Morphological Study of Chitosan/Poly (Vinyl Alcohol) Nanofibers Prepared by Electrospinning, Collected on Reticulated Vitreous Carbon
Abstract
:1. Introduction
2. Results and Discussions
2.1. Electrospinning Method: Experimental Parameters
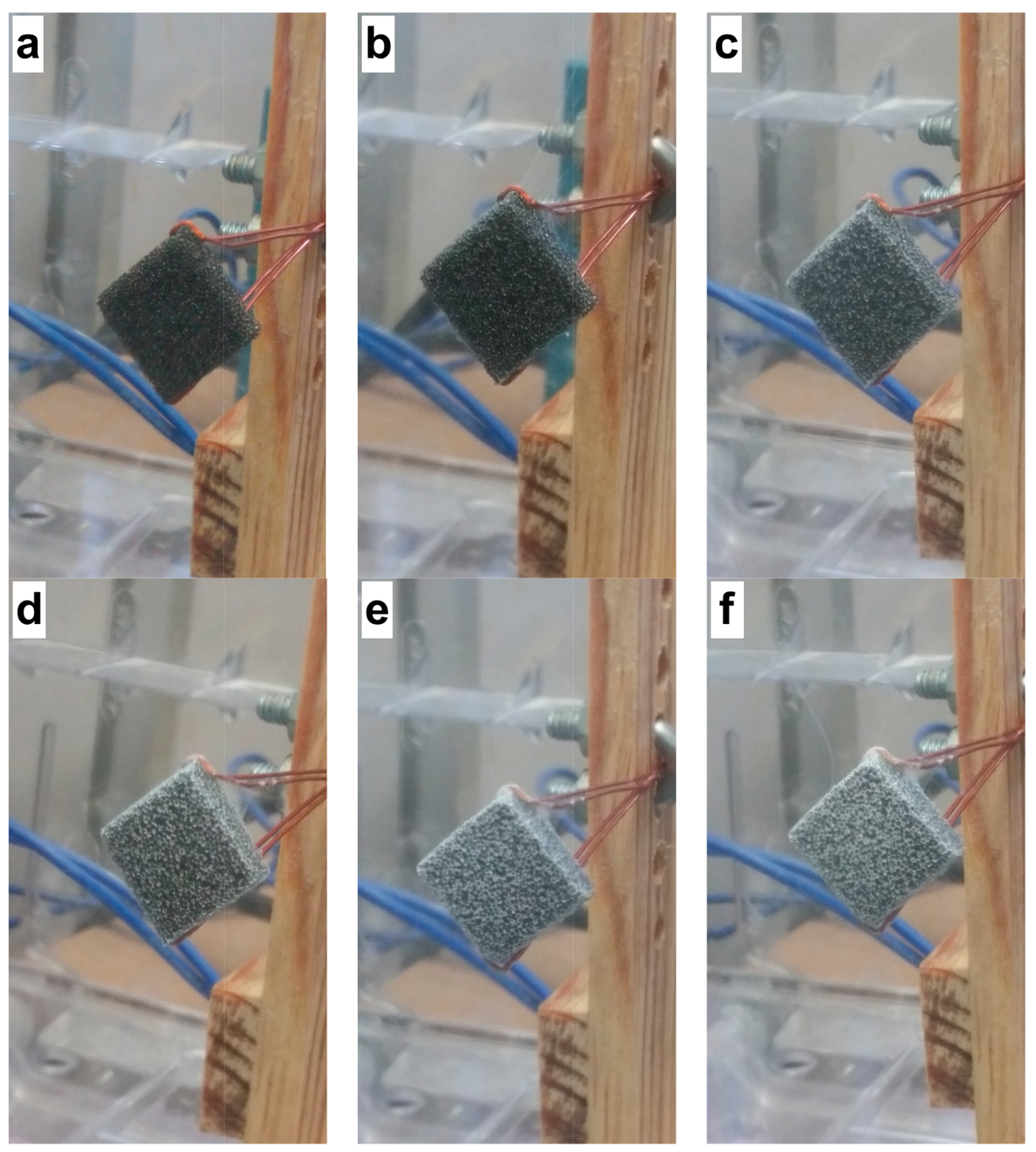
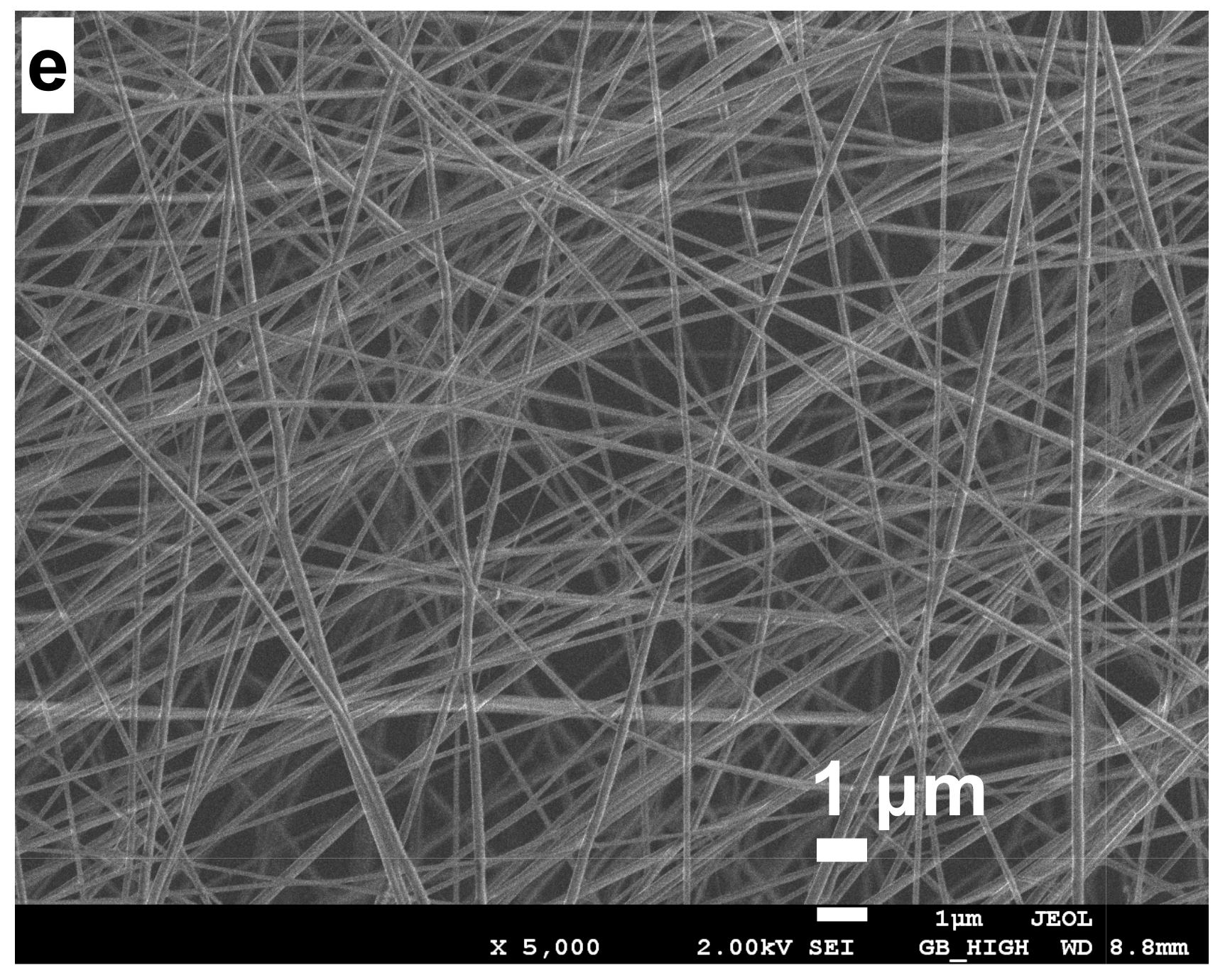
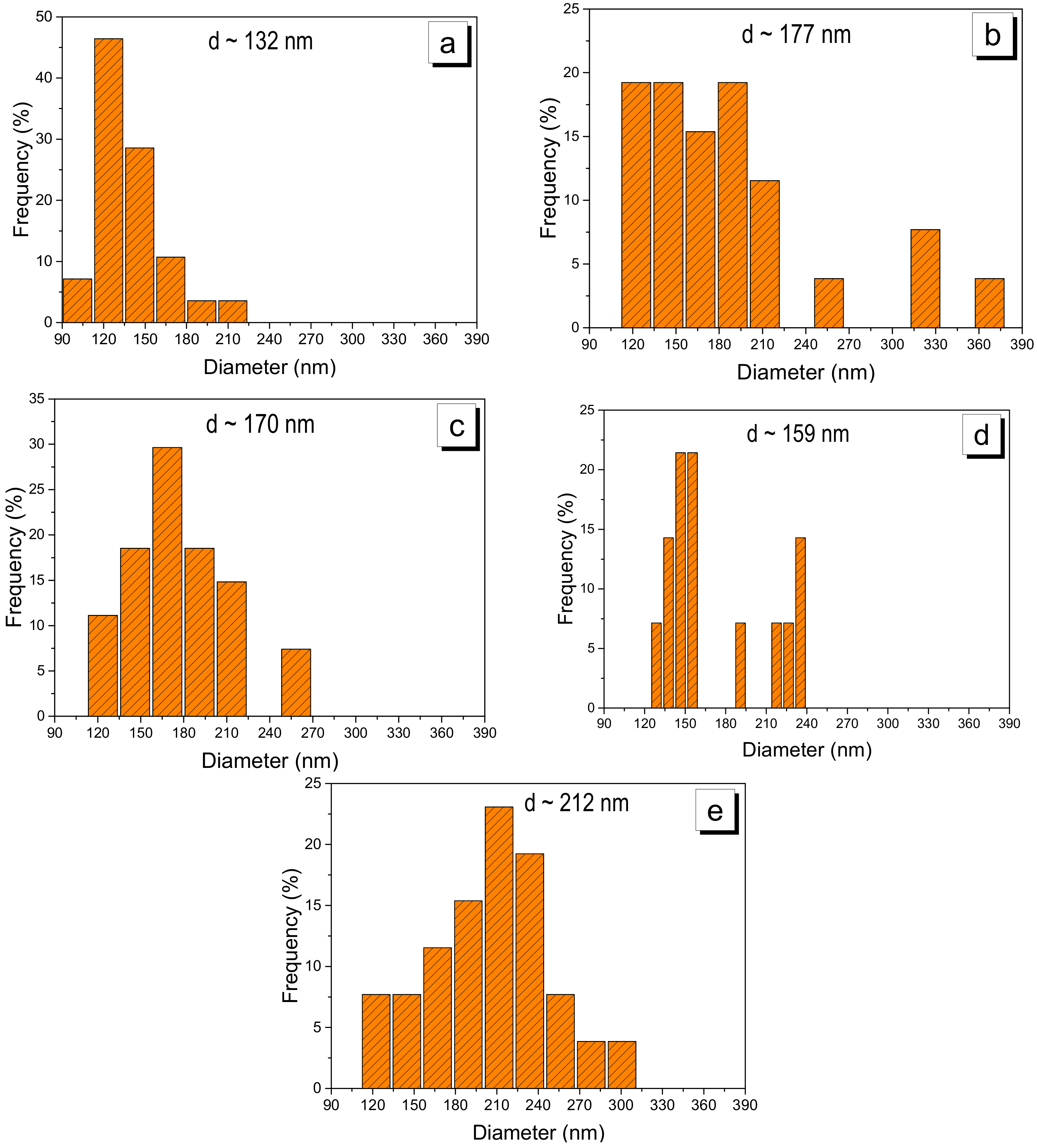
2.2. Electrospun CS/PVA Samples Collected on Reticulated Vitreous Carbon
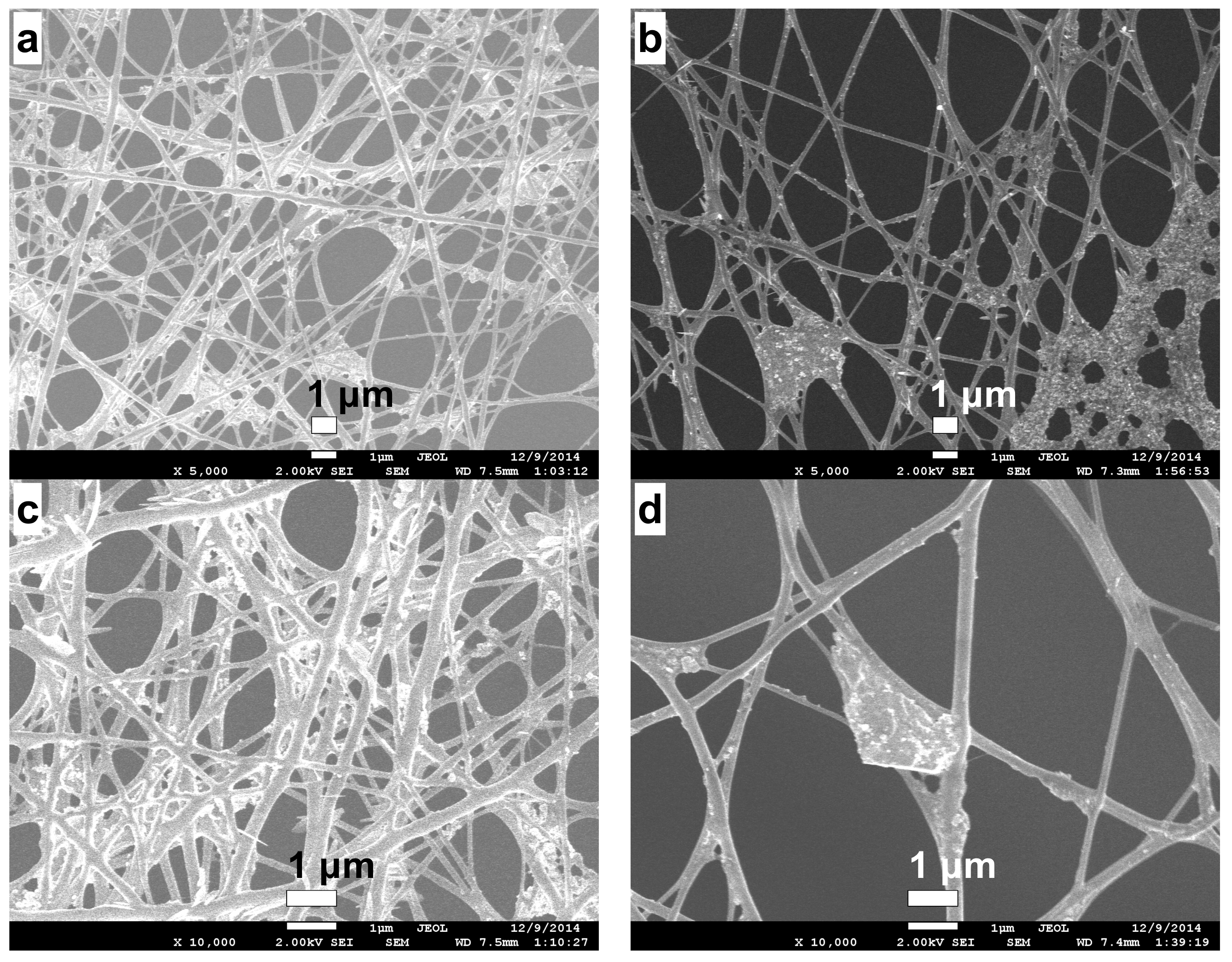
2.3. Morphology of the RVC Support and CS/PVA Fibers on RVC
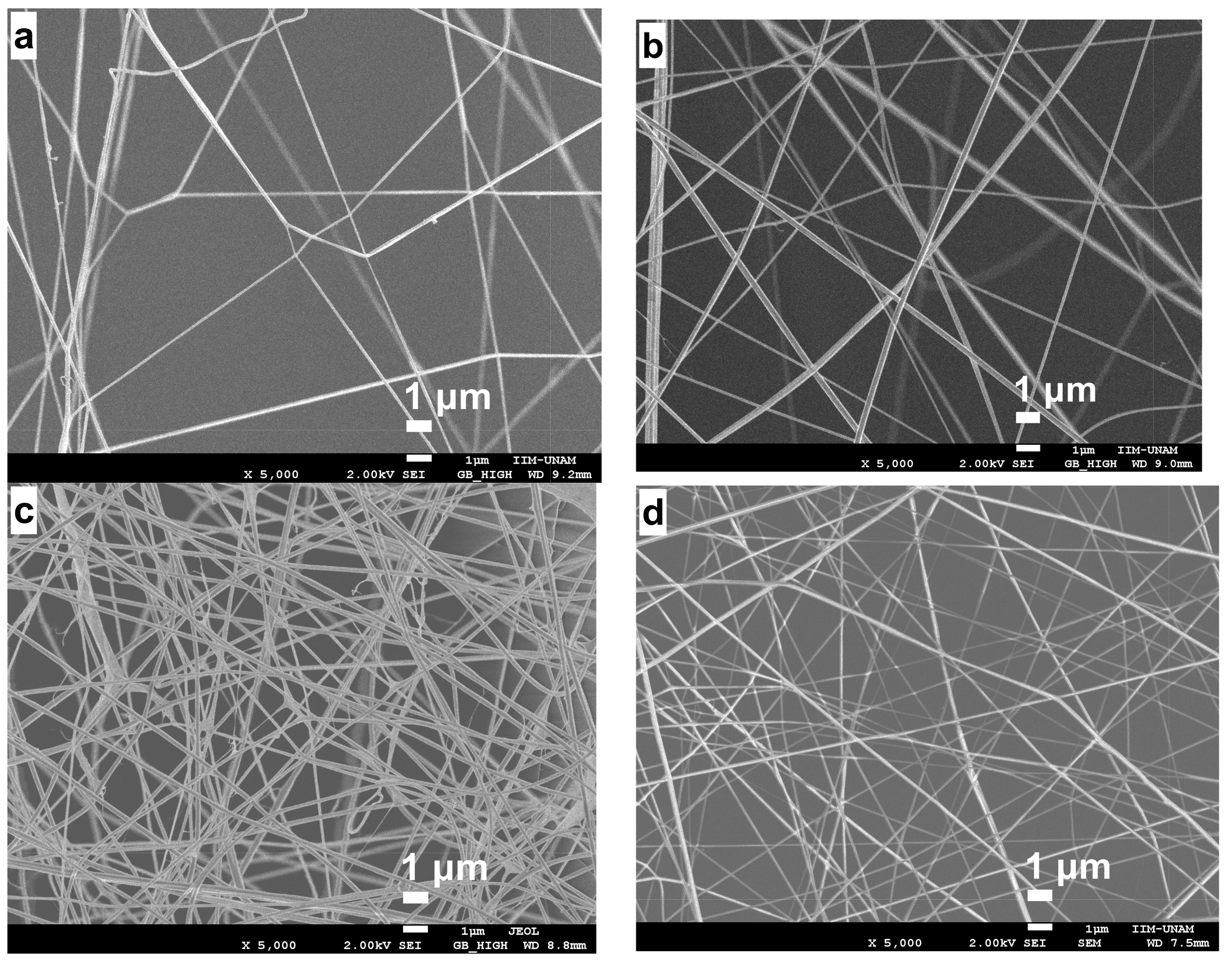
2.4. Stabilization of CS/PVA Nanofibers
2.5. Solution Viscosity
3. Materials and Methods
3.1. Materials
3.2. Preparation of the CS/PVA Solution
3.3. Electrospinning Process
3.4. Alkaline Treatment with NaOH
