Schisandra chinensis Fructus and Its Active Ingredients as Promising Resources for the Treatment of Neurological Diseases
Abstract
:1. Introduction
2. Literature and Data Search Methodology
3. Biological Function Enrichment of SCF
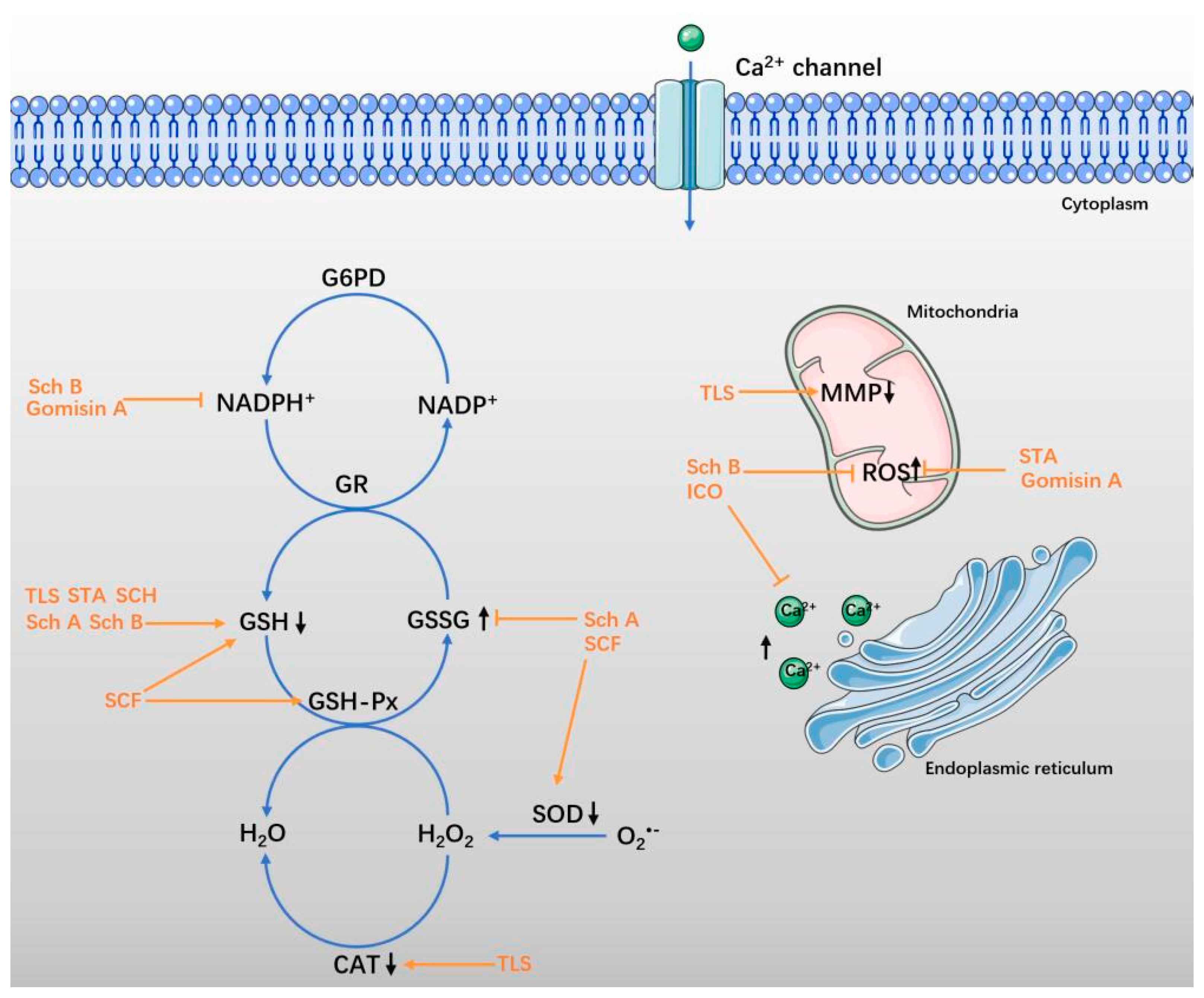
4. Antioxidative Effect in Neurological Diseases
4.1. SCF and Total Lignans of SCF
4.2. Sch A and Sch B
4.3. STA and SCH
4.4. ICO and Gomisin A
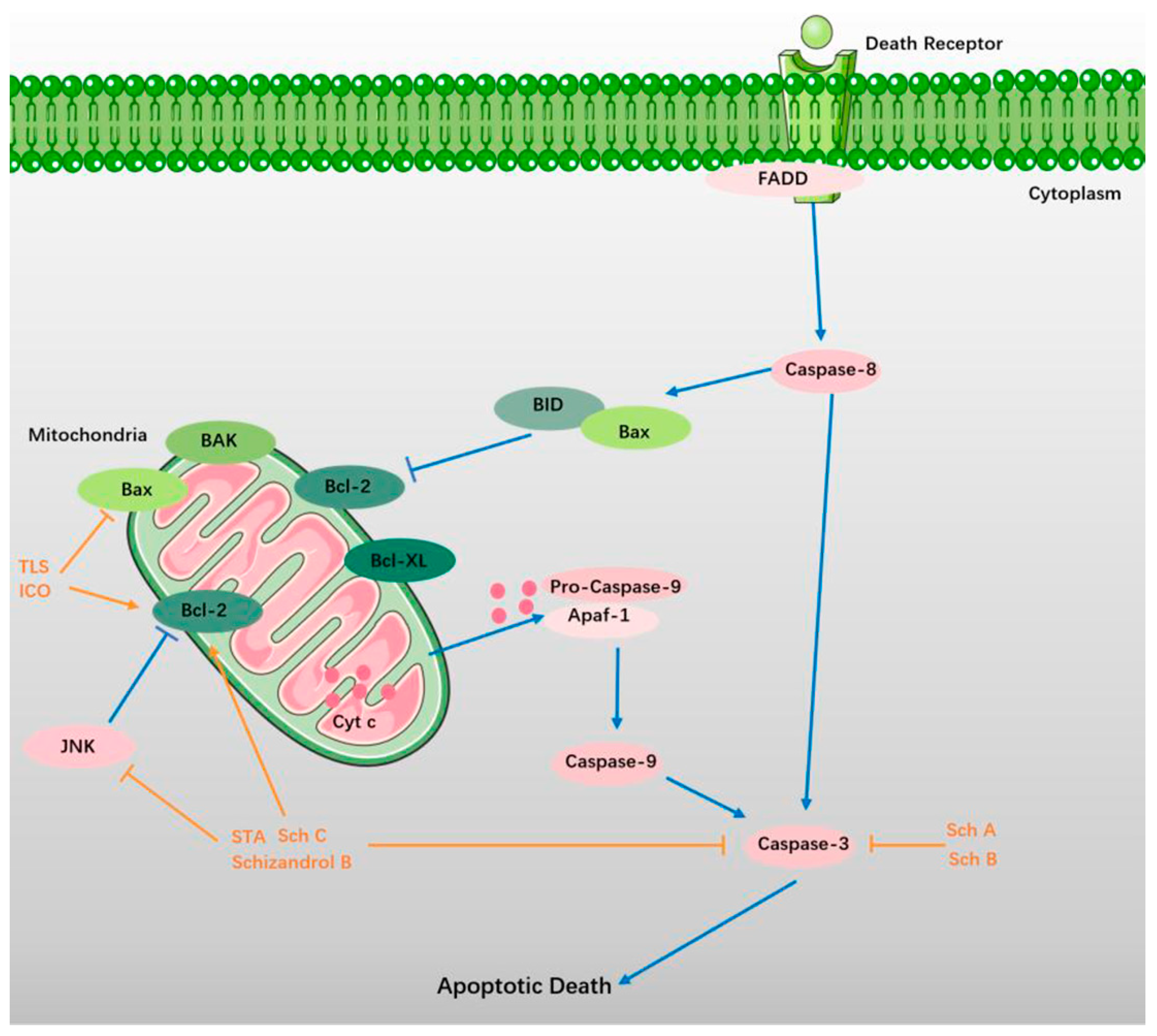
5. Suppression of Apoptosis
5.1. TLS
5.2. Sch A and Sch B
5.3. STA, Sch C, and Schizandrol B
5.4. ICO and Gomisin A
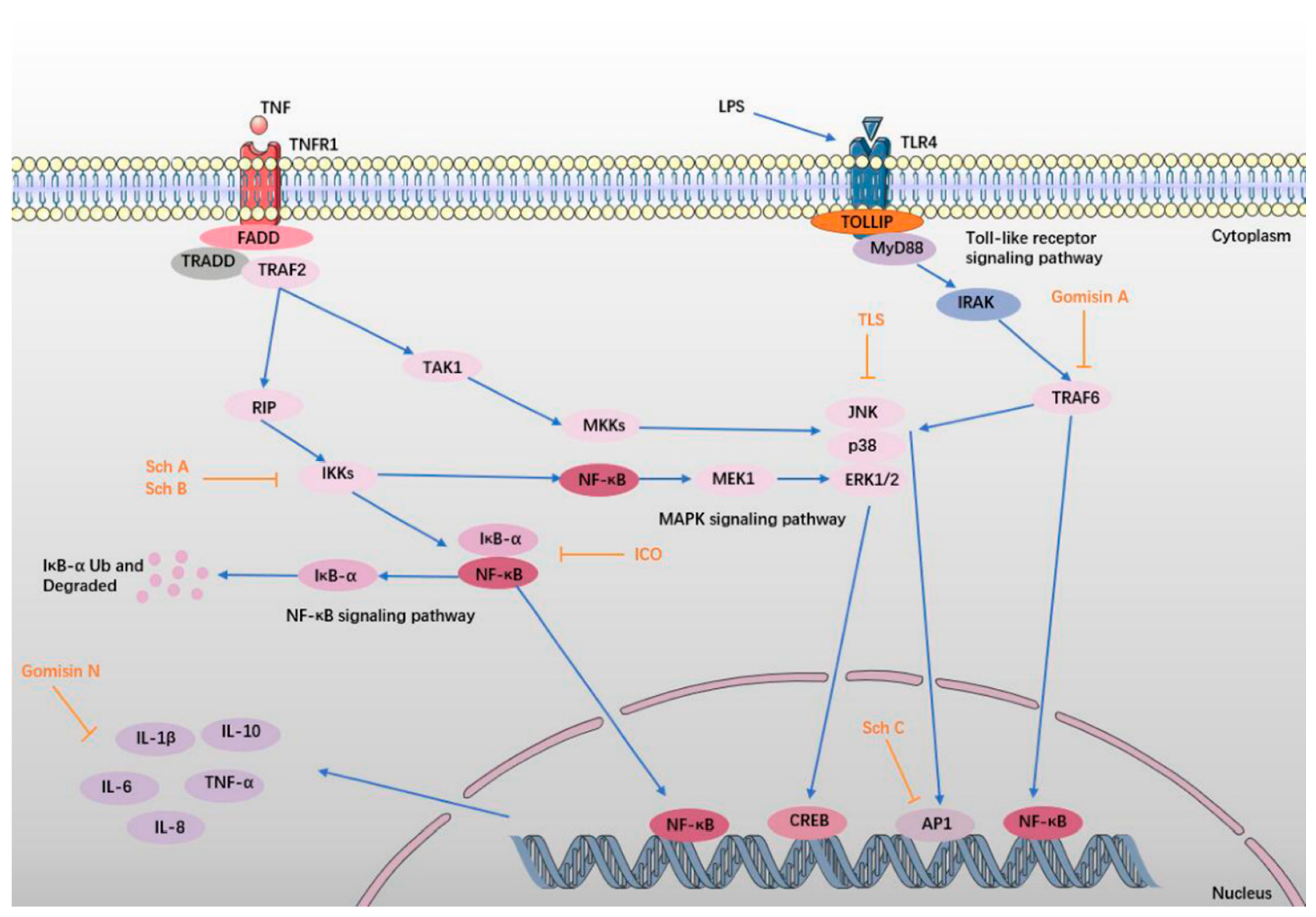
6. Anti-Inflammatory Effect
6.1. TLS
6.2. Sch A, Sch B, and Sch C
6.3. ICO, Gomisin A, and Gomisin N
7. Regulation of Neurotransmitters
8. Modulation of BDNF Related Pathways
9. Conclusions and Perspectives for Future Work
Acknowledgments
Conflicts of Interest
References
- Birbeck, G.L.; Meyer, A.C.; Ogunniyi, A. Nervous system disorders across the life course in resource-limited settings. Nature 2015, 527, S167–S171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatib, R.; Jawaada, A.M.; Arevalo, Y.A.; Hamed, H.K.; Mohammed, S.H.; Huffman, M.D. Implementing Evidence-Based Practices for Acute Stroke Care in Low- and Middle-Income Countries. Curr. Atheroscler. Rep. 2017, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- National Center for Cardiovascular Disease. Report on Cardiovascular Diseases in China (2016); Encyclopedia of China Publishing House: Beijing, China, 2017. [Google Scholar]
- Lindley, R.I. Stroke Prevention in the Very Elderly. Stroke 2018, 49, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.E.; Howard, V.J.; Jimenez, M.; Rexrode, K.M.; Acelajado, M.C.; Kleindorfer, D.; Chaturvedi, S. Impact of Conventional Stroke Risk Factors on Stroke in Women: An Update. Stroke 2018, 49, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Demel, S.L.; Kittner, S.; Ley, S.H.; McDermott, M.; Rexrode, K.M. Stroke Risk Factors Unique to Women. Stroke 2018, 49, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V.; Zarow, C.; Borys, E.; Whitman, J.D.; Tung, S.; Ellis, W.G.; Zheng, L.; Chui, H.C. Review: Vascular dementia: Clinicopathologic and genetic considerations. Neuropathol. Appl. Neurobiol. 2018, 44, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Meyyazhagan, A.; Carril, J.C.; Cacabelos, P.; Teijido, O. Pharmacogenetics of Vascular Risk Factors in Alzheimer’s Disease. J. Pers. Med. 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, D.F. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A 2009, 1216, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lai, H.; Jia, X.; Liu, J.; Zhang, Z.; Qi, Y.; Zhang, J.; Song, J.; Wu, C.; Zhang, B.; et al. Comprehensive chemical analysis of Schisandra chinensis by HPLC-DAD-MS combined with chemometrics. Phytomedicine 2013, 20, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yang, Y.; Liu, Y.; Liu, Z.; Zhou, H.; Hu, H. Two-steps extraction of essential oil, polysaccharides and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Pharm. Biomed. Anal. 2014, 96, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, H.; Guo, F.; Yu, Y.; Wei, J.; Geng, Y.; Wang, S.; Li, S.; Yang, H. Identification of active components in Yixinshu Capsule with protective effects against myocardial dysfunction on human induced pluripotent stem cell-derived cardiomyocytes by an integrative approach. Mol. Biosyst. 2017, 13, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Z.; Meng, F. Schisandrin B Ameliorates Myocardial Ischemia/Reperfusion Injury Through Attenuation of Endoplasmic Reticulum Stress-Induced Apoptosis. Inflammation 2017, 40, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Wat, E.; Ng, C.F.; Wong, E.C.; Koon, C.M.; Lau, C.P.; Cheung, D.W.; Fung, K.P.; Lau, C.B.; Leung, P.C. The hepatoprotective effect of the combination use of Fructus Schisandrae with statin—A preclinical evaluation. J. Ethnopharmacol. 2016, 178, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Casarin, E.; Dall’Acqua, S.; Smejkal, K.; Slapetova, T.; Innocenti, G.; Carrara, M. Molecular mechanisms of antiproliferative effects induced by Schisandra-derived dibenzocyclooctadiene lignans (+)-deoxyschisandrin and (−)-gomisin N in human tumour cell lines. Fitoterapia 2014, 98, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Grandi, N.; Del Vecchio, C.; Mandas, D.; Corona, A.; Piano, D.; Esposito, F.; Parolin, C.; Tramontano, E. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. J Microbiol. 2015, 53, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, F.; Wang, Y.; Li, C.; Zhang, X.; Li, H.; Diao, L.; Gu, J.; Wang, W.; Li, D.; et al. BATMAN-TCM: A Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Zhang, F.; Gao, S.; Chen, L.; Feng, G.; Yin, J.; Chen, W. Schisandra chinensis extract decreases chloroacetaldehyde production in rats and attenuates cyclophosphamide toxicity in liver, kidney and brain. J. Ethnopharmacol. 2018, 210, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Tan, J.Y. A UPLC-TOF/MS-based metabolomics study of rattan stems of Schisandra chinensis effects on Alzheimer’s disease rats model. Biomed. Chromatogr. 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Xu, M.; Wan, S.; Wang, M.; Wu, B.; Xiao, F.; Bi, K.; Jia, Y. Schisandra chinensis produces the antidepressant-like effects in repeated corticosterone-induced mice via the BDNF/TrkB/CREB signaling pathway. Psychiatry Res. 2016, 243, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; He, B.; Wan, S.; Xu, M.; Yang, H.; Xiao, F.; Bi, K.; Jia, Y. Antidepressant-like effects and cognitive enhancement of Schisandra chinensis in chronic unpredictable mild stress mice and its related mechanism. Sci. Rep. 2017, 7, 6903. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Z.; Yang, Y.; Yang, X.; Jang, E.Y.; Schilaty, N.D.; Hedges, D.M.; Kim, S.C.; Cho, I.J.; Zhao, R. Effects of the aqueous extract of Schizandra chinensis fruit on ethanol withdrawal-induced anxiety in rats. Chin. Med. J. 2014, 127, 1935–1940. [Google Scholar] [PubMed]
