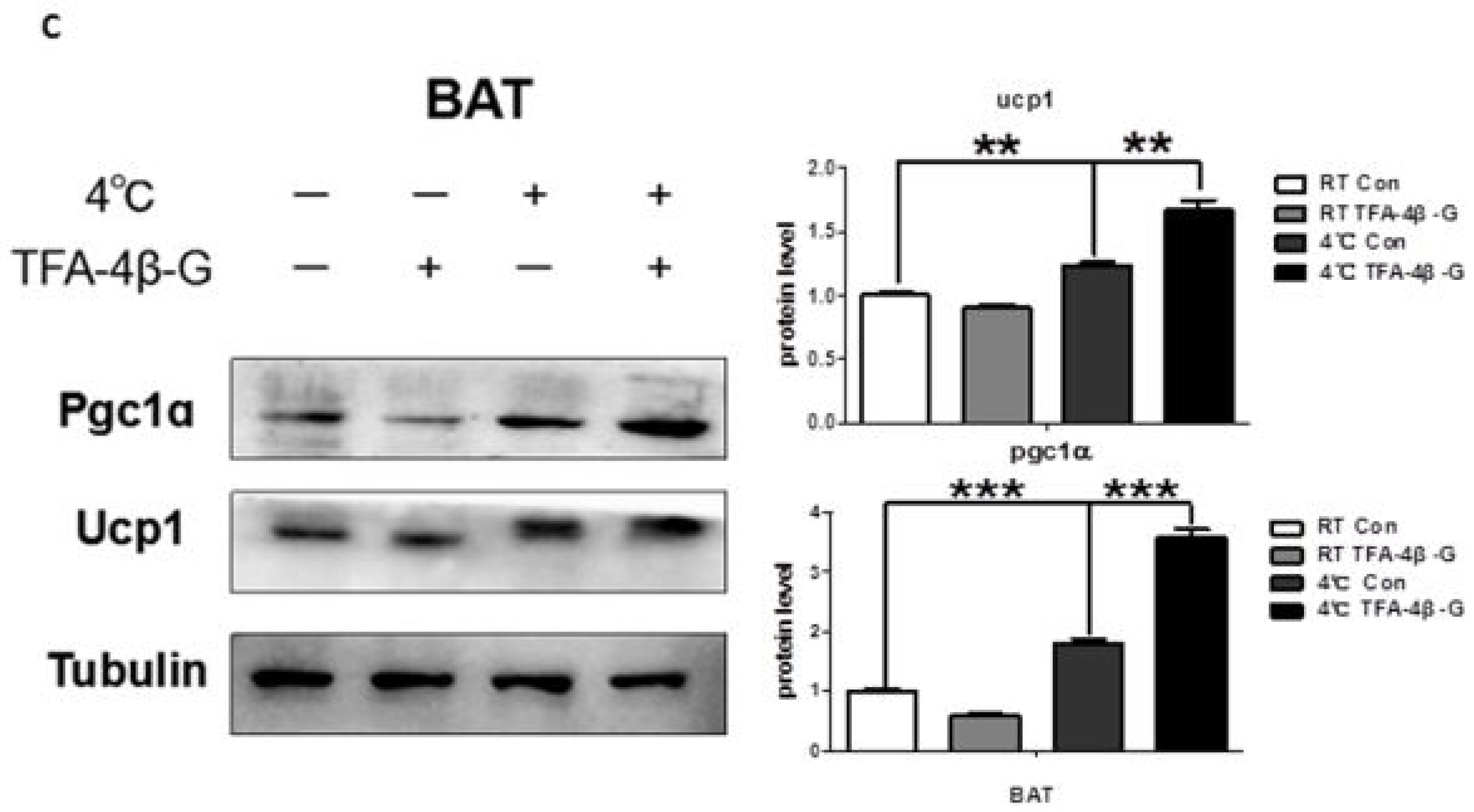
2.4. Trans-Ferulic Acid-4-β-Glucoside Activates Brown Adipose Tissue and Promotes Thermogenesis
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Q-PCR
4.3. Western Blot
4.4. Blood Biochemistry Analysis
4.5. Liver Glycogen Staining
4.6. Brown Adipose Tissue HE Staining
4.7. Oxidative Stress Index of Liver Tissue
4.7.1. Measurement of H2O2
4.7.2. Measurement of GSH-px
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TFA-4β-G | Trans-ferulic acid-4-β-glucoside |
| NST | non-shivering thermogenesis |
| UCP1 | uncoupled protein 1 |
| MDA | malondialdehyde |
| TBARS | thiobarbituric acid-reactive substances |
| HPS | hydroperoxides |
| ROS | reactive oxygen species |
References
- Lichtenbelt, W.; Kingma, B.; van der Lans, A.; Schellen, L. Cold exposure—An approach to increasing energy expenditure in humans. Trends Endocrinol. Metab. 2014, 25, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Eyolfson, D.A.; Tikuisis, P.; Xu, X.; Weseen, G.; Giesbrecht, G.G. Measurement and prediction of peak shivering intensity in humans. Eur. J. Appl. Physiol. 2001, 84, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, K.; Landau, B.R.; Wajngot, A.; Chandramouli, V.; Efendic, S.; Brunengraber, H.; Wahren, J. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 1999, 48, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- van der Lans, A.A.; Hoeks, J.; Brans, B.; Vijgen, G.H.; Visser, M.G.; Vosselman, M.J.; Hansen, J.; Jorgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, T.R. Chamber cold acclimatization in man. J. Appl. Physiol. 1961, 16, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Tingelstad, H.C.; Mantha, O.L.; Gosselin, C.; Haman, F. Maintaining thermogenesis in cold exposed humans: Relying on multiple metabolic pathways. Compr. Physiol. 2014, 4, 1383–1402. [Google Scholar] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blagojevic, D.P.; Grubor-Lajsic, G.N.; Spasic, M.B. Cold defence responses: The role of oxidative stress. Front. Biosci. 2011, 3, 416–427. [Google Scholar] [CrossRef]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Oxidative stress in cold-induced hyperthyroid state. J. Exp. Biol. 2010, 213 Pt 17, 2899–2911. [Google Scholar] [CrossRef] [Green Version]
- Lomakina, L.V. Effects of low temperature on lipid peroxidation and intensity of proteolysis in rat brain and liver. Ukrainskii Biokhimicheskii Zhurnal 1980, 52, 305–308. [Google Scholar] [PubMed]
- Kaushik, S.; Kaur, J. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clinica chimica acta. Int. J. Clin. Chem. 2003, 333, 69–77. [Google Scholar]