3.5. Viscometric Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fong, H.; Reneker, D.H. Structure Formation in Polymeric Fibers; Hanser Gardner Publishers: Munich, Germany, 2001; pp. 225–246. [Google Scholar]
- Huang, Z.; Zhang, Y.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Gibson, P.; Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf. A 2001, 187–188, 469–481. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Zivanovic, S. Morphological and surface properties of electrospun chitosan nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Kwon, O.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Homayoni, H.; Hosseini Ravandi, S.A.; Valizadeh, M. Electrospinning of chitosan nanofibers: Processing optimization. Carbohydr. Polym. 2009, 77, 656–661. [Google Scholar] [CrossRef]
- Li, L.; Hsieh, Y.-L. Chitosan bicomponent nanofibers and nanoporous fibers. Carbohydr. Res. 2006, 341, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, J.Y.; Lee, Y.M.; Kim, K.Y. Properties and swelling characteristics of cross-linked poly (vinyl alcohol)/chitosan blend membrane. J. Appl. Polym. Sci. 1992, 45, 1711–1717. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe(III) and Cr(VI) ions. Carbohydr. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Jawalkar, S.; Raju, K.; Halligudi, S.; Sairam, M.; Aminabhavi, T. Molecular modeling simulation to predict compatibility of poly (vinyl alcohol) and chitosan blends: A comparison with experiments. J. Phys. Chem. B 2007, 111, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Prabhakar, M.; Prasad, V.; Rao, M.; Reddy, A.; Rao, C.; Subha, M. Compatibility