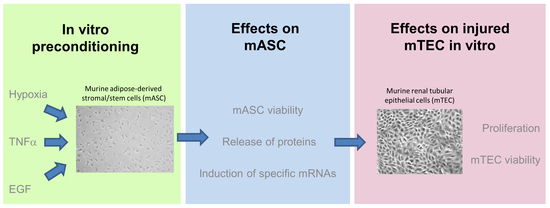
Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells
Abstract
:1. Introduction
2. Results
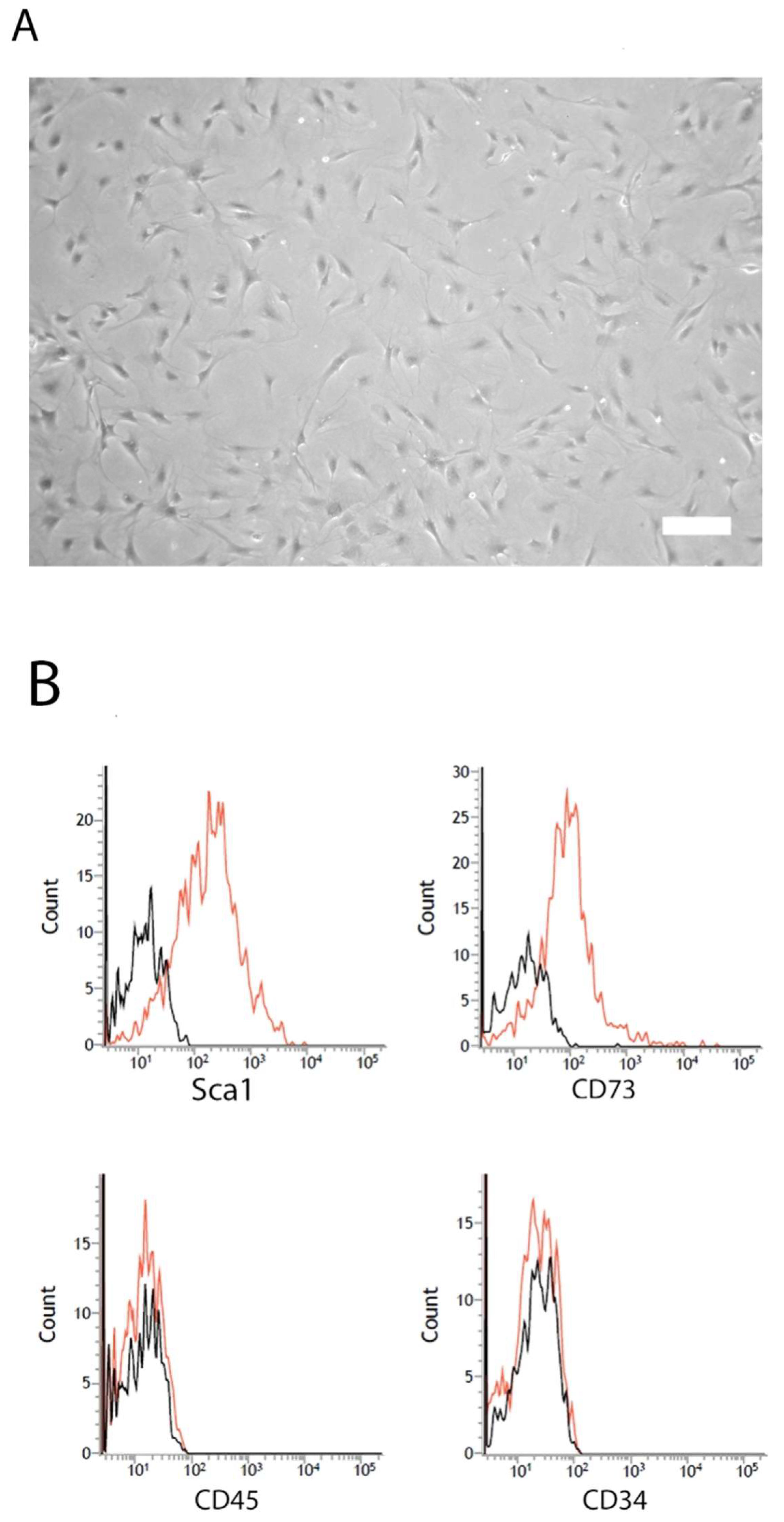
2.1. Characterization of mASC
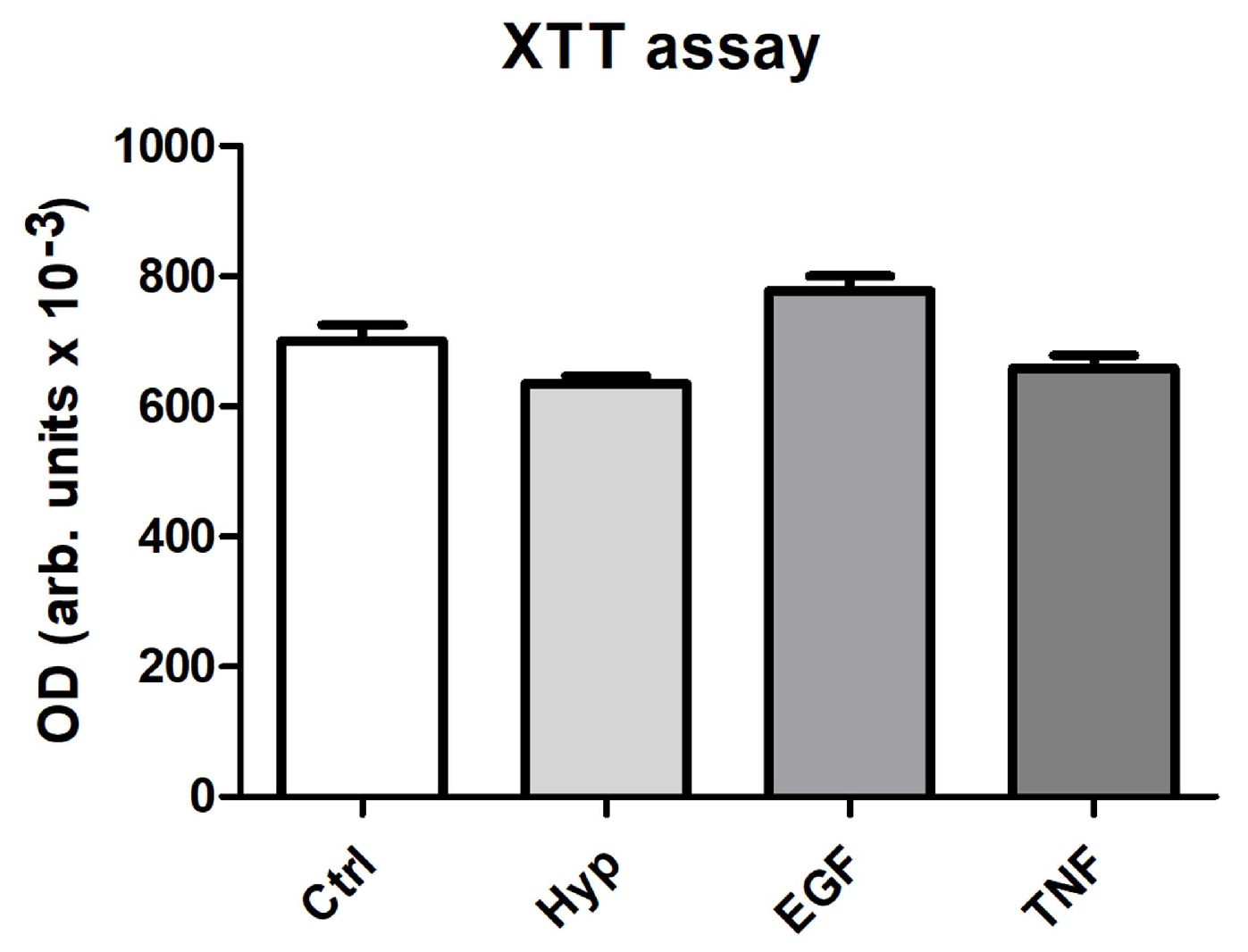
2.2. Measurement of Cell Viability after Preconditioning
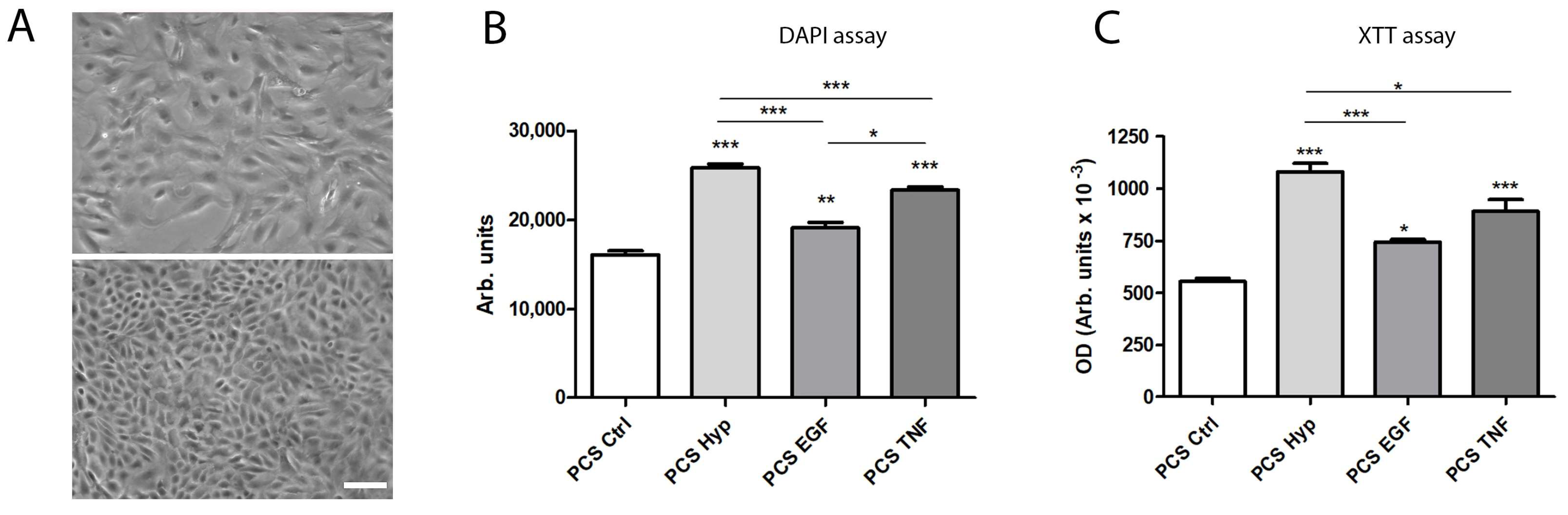
2.3. Effects of Preconditioning Regimens
2.4. Effects of Preconditioned Culture Supernatants on Epithelial Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Isolation and Culture
4.3. Preconditioning Regimens
4.4. Cell Viability and Proliferation Assays
4.5. Protein Array
4.6. PCR
4.7. Effect of Preconditioned Medium on the Proliferation of mTEC
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| bFGF | Basic fibroblast growth factor |
| CM | Conditioned medium |
| EGF | Epidermal growth factor |
| HGF | Hepatocyte growth factor |
| Hyp | Hypoxic environment |
| IL | Interleukin |
| mASC | Murine adipose-derived stromal/stem cells |
| MMP12 | Matrix metalloproteinase-12 |
| MSC | Mesenchymal stromal/stem cells |
| PCR | Quantitative real-time polymerase chain reaction |
| TNFα | Tumor necrosis factor-alpha |
| VEGF | Vascular endothelial growth factor |
References
- Caplan, A.I. All MSCs are pericytes? Cell Stem Cell 2008, 3, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.T.-F.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative Analysis of Paracrine Factor Expression in Human Adult Mesenchymal Stem Cells Derived from Bone Marrow, Adipose, and Dermal Tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, F.F.; Rocco, P.R.M. Stem-cell extracellular vesicles and lung repair. Stem Cell Investig. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Trans. Med. Hemother. 