- Wei, B.; Li, Q.; Fan, R.; Su, D.; Chen, X.; Jia, Y.; Bi, K. Determination of monoamine and amino acid neurotransmitters and their metabolites in rat brain samples by UFLC-MS/MS for the study of the sedative-hypnotic effects observed during treatment with S. chinensis. J. Pharm. Biomed. Anal. 2014, 88, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total Lignans of Schisandra chinensis Ameliorates Abeta1-42-Induced Neurodegeneration with Cognitive Impairment in Mice and Primary Mouse Neuronal Cells. PLoS ONE 2016, 11, e0152772. [Google Scholar] [CrossRef]
- Yan, T.; Shang, L.; Wang, M.; Zhang, C.; Zhao, X.; Bi, K.; Jia, Y. Lignans from Schisandra chinensis ameliorate cognition deficits and attenuate brain oxidative damage induced by D-galactose in rats. Metab. Brain Dis. 2016, 31, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.P.; Wang, S.Q.; Wang, Z.; Yu, C.R.; Chen, J.G.; Yu, C.Y. Effect of Schisandra chinensis lignans on neuronal apoptosis and p-AKT expression of rats in cerebral ischemia injury model. Zhongguo Zhong Yao Za Zhi 2014, 39, 1680–1684. [Google Scholar] [PubMed]
- Hu, D.; Yang, Z.; Yao, X.; Wang, H.; Han, N.; Liu, Z.; Wang, Y.; Yang, J.; Yin, J. Dibenzocyclooctadiene lignans from Schisandra chinensis and their inhibitory activity on NO production in lipopolysaccharide-activated microglia cells. Phytochemistry 2014, 104, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, C.; Han, N.; Miao, L.; Wang, D.; Liu, Z.; Wang, H.; Yin, J. Deoxyschizandrin isolated from the fruits of Schisandra chinensis ameliorates Aβ1−42-induced memory impairment in mice. Planta Med. 2012, 78, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Li, G.C.; Shi, Y.W.; Zhang, X.C.; Li, J.L.; Wang, Z.W.; Ding, F.; Liang, X.M. Neuroprotective effect of schizandrin A on oxygen and glucose deprivation/reperfusion-induced cell injury in primary culture of rat cortical neurons. J. Physiol. Biochem. 2014, 70, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zeng, K.; Liao, L.; Yu, Q.; Tu, P.; Wang, X. Schizandrin A Inhibits Microglia-Mediated Neuroninflammation through Inhibiting TRAF6-NF-kappaB and Jak2-Stat3 Signaling Pathways. PLoS ONE. 2016, 11, e0149991. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chiu, P.Y.; Ko, K.M. Schisandrin B enhances cerebral mitochondrial antioxidant status and structural integrity, and protects against cerebral ischemia/reperfusion injury in rats. Biol. Pharm. Bull. 2008, 31, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dong, Y.; Wan, S.; Yan, T.; Cao, J.; Wu, L.; Bi, K.; Jia, Y. Schisantherin B ameliorates Abeta1-42-induced cognitive decline via restoration of GLT-1 in a mouse model of Alzheimer’s disease. Physiol. Behav. 2016, 167, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Zhang, T.; Fu, H.; Liu, G.X.; Wang, X.M. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-kappaB signaling pathway in lipopolysaccharide-induced microglia. Eur. J. Pharmacol. 2012, 692, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Thandavarayan, R.A.; Arumugam, S.; Mizuno, M.; Nawa, H.; Suzuki, K.; Ko, K.M.; Krishnamurthy, P.; Watanabe, K.; Konishi, T. Schisandrin B Ameliorates ICV-Infused Amyloid beta Induced Oxidative Stress and Neuronal Dysfunction through Inhibiting RAGE/NF-kappaB/MAPK and Up-Regulating HSP/Beclin Expression. PLoS ONE 2015, 10, e0142483. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zheng, J.X.; Zhuang, Y.S.; Zhou, Z.K.; Zhao, J.H.; Yang, L. Anti-Inflammatory Effects of Schisandrin B on LPS-Stimulated BV2 Microglia via Activating PPAR-gamma. Inflammation 2017, 40, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Jung, C.H.; Lee, D.H. Neuroprotective effects of Schisandrin B against transient focal cerebral ischemia in Sprague-Dawley rats. Food Chem Toxicol. 2012, 50, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, X.; Shi, Y.; Wang, D.; Gu, Y.; Li, S.; Liang, X.; Wang, Z.; Wang, C. Protection of seven dibenzocyclooctadiene lignans from Schisandra chinensis against serum and glucose deprivation injury in SH-SY5Y cells. Cell Biol. Int. 2015, 39, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, S.J.; Park, T.G.; Rajasekar, S.; Lee, S.J.; Choi, Y.W. Schizandrin C exerts anti-neuroinflammatory effects by upregulating phase II detoxifying/antioxidant enzymes in microglia. Int. Immunopharmacol. 2013, 17, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Sa, F.; Chong, C.M.; Wang, Y.; Zhou, Z.Y.; Chang, R.C.; Chan, S.W.; Hoi, P.M.; Yuen Lee, S.M. Schisantherin A protects against 6-OHDA-induced dopaminergic neuron damage in zebrafish and cytotoxicity in SH-SY5Y cells through the ROS/NO and AKT/GSK3beta pathways. Oxid. Med. Cell. Longev. 