studies of chitosan/PVA blend in 2% aqueous acetic acid solution at 30 °C. Carbohydr. Polym. 2010, 82, 251–255. [Google Scholar] [CrossRef]
- Lewandowska, K. Viscometric Studies in dilute solution mixtures of chitosan and microcrystalline chitosan with poly (vinyl alcohol). J. Solut. Chem. 2013, 42, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Paipitak, K.; Pornpra, T.; Mongkontalang, P.; Techitdheera, W.; Pecharapa, W. Characterization of PVA-chitosan Nanofibers prepared by electrospinning. Procedia Eng. 2011, 8, 101–105. [Google Scholar] [CrossRef]
- Zargarian, S.S.H.; Haddadi-Asl, V. A nanofibrous composite scaffold of PCL/hydroxyapatite-chitosan/PVA prepared by electrospinning. Iran. Polym. J. 2010, 19, 457–468. [Google Scholar]
- Kizling, K.; Dzwonek, M.; Olszewski, B.; Bącal, P.; Tymecki, L.; Więckowska, A.; Stolarczyk, K.; Bilewicz, R. Reticulated vitreous carbon as a scaffold for enzymatic fuel cell designing. Biosens. Bioelectron. 2017, 95, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F.C.; Arenas, L.F.; Ponce de León, C.; Reade, G.W.; Whyte, I.; Mellor, B.G. The continued development of reticulated vitreous carbon as a versatile electrode material: Structure, properties and applications. Electrochim. Acta 2016, 215, 566–591. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-J.; Ge, D.; Xu, Z.-K. Preparation and characterization of stable chitosan nanofibrous membrane for lipase immobilization. Eur. Polym. J. 2007, 43, 3710–3718. [Google Scholar] [CrossRef]