2016, 43, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doorn, J.; Moll, G.; Le Blanc, K.; van Blitterswijk, C.; de Boer, J. Therapeutic applications of mesenchymal stromal cells: Paracrine effects and potential improvements. Tissue Eng. Part B Rev. 2012, 18, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Taylor, D.A.; Geng, Y.-J.; de Caterina, R.; Shelat, H.; Perin, E.C.; Willerson, J.T. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ. Res. 2013, 113, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, T.; Huang, W.; Wang, T.; Qian, J.; Xu, M.; Kranias, E.G.; Wang, Y.; Fan, G.-C. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells 2009, 27, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, W.; Zheng, W.; Ma, Y.; Lin, L.; Tang, T.; Liu, J.; Yu, J.; Zhou, X.; Hu, J. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology 2007, 107, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-L.; Zhang, Y.-J.; Li, M.-H.; Zhang, X.-L.; Chen, S.-L. Mesenchymal stem cells with overexpression of midkine enhance cell survival and attenuate cardiac dysfunction in a rat model of myocardial infarction. Stem Cell Res. Therapy 2014, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overath, J.M.; Gauer, S.; Obermüller, N.; Schubert, R.; Schäfer, R.; Geiger, H.; Baer, P.C. Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp. Cell Res. 2016, 342, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, S.L.; Hsiao, S.T.-F.; Peshavariya, H.M.; Lim, S.Y.; Dusting, G.J.; Dilley, R.J. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012, 21, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Beegle, J.; Lakatos, K.; Kalomoiris, S.; Stewart, H.; Isseroff, R.R.; Nolta, J.A.; Fierro, F.A. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 2015, 33, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, L.; Huo, Y.; Yang, Y.; Wang, Y. Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. BioMed Res. Int. 2014, 2014, 462472. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, S.-M.; Sung, J.-H. Cellular and molecular stimulation of adipose-derived stem cells under hypoxia. Cell Biol. Int. 2014, 38, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, M.E.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004, 10, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Jung, Y.H.; Oh, S.Y.; Yong, M.S.; Ryu, J.M.; Han, H.J. Netrin-1 induces MMP-12-dependent E-cadherin degradation via the distinct activation of PKCα and FAK/Fyn in promoting mesenchymal stem cell motility. Stem Cells Dev. 2014, 23, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.M.; Klose, K.; Bieback, K.; Korinth, D.; Schneider, M.; Seifert, M.; Choi, Y.-H.; Kurtz, A.; Falk, V.; Stamm, C. Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS ONE 2015, 10, e0138477. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Fan, V.H.; Griffith, L.G.; Blair, H.C.; Wells, A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kawasaki, H.; Wells, A. Epidermal Growth Factor (EGF) Treatment on Multipotential Stromal Cells (MSCs). Possible Enhancement of Therapeutic Potential of MSC. J. Biomed. Biotechnol. 2010, 2010, 795385. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C.; Schubert, R.; Bereiter-Hahn, J.; Plösser, M.; Geiger, H. Expression of a functional epidermal growth factor receptor on human adipose-derived mesenchymal stem cells and its signaling mechanism. Eur. J. Cell Biol. 2009, 88, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kerpedjieva, S.S.; Kim, D.S.; Barbeau, D.J.; Tamama, K. EGFR