2015, 170, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, X.; Xu, X.; Mao, X.; Liu, Z.; Li, H.; Guo, L.; Bi, K.; Jia, Y. Schisantherin A recovers Abeta-induced neurodegeneration with cognitive decline in mice. Physiol. Behav. 2014, 132, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Sa, F.; Zhang, L.Q.; Chong, C.M.; Guo, B.J.; Li, S.; Zhang, Z.J.; Zheng, Y.; Hoi, P.M.; Lee, S.M. Discovery of novel anti-parkinsonian effect of schisantherin A in in vitro and in vivo. Neurosci. Lett. 2015, 593, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Cao, Y.; He, R.; Han, N.; Liu, Z.; Miao, L.; Yin, J. Schizandrin, an antioxidant lignan from Schisandra chinensis, ameliorates Abeta1-42-induced memory impairment in mice. Oxid. Med. Cell. Longev. 2012, 2012, 721721. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.B.; Liu, M.Y.; Chen, Z.X.; Wei, M.J. Schisandrin ameliorates cognitive impairment and attenuates Abeta deposition in APP/PS1 transgenic mice: Involvement of adjusting neurotransmitters and their metabolite changes in the brain. Acta Pharmacol. Sin. 2018. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Son, B.G.; Park, Y.H.; Kim, C.M.; Park, G.; Choi, Y.W. The neuroprotective effects of alpha-iso-cubebene on dopaminergic cell death: Involvement of CREB/Nrf-2 signaling. Neurochem. Res. 2014, 39, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Choi, S.M.; Kim, J.E.; Sung, J.E.; Lee, H.A.; Choi, Y.H.; Bae, C.J.; Choi, Y.W.; Hwang, D.Y. alpha-Isocubebenol alleviates scopolamine-induced cognitive impairment by repressing acetylcholinesterase activity. Neurosci. Lett. 2017, 638, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, S.J.; Park, N.J.; Joo, W.H.; Lee, S.J.; Choi, Y.W. alpha-Iso-cubebene exerts neuroprotective effects in amyloid beta stimulated microglia activation. Neurosci. Lett. 2013, 555, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, D.; Zhang, L.; Lian, G.; Zhao, S.; Wang, C.; Yin, J.; Wu, C.; Yang, J. Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-kappaB/MAPKs pathway. Food Chem. Toxicol. 2014, 63, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Zhang, F.; Gao, S.; Chen, L.; Feng, G.; Yin, J.; Chen, W. Time- and NADPH-Dependent Inhibition on CYP3A by Gomisin A and the Pharmacokinetic Interactions between Gomisin A and Cyclophosphamide in Rats. Molecules 2017, 22, 1298. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Hiraki, Y.; Nishida, S.; Inatomi, Y.; Yabe, T. Gomisin N ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J. Pharmacol. Sci. 2016, 132, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.X.; Yang, L.P.; Yang, Z.L.; Xiao, W.L.; Sun, H.D.; Wu, G.S.; Luo, H.R. Effect of nigranoic acid on Ca(2)(+) influx and its downstream signal mechanism in NGF-differentiated PC12 cells. J. Ethnopharmacol. 2014, 153, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Skovierova, H.; Vidomanova, E.; Mahmood, S.; Sopkova, J.; Drgova, A.; Cervenova, T.; Halasova, E.; Lehotsky, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pahlajani, S.; De Sanctis, V.; Stern, J.N.H.; Najjar, A.; Chong, D. Neurovascular Unit Dysfunction and Blood-Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front. Psychiatry 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Bar-Or, R.; Rael, L.T.; Brody, E.N. Oxidative stress in severe acute illness. Redox Biol. 2015, 4, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Chomova, M.; Zitnanova, I. Look into brain energy crisis and membrane pathophysiology in ischemia and reperfusion. Stress 2016, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 1203285. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Dean, O.M.; Turner, A.; Sureda, A.; Daglia, M.; Nabavi, S.M. Oxidative stress and post-stroke depression: Possible therapeutic role of polyphenols? Curr. Med. Chem. 2015, 22, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Gu, L. The Interrelation between Reactive Oxygen Species and Autophagy in Neurological Disorders. Oxid. Med. Cell. Longev. 2017, 2017, 8495160. [Google Scholar] [CrossRef] [PubMed]
- Prentice, H.; Modi, J.P.; Wu, J.Y. Mechanisms of Neuronal Protection against Excitotoxicity, Endoplasmic Reticulum Stress, and Mitochondrial Dysfunction in Stroke and Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 964518. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, N.; Okada, A.; Tsukahara, H. Effects of Therapeutic Hypothermia for Neuroprotection from the Viewpoint of Redox Regulation. J. Immunol. Res. 2017, 71, 1–9. [Google Scholar] [CrossRef]