- Skaugrud, O. Chitosan-New biopolymer for cosmetics and drugs. Drug Cosmet. Ind. 1991, 148, 24–29. [Google Scholar]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]

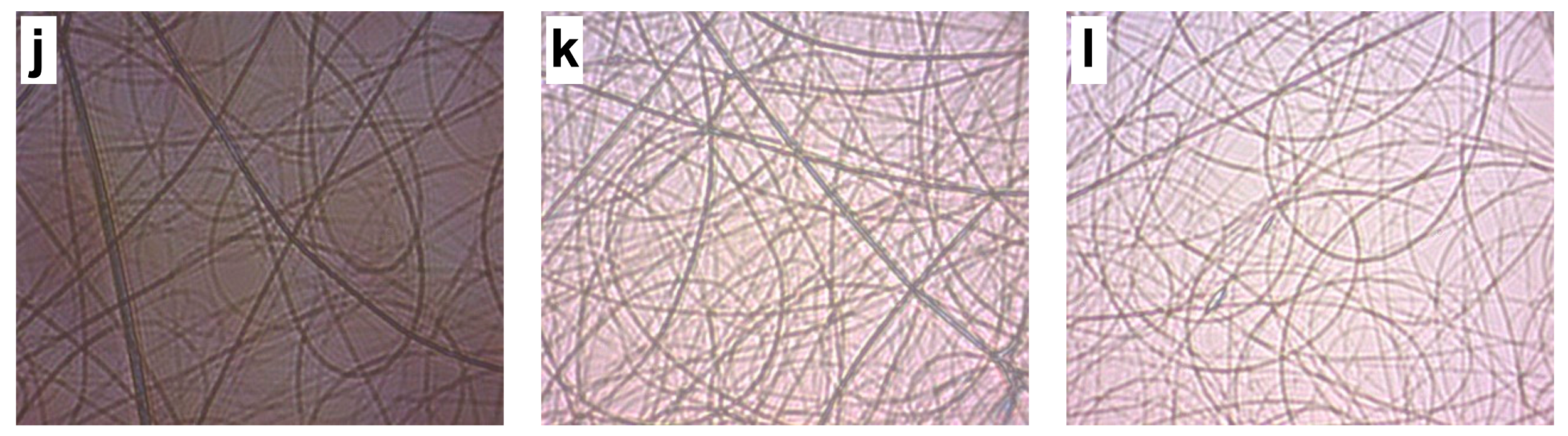
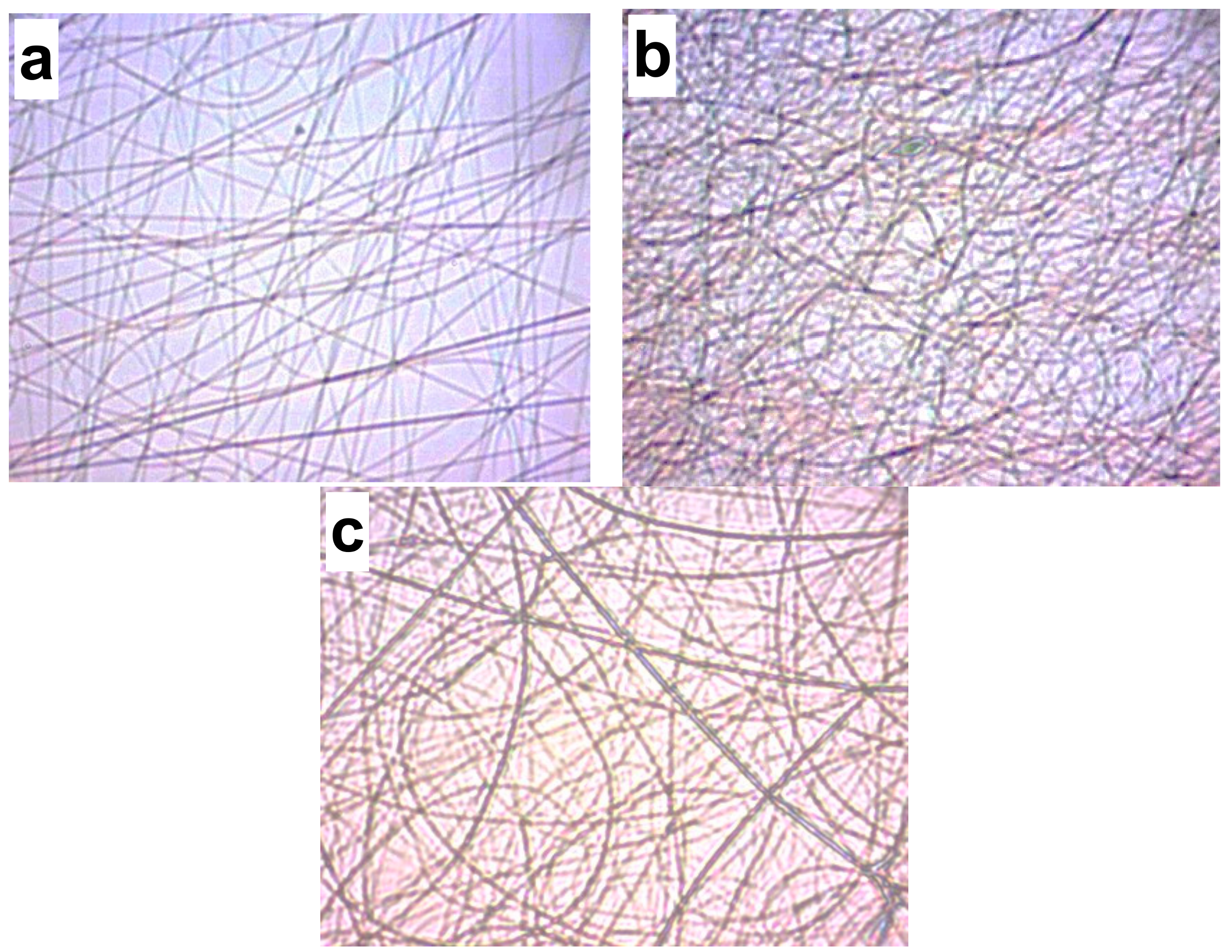



| 2 wt. CS/8 wt % PVA Solution | Image | Flow Rate | Distance | Voltage | Observations * |
|---|---|---|---|---|---|
| Id. | mL/h | cm | kV | ||
| Flow rate | a | 0.12 | 15 | 14 | Non-uniform fibers |
| b | 0.13 | 15 | 14 | Non-uniform fibers with nodules | |
| Voltage | c | 0.13 | 15 | 15 | Non-uniform fibers with nodules |
| d | 0.13 | 15 | 14 | Non-uniform fibers | |
| Tip–target distance | e | 0.12 | 20 | 14 | Non-uniform fibers |
| f | 0.12 | 25 | 14 | Non-uniform fibers | |
| g | 0.13 | 20 | 16 | Non-uniform fibers | |
| h | 0.12 | 25 | 16 | Non-uniform fibers | |