- Venditti, P.; De Rosa, R.; Portero-Otin, M.; Pamplona, R.; Di Meo, S. Cold-induced hyperthyroidism produces oxidative damage in rat tissues and increases susceptibility to oxidants. Int. J. Biochem. Cell Biol. 2004, 36, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Nogueiras, R.; Dieguez, C.; Rahmouni, K.; Lopez, M. Traveling from the hypothalamus to the adipose tissue: The thermogenic pathway. Redox Biol. 2017, 12, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Castellani, J.W.; Young, A.J. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton. Neurosci. Basic Clin. 2016, 196, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepa-Kishi, D.M.; Katsnelson, G.; Bikopoulos, G.; Iqbal, A.; Ceddia, R.B. Cold acclimation reduces hepatic protein Kinase B and AMP-activated protein kinase phosphorylation and increases gluconeogenesis in Rats. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yu, G.Z. Recognition and use of Aconitum carmichaeli from the pre-Qin period to Tang Dynasty-Also on rules in evolution of traditional Chinese medicine. China J. Chin. Mater. Med. 2017, 42, 4674–4678. [Google Scholar]
- Singhuber, J.; Zhu, M.; Prinz, S.; Kopp, B. Aconitum in traditional Chinese medicine: A valuable drug or an unpredictable risk? J. Ethnopharmacol. 2009, 126, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Guo, Z.Z.; Zhu, Y.F.; Huang, Z.J.; Gong, X.; Li, Y.H.; Son, W.J.; Li, X.Y.; Lou, Y.M.; Zhu, L.J.; et al. A systematic review of pharmacokinetic studies on herbal drug Fuzi: Implications for Fuzi as personalized medicine. Phytomed. Int. J. Phytother. Phytopharmacol. 2018, 44, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Yu, J.; Fang, Q.J.; Lian, J.B.; Wang, R.X.; He, R.L.; Lin, M.J. Sodium ferulate protects against daunorubicin-induced cardiotoxicity by inhibition of mitochondrial apoptosis in juvenile rats. J. Cardiovasc. Pharmacol. 2014, 63, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Bruton, J.D.; Aydin, J.; Yamada, T.; Shabalina, I.G.; Ivarsson, N.; Zhang, S.J.; Wada, M.; Tavi, P.; Nedergaard, J.; Katz, A.; et al. Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J. Physiol. 2010, 588 Pt 21, 4275–4288. [Google Scholar] [CrossRef]
- Wang, B.H.; Ou-Yang, J.P. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc. Drug Rev. 2005, 23, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Grune, T.; Stolzing, A.; Jakstadt, M.; McLaren, J.S.; Speakman, J.R. The consequences of acute cold exposure on protein oxidation and proteasome activity in short-tailed field voles, microtus agrestis. Free Radical Biol. Med. 2002, 33, 259–265. [Google Scholar] [CrossRef]