- Morris, G.; Anderson, G.; Dean, O.; Berk, M.; Galecki, P.; Martin-Subero, M.; Maes, M. The glutathione system: A new drug target in neuroimmune disorders. Mol. Neurobiol. 2014, 50, 1059–1084. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.M. Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem. Res. 2015, 40, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Denton, T.T. Alternative functions of the brain transsulfuration pathway represent an underappreciated aspect of brain redox biochemistry with significant potential for therapeutic engagement. Free Radic. Biol. Med. 2015, 78, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Lee, S.J.; Han, C.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Oxidative/nitrosative stress and antidepressants: Targets for novel antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Muyderman, H.; Chen, T. Mitochondrial dysfunction in amyotrophic lateral sclerosis—A valid pharmacological target? Br. J. Pharmacol. 2014, 171, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch. Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-C.; Zheng, Q.; Tan, H.; Zhang, B.; Li, X.; Yang, Y.; Yu, J.; Liu, Y.; Chai, H.; Wang, X.; et al. TMCO1 Is an ER Ca2+ Load-Activated Ca2+ Channel. Cell 2016, 165, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Paula-Lima, A.C.; Adasme, T.; Hidalgo, C. Contribution of Ca2+ release channels to hippocampal synaptic plasticity and spatial memory: Potential redox modulation. Antioxid. Redox Signal. 2014, 21, 892–914. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Autophagy and ethanol neurotoxicity. Autophagy 2014, 10, 2099–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilyeva, L.N.; Podgornaya, O.I.; Bespalov, V.G. Nucleosome fracton of extracellular dna as the index of apoptosis. Tsitologiia 2015, 57, 87–94. [Google Scholar] [PubMed]
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc. Pharmacol. 2017, 15, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.C.; Ma, L.S.; Chu, Z.H.; Xu, H.; Wu, W.Q.; Liu, F. Regulation of microglial activation in stroke. Acta Pharmacol. Sin. 2017, 38, 445–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulamek-Koziol, M.; Pluta, R.; Januszewski, S.; Kocki, J.; Bogucka-Kocka, A.; Czuczwar, S.J. Expression of Alzheimer’s disease risk genes in ischemic brain degeneration. Pharmacol. Rep. 2016, 68, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Vemuganti, R. Mechanisms of Parkinson’s disease-related proteins in mediating secondary brain damage after cerebral ischemia. J. Cereb. Blood Flow Metab. 2017, 37, 1910–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, T.M.; Dawson, V.L. Mitochondrial Mechanisms of Neuronal Cell Death: Potential Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.; Hagberg, H. Role of mitochondria in apoptotic and necroptotic cell death in the developing brain. Clin. Chim. Acta 2015, 451, 35–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia de la Cadena, S.; Massieu, L. Caspases and their role in inflammation and ischemic neuronal death. Focus on caspase-12. Apoptosis 2016, 21, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, M.P.; Nikoletopoulou, V.; Barde, Y.A. Cell biology in neuroscience: Death of developing neurons: New insights and implications for connectivity. J. Cell Biol. 2013, 203, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Williams, D.W. More alive than dead: Non-apoptotic roles for caspases in neuronal development, plasticity and disease. Cell Death Differ. 2017, 24, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuster, M.J.; Garcia-Sevilla, J.A. Monoamine receptor agonists, acting preferentially at presynaptic autoreceptors and heteroreceptors, downregulate the cell fate adaptor FADD in rat brain cortex. Neuropharmacology 2015, 89, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuster, M.J.; Diez-Alarcia, R.; Ferrer-Alcon, M.; La Harpe, R.; Meana, J.J.; Garcia-Sevilla, J.A. FADD adaptor and PEA-15/ERK1/2 partners in major depression and schizophrenia postmortem brains: Basal contents and effects of psychotropic treatments. Neuroscience 2014, 277, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Mengying, Z.; Yiyue, X.; Tong, P.; Yue, H.; Limpanont, Y.; Ping, H.; Okanurak, K.; Yanqi, W.; Dekumyoy, P.; Hongli, Z.; et al. Apoptosis and necroptosis of mouse hippocampal and parenchymal astrocytes, microglia and neurons caused by Angiostrongylus cantonensis infection. Parasites Vectors 2017, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Kole, A.J.; Annis, R.P.; Deshmukh, M. Mature neurons: Equipped for survival. Cell Death Dis. 2013, 4, e689. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.E.; Schlamp, C.L.; Nickells, R.W. BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin. Eye Res. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jazvinscak Jembrek, M.; Hof, P.R.; Simic, G. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Abeta Accumulation. Oxid. Med. Cell. Longev. 2015, 2015, 346783. [Google Scholar] [CrossRef] [PubMed]
- Aouacheria, A.; Baghdiguian, S.; Lamb, H.M.; Huska, J.D.; Pineda, F.J.; Hardwick, J.M. Connecting mitochondrial dynamics and life-or-death events via Bcl-2 family proteins. Neurochem. Int. 2017, 109, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Lu, Y.; Tian, H.; Duan, C.; Lu, L.; Gao, G.; Wu, X.; Wang, X.; Yang, H. Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone-induced Parkinson disease models. Autophagy 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Samhan-Arias, A.K.; Fortalezas, S.; Cordas, C.M.; Moura, I.; Moura, J.J.G.; Gutierrez-Merino, C. Cytochrome b5 reductase is the component from neuronal synaptic plasma membrane vesicles that generates superoxide anion upon stimulation by cytochrome c. Redox Biol. 2018, 15, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Geden, M.J.; Deshmukh, M. Axon degeneration: Context defines distinct pathways. Curr. Opin. Neurobiol. 2016, 39, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Lin, T.; Wang, D.; Peng, R.; Wang, S.; Gao, Y.; Xu, X.; Li, Y.; Wang, S.; Zhao, L.; et al. Neural cell apoptosis induced by microwave exposure through mitochondria-dependent caspase-3 pathway. Int. J. Med. Sci. 2014, 11, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, V.Z.; Wong, G.K.C. Neuroinflammation responses after subarachnoid hemorrhage: A review. J. Clin. Neurosci. 2017, 42, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef] [PubMed]