ligands drive multipotential stromal cells to produce multiple growth factors and cytokines via early growth response-1. Stem Cells Dev. 2012, 21, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, J.; Kim, M.Y.; Bae, Y.-S.; Ryu, S.H.; Lee, T.G.; Kim, J.H. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res. 2010, 9, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C.; Bereiter-Hahn, J. Epithelial cells in culture: Injured or differentiated cells? Cell Biol. Int. 2012, 36, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Griesche, N.; Luttmann, W.; Luttmann, A.; Stammermann, T.; Geiger, H.; Baer, P.C. A simple modification of the separation method reduces heterogeneity of adipose-derived stem cells. Cells Tissues Org. 2010, 192, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C.; Nockher, W.A.; Haase, W.; Scherberich, J.E. Isolation of proximal and distal tubule cells from human kidney by immunomagnetic separation. Technical note. Kidney Int. 1997, 52, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.A.; Watkins, M.L.; Li, L.; Herter, P.; Haxelmans, S.; Schlatter, E. A simplified method for isolation of large numbers of defined nephron segments. Am. J. Physiol. 1997, 273, F650–F657. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.; Griesche, N.; Geiger, H. Differentiation of adipose-derived stem cells towards the epithelial lineage. J. Stem Cells Regener. Med. 2010, 6, 61. [Google Scholar]
- Blaheta, R.A.; Franz, M.; Auth, M.K.; Wenisch, H.J.; Markus, B.H. A rapid non-radioactive fluorescence assay for the measurement of both cell number and proliferation. J. Immunol. Methods 1991, 142, 199–206. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Forward | Primer Reverse | Product Length (bp) | NCBI Reference Sequence |