| i | 0.13 | 20 | 14 | Non-uniform fibers | |
| j | 0.13 | 25 | 14 | Non-uniform fibers | |
| k | 0.13 | 20 | 16 | Uniform fibers | |
| l | 0.13 | 25 | 16 | Non-uniform fibers with nodules |
| Parameter | 15 min | 30 min | 1 h | 1.5 h | 2 h |
|---|---|---|---|---|---|
| Diameter (nm) | 132 | 177 | 170 | 159 | 212 |
| Standard deviation (nm) | ±20 | ±9 | ±7 | ±11 | ±6 |
| Parameter | Before | After |
|---|---|---|
| Diameter (nm) | 159 ± 11 | 115 ± 9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Alvarado, D.I.; Guzmán-Pantoja, J.; Páramo-García, U.; Maciel-Cerda, A.; Martínez-Orozco, R.D.; Vera-Graziano, R. Morphological Study of Chitosan/Poly (Vinyl Alcohol) Nanofibers Prepared by Electrospinning, Collected on Reticulated Vitreous Carbon. Int. J. Mol. Sci. 2018, 19, 1718. https://doi.org/10.3390/ijms19061718
Sanchez-Alvarado DI, Guzmán-Pantoja J, Páramo-García U, Maciel-Cerda A, Martínez-Orozco RD, Vera-Graziano R. Morphological Study of Chitosan/Poly (Vinyl Alcohol) Nanofibers Prepared by Electrospinning, Collected on Reticulated Vitreous Carbon. International Journal of Molecular Sciences. 2018; 19(6):1718. https://doi.org/10.3390/ijms19061718
Chicago/Turabian StyleSanchez-Alvarado, Diana Isela, Javier Guzmán-Pantoja, Ulises Páramo-García, Alfredo Maciel-Cerda, Reinaldo David Martínez-Orozco, and Ricardo Vera-Graziano. 2018. "Morphological Study of Chitosan/Poly (Vinyl Alcohol) Nanofibers Prepared by Electrospinning, Collected on Reticulated Vitreous Carbon" International Journal of Molecular Sciences 19, no. 6: 1718. https://doi.org/10.3390/ijms19061718