- Ziemka-Nalecz, M.; Jaworska, J.; Zalewska, T. Insights Into the Neuroinflammatory Responses After Neonatal Hypoxia-Ischemia. Med. Gas Res. 2017, 76, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Mou, R.T.; Feng, D.X.; Wang, Z.; Chen, G. The role of nitric oxide in stroke. Med. Gas Res. 2017, 7, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Xiong, X.Y.; Chen, J.; Wang, Y.C.; Duan, W.; Yang, Q.W. Function and mechanism of toll-like receptors in cerebral ischemic tolerance: From preconditioning to treatment. J. NeuroInflamm. 2015, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Kollias, G.; Kontoyiannis, D. Role of TNF/TNFR in autoimmunity: Specific TNF receptor blockade may be advantageous to anti-TNF treatments. Cytokine Growth Factor Rev. 2002, 13, 315–321. [Google Scholar] [CrossRef]
- Tortarolo, M.; Lo Coco, D.; Veglianese, P.; Vallarola, A.; Giordana, M.T.; Marcon, G.; Beghi, E.; Poloni, M.; Strong, M.J.; Iyer, A.M.; et al. Amyotrophic Lateral Sclerosis, a Multisystem Pathology: Insights into the Role of TNFalpha. Med. Inflamm. 2017, 2017, 2985051. [Google Scholar] [CrossRef] [PubMed]
- Snow, W.M.; Albensi, B.C. Neuronal Gene Targets of NF-kappaB and Their Dysregulation in Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, Y.; Lin, W.; Zhang, Q.; Zhao, J.; Liu, F.T.; Tang, Y.L.; Xiao, B.G.; Wang, J. Trehalose alleviates PC12 neuronal death mediated by lipopolysaccharide-stimulated BV-2 cells via inhibiting nuclear transcription factor NF-kappaB and AP-1 activation. Neurotox. Res. 2014, 26, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Jang, B.C. Prednisone inhibits the IL-1beta-induced expression of COX-2 in HEI-OC1 murine auditory cells through the inhibition of ERK-1/2, JNK-1 and AP-1 activity. Int. J. Mol. Med. 2014, 34, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Du, K.; Cai, Q.; Ma, L.; Jiao, Z.; Tan, J.; Xu, Z.; Li, J.; Luo, W.; Chen, J.; et al. Lead induces COX-2 expression in glial cells in a NFAT-dependent, AP-1/NFkappaB-independent manner. Toxicology 2014, 325, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, L.; Cheng, Z.; Cai, J.; Niu, Y.; Meng, W.; Zhao, Q. Kukoamine A Prevents Radiation-Induced Neuroinflammation and Preserves Hippocampal Neurogenesis in Rats by Inhibiting Activation of NF-kappaB and AP-1. Neurotox. Res. 2017, 31, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Stranahan, A.M.; Suchecki, D.; Yunes, R. Neuroendocrine Regulation of Anxiety: Beyond the Hypothalamic-Pituitary-Adrenal Axis. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.P.; Reichel, M.; Muhle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Shimizu, S.; Tokudome, K.; Kunisawa, N.; Sasa, M. New insight into the therapeutic role of the serotonergic system in Parkinson’s disease. Prog. Neurobiol. 2015, 134, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, K.; Benson, C.; Kerr, B.; Aricioglu, F.; Cetin, M.; Dursun, S.; Baker, G. Involvement of Neuroactive Steroids in Pain, Depression and Anxiety. Mod. Trends Pharmacopsychiatr. 2015, 30, 94–102. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Greiner, R.; Johnson, S.K.; Strawn, J.R.; Dobson, E.T.; Giles, L.L. Primary Pediatric Care Psychopharmacology: Focus on Medications for ADHD, Depression, and Anxiety. J. Sci. Food Agric. 2017, 47, 3–14. [Google Scholar] [CrossRef]
- Wiebking, C.; Duncan, N.W.; Tiret, B.; Hayes, D.J.; Marjanska, M.; Doyon, J.; Bajbouj, M.; Northoff, G. GABA in the insula—A predictor of the neural response to interoceptive awareness. Neuroimage 2014, 86, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Schatzberg, A.F. Development of New Psychopharmacological Agents for Depression and Anxiety. Psychiatr. Clin. N. Am. 2015, 38, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Dincheva, I.; Lynch, N.B.; Lee, F.S. The Role of BDNF in the Development of Fear Learning. Depress Anxiety. 2016, 33, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Ketamine, magnesium and major depression—From pharmacology to pathophysiology and back. J. Psychiatr. Res. 2013, 47, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Mendez-David, I.; Hen, R.; Gardier, A.M.; David, D.J. Adult hippocampal neurogenesis: An actor in the antidepressant-like action. Ann. Pharm. Fr. 2013, 71, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Renoir, T.; Hannan, A.J. Gene-environment interactions informing therapeutic approaches to cognitive and affective disorders. Neuropharmacology 2017. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, W.K.; Noda, Y.; Barr, M.S.; Vila-Rodriguez, F.; Rajji, T.K.; Fitzgerald, P.B.; Downar, J.; Mulsant, B.H.; Vigod, S.; Daskalakis, Z.J.; et al. Neurobiological predictors of response to dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation in depression: A systematic review. Depress. Anxiety 2015, 32, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.; Stein, D.J.; de Almeida, R.M. Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: A systematic review of the last decade. Trends Psychiatry Psychother. 