|---|---|---|---|---|
| VEGF | ATG AAC TTT CTG CTC TCT TG | CTT CTG CTC TCC TTC TGT C | 105 | NM_001025250 |
| bFGF | AAC TAC AAC TCC AAG CAG AA | CGT TCA AAG AAG AAA CAC TC | 136 | NM_008006 |
| IL-11 | CTT CAG ACC CTC GAG CAG AT | CGT CAG CTG GGA ATT TGT CT | 108 | NM_008350.4 |
| IL-10 | TCC CCT GTG AAA ATA AGA G | CAG TTG ATG AAG ATG TCA AA | 112 | NM_010548.2 |
| MMP12 | CTC TGC TGA AAG GAG TCTG | AAT TCT GTC CTT TCC ATA ATC | 146 | NM_008605 |
| HGF | CCT TTG CTT TGA TTC TTTC | TTC TTC TTT TCT TCT GTC CTT | 177 | NM_001289458 |
| β-Actin | CCA CCA TGT ACC CAG GCA TT | AGG GTG TAA AAC GCA GCT CA | 253 | NM_007393 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baer, P.C.; Overath, J.M.; Urbschat, A.; Schubert, R.; Koch, B.; Bohn, A.A.; Geiger, H. Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 1719. https://doi.org/10.3390/ijms19061719
Baer PC, Overath JM, Urbschat A, Schubert R, Koch B, Bohn AA, Geiger H. Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells. International Journal of Molecular Sciences. 2018; 19(6):1719. https://doi.org/10.3390/ijms19061719
Chicago/Turabian StyleBaer, Patrick C., Jürgen M. Overath, Anja Urbschat, Ralf Schubert, Benjamin Koch, Asanke A. Bohn, and Helmut Geiger. 2018. "Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells" International Journal of Molecular Sciences 19, no. 6: 1719. https://doi.org/10.3390/ijms19061719
APA StyleBaer, P. C., Overath, J. M., Urbschat, A., Schubert, R., Koch, B., Bohn, A. A., & Geiger, H. (2018). Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells. International Journal of Molecular Sciences, 19(6), 1719. https://doi.org/10.3390/ijms19061719