- Griggio, M.A. Thermogenic mechanisms in cold-acclimated animals. Braz. J. Med. Biol. Res. 1988, 21, 171–176. [Google Scholar] [PubMed]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Sacco, J.; Adeli, K. Autophagy: Emerging roles in lipid homeostasis and metabolic control. Biochim. Biophys. Acta 2013, 1831, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, X.; Jin, S. Autophagy in adipose tissue biology. Pharmacol. Res. 2012, 66, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Hussain, N.A.; Zhang, Q.; Chang, C.; Li, H.; Fu, Y.; Cao, L.; Han, W.; Stunkel, W.; Xu, F. miRNA-32 Drives Brown Fat Thermogenesis and Trans-activates Subcutaneous White Fat Browning in Mice. Cell Rep. 2017, 19, 1229–1246. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Cui, X.; Chen, Q.; Yang, X.; Fang, F.; Zhang, J.; Liu, G.; Jin, W.; Chang, Y. Cold-Inducible SIRT6 Regulates Thermogenesis of Brown and Beige Fat. Cell Rep. 2017, 20, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xiujuan, S.; Juan, W.; Song, J.; Lei, X.; Guotong, X.; Lixia, L. Comprehensive experiment-clinical biochemistry: Determination of blood glucose and triglycerides in normal and diabetic rats. Biochem. Mol. Biol. Educ. A Bimon. Publ. Int. Union Biochem. Mol. Biol. 2015, 43, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.A.; Campbell-Thompson, M. Periodic Acid-Schiff Staining with Diastase. Methods Mol. Biol. 2017, 1639, 145–149. [Google Scholar] [PubMed]
- Bauters, D.; Cobbaut, M.; Geys, L.; Van Lint, J.; Hemmeryckx, B.; Lijnen, H.R. Loss of ADAMTS5 enhances brown adipose tissue mass and promotes browning of white adipose tissue via CREB signaling. Mol. Metab. 2017, 6, 715–724. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, C.; Lu, H.; Liu, Y.; Zhang, J.; Wang, J.; Luo, W.; Zhang, W.; Chen, J. Trans-Ferulic Acid-4-β-Glucoside Alleviates Cold-Induced Oxidative Stress and Promotes Cold Tolerance. Int. J. Mol. Sci. 2018, 19, 2321. https://doi.org/10.3390/ijms19082321
Xue C, Lu H, Liu Y, Zhang J, Wang J, Luo W, Zhang W, Chen J. Trans-Ferulic Acid-4-β-Glucoside Alleviates Cold-Induced Oxidative Stress and Promotes Cold Tolerance. International Journal of Molecular Sciences. 2018; 19(8):2321. https://doi.org/10.3390/ijms19082321
Chicago/Turabian StyleXue, Chong, Huanyu Lu, Ying Liu, Jianbin Zhang, Jiye Wang, Wenjing Luo, Wenbin Zhang, and Jingyuan Chen. 2018. "Trans-Ferulic Acid-4-β-Glucoside Alleviates Cold-Induced Oxidative Stress and Promotes Cold Tolerance" International Journal of Molecular Sciences 19, no. 8: 2321. https://doi.org/10.3390/ijms19082321