2015, 37, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Morrison, F.G.; Ressler, K.J. From the neurobiology of extinction to improved clinical treatments. Depress. Anxiety 2014, 31, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Barthas, F.; Barrot, M. Emotional consequences of neuropathic pain: Insight from preclinical studies. Neurosci. Biobehav. Rev. 2014, 47, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Han, N.; Yao, X.; Liu, Z.; Wang, Y.; Yang, J.; Yin, J. Structure-activity relationship study of dibenzocyclooctadiene lignans isolated from Schisandra chinensis on lipopolysaccharide-induced microglia activation. Planta Med. 2014, 80, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Lai, Y.C.; Chang, C.L. High throughput screening and antioxidant assay of dibenzo[a,c]cyclooctadiene lignans in modified-ultrasonic and supercritical fluid extracts of Schisandra chinensis Baill by liquid chromatography—Mass spectrometry and a free radical-scavenging method. J. Sep. Sci. 2008, 31, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Morizawa, Y.M.; Hirayama, Y.; Ohno, N.; Shibata, S.; Shigetomi, E.; Sui, Y.; Nabekura, J.; Sato, K.; Okajima, F.; Takebayashi, H.; et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Delekate, A.; Fuchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016, 7, 10523. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.T.; Choi, J.P.; Kotzin, J.J.; Yang, Y.; Hong, C.C.; Hobson, N.; Girard, R.; Zeineddine, H.A.; Lightle, R.; Moore, T.; et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 2017, 545, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, O.S.; Deng, H.; Liu, D.; Zhang, Y.; Wei, R.; Deng, Y.; Zhang, F.; Louvi, A.; Turk, B.E.; Boggon, T.J.; et al. Structure and vascular function of MEKK3-cerebral cavernous malformations 2 complex. Nat. Commun. 2015, 6, 7937. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.B.; Kolodziejczyk, K.; Kougioumtzidou, E.; Attwell, D. Proton-gated Ca(2+)-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 2016, 529, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ting, S.M.; Liu, C.H.; Sun, G.; Kruzel, M.; Roy-O’Reilly, M.; Aronowski, J. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat. Commun. 2017, 8, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, H.S.; Chang, C.Y.; Jeon, S.B.; Yoon, H.J.; Ahn, Y.H.; Kim, H.S.; Kim, I.H.; Jeon, S.H.; Johnson, R.S.; Park, E.J. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat. Commun. 2015, 6, 6340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, M.; Gladbach, A.; van Eersel, J.; Ittner, A.; Przybyla, M.; van Hummel, A.; Chua, S.W.; van der Hoven, J.; Lee, W.S.; Muller, J. Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat. Commun. 2017, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, X.; Hu, W.; Ma, J.; Hou, W.; Zhang, X.; Wang, X.; Gao, J.; Shen, Y.; Lv, J.; et al. Histamine H3 receptors aggravate cerebral ischaemic injury by histamine-independent mechanisms. Nat. Commun. 2014, 5, 3334. [Google Scholar] [CrossRef] [PubMed]

| SCF and Its Active Ingredients | Pharmacological Activity | Biological Analysis | Key References |
|---|---|---|---|
| SCF | Anti-oxidant | GSH antioxidant response | [20,21] |
| Modulate BDNF related pathways | BDNF, TrkB/CREB/ERK and PI3K/Akt/GSK-3β pathways | [22,23] | |
| Regulate neurotransmitters | NE activity | [24] | |
| Neurotransmitters activities | [25] | ||
| TLS | Anti-oxidant | Mitochondrial function | [26] |
| GSH antioxidant response | [27] | ||
| Anti-apoptosis | Bcl-2 expression | [26] | |
| Bcl-2 and Bax expression | [28] | ||
| Anti-inflammatory | NO activity | [29] | |
| MAPKs signaling | [26] | ||
| Sch A | Anti-oxidant | GSH antioxidant response | [30] |
| Anti-apoptosis | ERK, JNK, Caspase-3 signaling | [31] | |
| Anti-inflammatory | TRAF6/IKKβ/NF-κB and Jak2/Stat3 signaling pathways | [32] | |
| Sch B | Anti-oxidant | ACh activity | [33] |
| GSH antioxidant response | [34] | ||
| GLT-1 and GSK3β activities | [35] | ||
| ROS, NADPH oxidase activity | [36] | ||
| Anti-apoptosis | Caspase-3, HSP70, beclin-1 expression | [37] | |
| Anti-inflammatory | RAGE, NF-κB, MAPKs signaling | [37] | |
| PPAR-γ activity | [38] | ||
| MyD88/IKK/NF-κB signaling pathway | [36] | ||
| TNF-α, IL-1β activities | [39] | ||
| Sch C | Anti-apoptosis | JNK/Caspase-3 signaling | [40] |
| Anti-inflammatory | cAMP/PKA/CREB and Nrf-2 signaling | [41] | |
| STA | Anti-oxidant | MAPKs, PI3K/Akt and GSK3β signaling | [42] |
| GSH antioxidant response | [43] | ||
| Anti-apoptosis | Bcl-2 expression and PI3K/Akt signaling | [44] | |
| JNK/Caspase-3 signaling | [40] | ||
| SCH | Anti-oxidant | GSH antioxidant response | [45] |
| Regulate neurotransmitters | Neurotransmitters and their metabolites effects | [46] | |
| Schizandrol B | Anti-apoptosis | JNK/Caspase-3 signaling | [40] |
| ICO | Anti-oxidant | ROS and calcium accumulation | [47] |
| Anti-apoptosis | CREB/Nrf-2 signaling | [47] | |
| Bcl-2 and Bax expression | [48] | ||
| Anti-inflammatory | NF-κB and MAPK signaling pathways | [49] | |
| Gomisin A | Anti-oxidant | ROS, NADPH oxidase activity | [50] |
| Anti-apoptosis | CYP3A activity | [51] | |
| Anti-inflammatory | TLR4 mediated NF-κB and MAPKs pathways | [50] | |
| Gomisin N | Anti-inflammatory | Inflammatory responses and neural activation | [52] |
| Nigranoic acid | Modulate BDNF related pathways | ERK1/2, Ca2+-CaMKII pathways, BDNF activity | [53] |
| SCF and Its Active Ingredients | Study Design | Study Type | Molecular and Cellular Mechanisms of Action | Dose Range | Minimal Active Concentration | Key Reference |
|---|---|---|---|---|---|---|
| SCF | CTX induced brain injury in rats | In vivo | Increases GSH content | 0.10–1.00 g/kg | 0.50 g/kg | [20] |
| Decreases MDA levels | ||||||
| intra-hippocampal Aβ1-42 induced AD in rats | In vivo | Increases SOD and GSH-Px activity | 200 mg/kg | 200 mg/kg | [21] | |
| TLS | Aβ1-42 induced AD in primary mouse neuronal cells | In vitro | Blocking the decrease of MMP | 10, 30, 100 μM | 10 μM | [26] |
| Aβ1-42 induced AD in mice | In vivo | Restroes T-AOC and MDA level | 50, 200 mg/kg | 50 mg/kg | ||
| Ameliorates the neurodegeneration in the hippocampus | ||||||
| D-galactose (D-gal)-induced neurotoxicity in rats | In vivo | Attenuates SOD, CAT, T-AOC decreasing | ― | ― | [27] | |
| Maintains GSH, MDA, NO levels | ||||||
| Sch A | Aβ1-42 induced AD in mice | In vivo | Increases SOD, GSH-Px, GSH levels | 4, 12, 36 mg/kg | 12 mg/kg | [30] |
| Decreases MDA, GSSG levels | ||||||
| Sch B | SP induced dementia in mice | In vivo | Suppresses AChE (acetylcholinesterase) activity | 10, 25, 50 mg/kg | 25 mg/kg | [33] |
| Maintaines ACh level | ||||||
| Occlusion (using aneurysm clips) induced cerebral I/R injury | In vivo | Increases GSH, α-TOC, Mn-SOD | 1, 10, 30 mg/kg | 1 mg/kg | [34] | |
| Decreases MDA, Ca2+, MPT | ||||||
| Aβ1-42 induced AD in mice | In vivo | Restroes GLT-1 and GSK3β activities | 0.15 mg/kg | [35] | ||
| Decreases hyperphosphorylated tau protein | ||||||
| Microglial-mediated inflammatory injury | In vitro | Inhibites ROS, NADPH oxidase activity | 5, 10, 20 μM | 5 μM | [36] | |
| STA | 6-OHDA-induced neural damage in SH-SY5Y cells | In vitro | Decreases cytotoxicity | 3, 6, 12, 25, 50, 100 μM | 14.8 μM (EC50) | [42] |
| Down-regulates ROS level | ||||||
| Inhibites NO, iNOS levels | ||||||
| Opposes ERK phosphorylation decreases | ||||||
| Up-ragulates p-Akt/t-Akt ratio | ||||||
| Preventes GSK3β dephosphorylation | ||||||
| 6-OHDA-induced neural damage in zebrafish | In vivo | Prevents dopaminergic neuron loss | 2.5, 5, 10 μM | 10 μM | ||
| Aβ1-42 induced AD in mice | In vivo | Restroes SOD, GSH-Px, MDA, GSH activites | 0.01–0.1 mg/kg | 0.1 mg/kg | [43] | |
| SCH | Aβ1-42 induced AD in mice | In vivo | Increases SOD, GSH-Px, GSH levels | 4, 12, 36 mg/kg | 36 mg/kg | [45] |
| Decreases MDA, GSSG levels | ||||||
| ICO | 6-OHDA-induced neural damage in SH-SY5Y cells | In vitro | Inhibites ROS | 20, 40, 80 µM | 40 µM | [47] |
| Inhibites calcuim accumulation | ||||||
| Increases NQO1, HO-1 levels | ||||||
| Gomisin A | LPS-stimulated N9 microglia | In vitro | Inhibites ROS, NADPH, gp91phox expression | 1–100 µM | 3 µM | [50] |
| SCF and Its Active Ingredients | Study Design | Study Type | Molecular and Cellular Mechanisms of Action | Dose Range | Minimal Active Concentration | Key Reference |
|---|---|---|---|---|---|---|
| TLS | Aβ1-42 induced AD in primary mouse neuronal cells | In vitro | Increase Bcl-2 expressions | 10, 30, 100 μM | 10 μM | [26] |
| Suture-occluded induced cerebral ischemia injury | In vivo | Inhibites Bax level | 25–100 mg/kg | 25 mg/kg | [28] | |
| Increases Bcl-2, p-Akt levles | ||||||
| Sch A | OGD/R-induced cell death in primary culture of rat cortical neurons | In vitro | Decreases Ca2+, LDH levels | 1.25, 2.5, 5 μg/mL | 1.25 μg/mL | [31] |
| Up-regulates C3aR, C5aR levels | ||||||
| H293T cell | Down-regulates ERK, JNK, p38, caspase-3 levels | |||||
| Sch B | Aβ-induced neuronal dysfunction in rats | In vivo | Inhibites Caspase-3, TUNEL positive cells | 25 or 50 mg/kg | 25 mg/kg | [37] |
| Up-regulates HSP70, beclin-1 | ||||||
| Sch C, Schizandrol B | Serum and glucose deprivation (SGD) injury in SH-SY5Y cells | In vitro | Inhibites LDH level | 2.5, 5.0 mg/mL | 2.5 mg/mL | [40] |
| Inhibites NLRP3, Caspase-1, IL-1β, NF-κB, plκB/lκB, pJNK1/2, JNK1/2, Caspase-3 expression | ||||||
| STA | MPP+ induced neural damage in SH-SY5Y cells | In vitro | Decreases cytotoxicity | 60 μM | 60 μM | [44] |
| Increases CREB, Bcl-2 expression | ||||||
| Activates PI3K and Akt levels | ||||||
| MPTP induced neural damage in mice (PD) | In vivo | Prevents TH-positive dopaminergic neurons loss | 30, 100, 300 mg/kg | 300 mg/kg | ||
| Serum and glucose deprivation (SGD) injury in SH-SY5Y cells | In vitro | Inhibites LDH level | 2.5, 5.0 mg/mL | 2.5 mg/mL | [40] | |
| Inhibites NLRP3, Caspase-1, IL-1β, NF-κB, plκB/lκB, pJNK1/2, JNK1/2, Caspase-3 expression | ||||||
| ICO | 6-OHDA-induced neural damage in SH-SY5Y cells | In vitro | Inhibites TUNEL positive cells | 20, 40, 80 µM | 40 µM | [47] |
| Inhibites the release of AIF | ||||||
| Stimulates the activation of PKA/PKB/CREB/Nrf-2 | ||||||
| SP induced memory impairment in mice (AD) | In vivo | Decreases AChE activity | 5, 10 mg/kg | 5 mg/kg | [48] | |
| Up-ragulates Bcl-2/Bax ratio | ||||||
| Attenuates the decrease of ERK phosphorylation | ||||||
| Gomisin A | CTX induced brain injury in rats | In vivo | Blocking CYP3A-mediated metabolism | 20.8 mg/kg | 20.8 mg/kg | [51] |
| Reducing CAA production |
| SCF and Its Active Ingredients | Study Design | Study Type | Molecular and Cellular Mechanisms of Action | Dose Range | Minimal Active Concentration | Key Reference |
|---|---|---|---|---|---|---|
| TLS | Aβ1-42 induced AD in primary mouse neuronal cells | In vitro | Decreases BACE1 activity | 10, 30, 100 μM | 10 μM | [26] |
| Inhibites JNK/p38 expression | ||||||
| LPS-induced inflammation in microglia (BV2 cells) | In vitro | Inhibites NO level | 1, 10 μM | 10 μM | [29] | |
| Sch A | LPS-induced inflammation in microglia (BV2 cells) | In vitro | Down-regulates the NO, TNF-α, IL-6 increasing | 10, 20, 50 μM | 10 μM | [32] |
| Microglia-mediated inflammatory injury in neurons | Inhibites iNOS, COX-2 levels | 10, 20, 50 μM | 20 μM | |||
| Inhibites TRAF6-IKKβ-NF-κB pathway | ||||||
| Inhibites Jak2-Stat3 pathway activation and Stat3 nuclear translocation | ||||||
| Sch B | Aβ-induced neuronal dysfunction in rats | In vivo | Inhibites iNOS, COX-2, IL-1β, IL-6, TNF-α levels and DNA damage | 25 or 50 mg/kg | 25 mg/kg | [37] |
| Inhibites RAGE, NF-κB, MAPKs | ||||||
| LPS-induced inflammation in microglia (BV2 cells) | In vitro | Down-regulates TNF-α, IL-6, IL-1β, and PGE2 levels | 12.5, 25, 50 μM | 12.5 μM | [38] | |
| Inhibites NF-κB activation | ||||||
| Up-ragulates the expression of PPAR-γ | ||||||
| Microglial-mediated inflammatory injury | In vitro | Down-regulates NO, TNF-α, PGE2, IL-1β, IL-6 levels | 5, 10, 20 μM | 5 μM | [36] | |
| Inhibites TLR 4, MyD88, IRAK-1, TRAF-6 interaction | ||||||
| Inhibites IKK, NF-κB levels | ||||||
| Intraluminal thread induced focal cerbral ischemia in rats | In vivo | Inhibites TNF-α, IL-1β, matrix metalloproteinase (MMP)-2, MMP-9, OX-42 levels | 10, 30 mg/kg | 10 mg/kg | [39] | |
| Sch C | LTA induced inflammation in mouse primary microglia | In vitro | Increases HO-1, NQO-1 levels | 1, 5, 10, 20 μM | 10 μM | [41] |
| Activates cAMP, PKA, CREB, Nrf-2 levels | ||||||
| Attenuates ddAdo, H-89 levels | ||||||
| Inhibites PGE2, NO, ROS, iNOS, COX-2, MMP-9 expressions | ||||||
| Suppresses NF-κB, AP-1, JAK-STATs, MAPK activation | ||||||
| ICO | Aβ-stimulated neuroinflammation in mouse primary microglia | In vitro | Inhibites PGE2, NO, ROS, MMP-9 levels | 25, 50, 100 µM | 100 µM | [49] |
| Inhibites iNOS, COX-2 levels | ||||||
| Inhibites IκB-α, NF-κB, MAPK activities | ||||||
| Gomisin A | LPS-stimulated inflammation N9 microglia | In vitro | Suppresses iNOS, COX-2 levels | 1–100 µM | 3 µM | [50] |
| Attenuates TNF-α, IL-1β and IL-6 levels | ||||||
| Inhibited TAK1-IKKa/b-IκB -NF- κB and MAPKs inflammatory signaling pathways | 30–100 µM | 30 µM | ||||
| Inhibited TLR4 expression | ||||||
| Gomisin N | LPS-induced inflammatory and depressive symptoms in mice | In vivo | Inhibites iNOS, COX-2, IL-1β, IL-6, TNF-α levels | 100 mg/kg | 100 mg/kg | [52] |
| Increases c-Fos immunopositive cells number | ||||||
| LPS-induced inflammation in microglia (BV2 cells) | In vitro | Inhibites iNOS, COX-2, IL-1β, IL-6, TNF-α levels | 1.56–50 µM | 25 µM |
| SCF and its Active Ingredients | Study Design | Study Type | Molecular and Cellular Mechanisms of Action | Dose Range | Minimal Active Concentration | Key Reference |
|---|---|---|---|---|---|---|
| SCF | Ethanol withdrawal induced anxiety-like behavior | In vivo | Decreases NE and its metabolite | [24] | ||
| PCPA induced insomnia in rat | In vivo | Reduces the elevation of GABA, NE, DA, DOPAC, HVA | 7.5 g/kg | 7.5 g/kg | [25] | |
| Increases 5-HT, 5-HIAA levels | ||||||
| SCH | APP/PS1 transgenic mice (induced AD) | In vivo | Ameliorated the cognitive impairment | 2 mg/kg | 2 mg/kg | [46] |
| Decreases Aβ deposition in the hippocampus | ||||||
| Regulates serotonin, 5-HIAA, DA, NE, γ-aminobutyric acid, glutamic acid, homovanillic acid, 3,4-dihydroxyphenylacetic acid and acetylcholine levels |
| SCF and Its Active Ingredients | Study Design | Study Type | Molecular and Cellular Mechanisms of Action | Dose Range | Minimal Active Concentration | Key Reference |
|---|---|---|---|---|---|---|
| SCF | Corticosterone induced depressive-like behavior in mice | In vivo | Up-ragulates BDNF/TrkB/CREB | 300, 600 mg/kg | 600 mg/kg | [22] |
| CUMS-induced depression and cognitive impairment in mice | In vivo | Increases BDNF levels in hippocampus | 600–1200 mg/kg | 600 mg/kg | [23] | |
| Up-regulates TrkB/CREB/ERK | ||||||
| Up-regulates PI3K/Akt/GSK-3β | ||||||
| Nigranoic acid | NGF-differentiated PC12 cells | In vitro | Increases BDNF, c-fos mRNA | 1, 10, 50 µM | 50 µM | [53] |
| Increases cytoplasmic Ca2+, NO levels | ||||||
| Activates ERK1/2, CaMKII levels |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Xu, L.; Yang, H. Schisandra chinensis Fructus and Its Active Ingredients as Promising Resources for the Treatment of Neurological Diseases. Int. J. Mol. Sci. 2018, 19, 1970. https://doi.org/10.3390/ijms19071970
Zhang M, Xu L, Yang H. Schisandra chinensis Fructus and Its Active Ingredients as Promising Resources for the Treatment of Neurological Diseases. International Journal of Molecular Sciences. 2018; 19(7):1970. https://doi.org/10.3390/ijms19071970
Chicago/Turabian StyleZhang, Minyu, Liping Xu, and Hongjun Yang. 2018. "Schisandra chinensis Fructus and Its Active Ingredients as Promising Resources for the Treatment of Neurological Diseases" International Journal of Molecular Sciences 19, no. 7: 1970. https://doi.org/10.3390/ijms19071970






