Cytoprotective Effect of Epigallocatechin Gallate (EGCG)-5′-O-α-Glucopyranoside, a Novel EGCG Derivative
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Effect of EGCG-5′Glu in Cell and Cell-free Systems
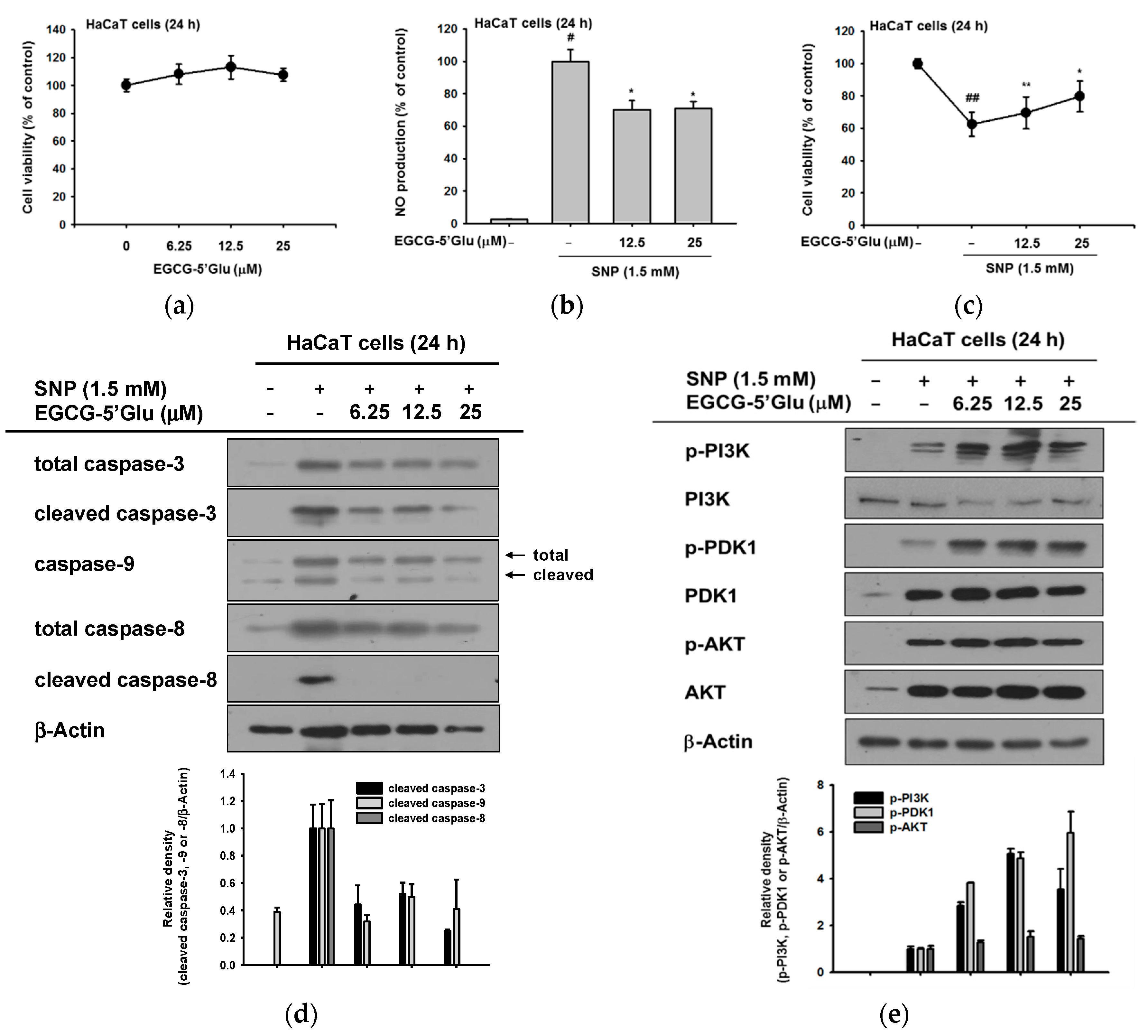
2.2. Cell Protective Effect of EGCG-5′Glu from SNP-Induced Radicals
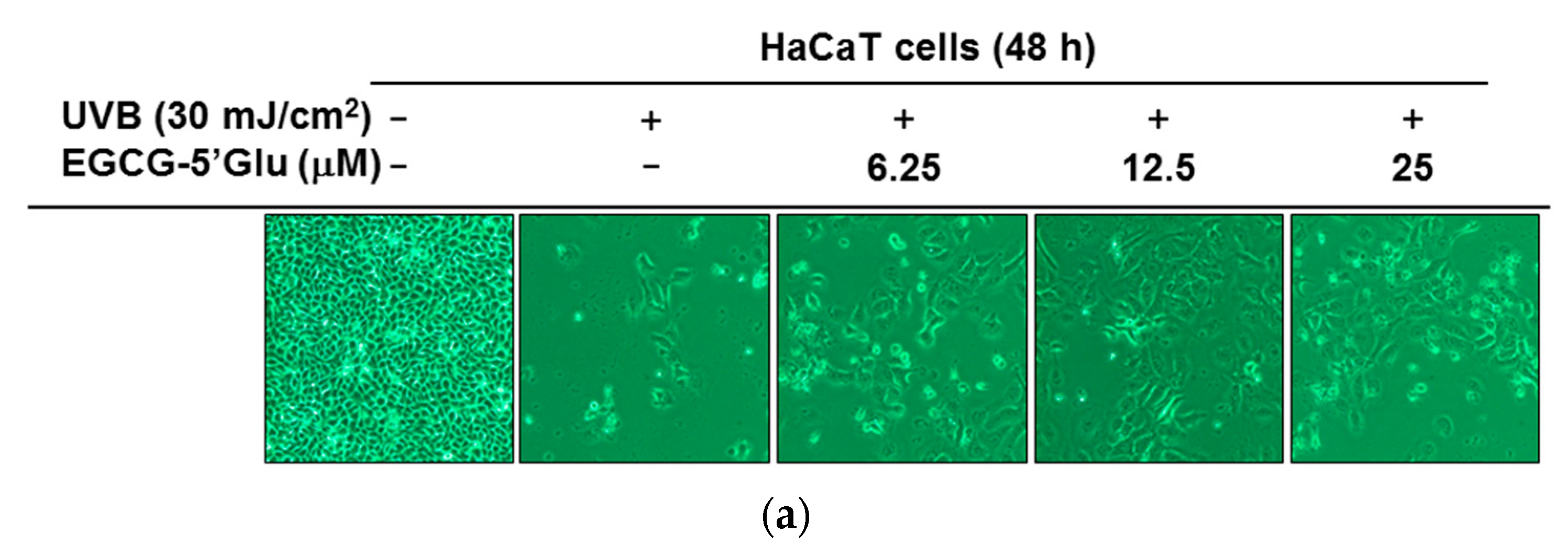
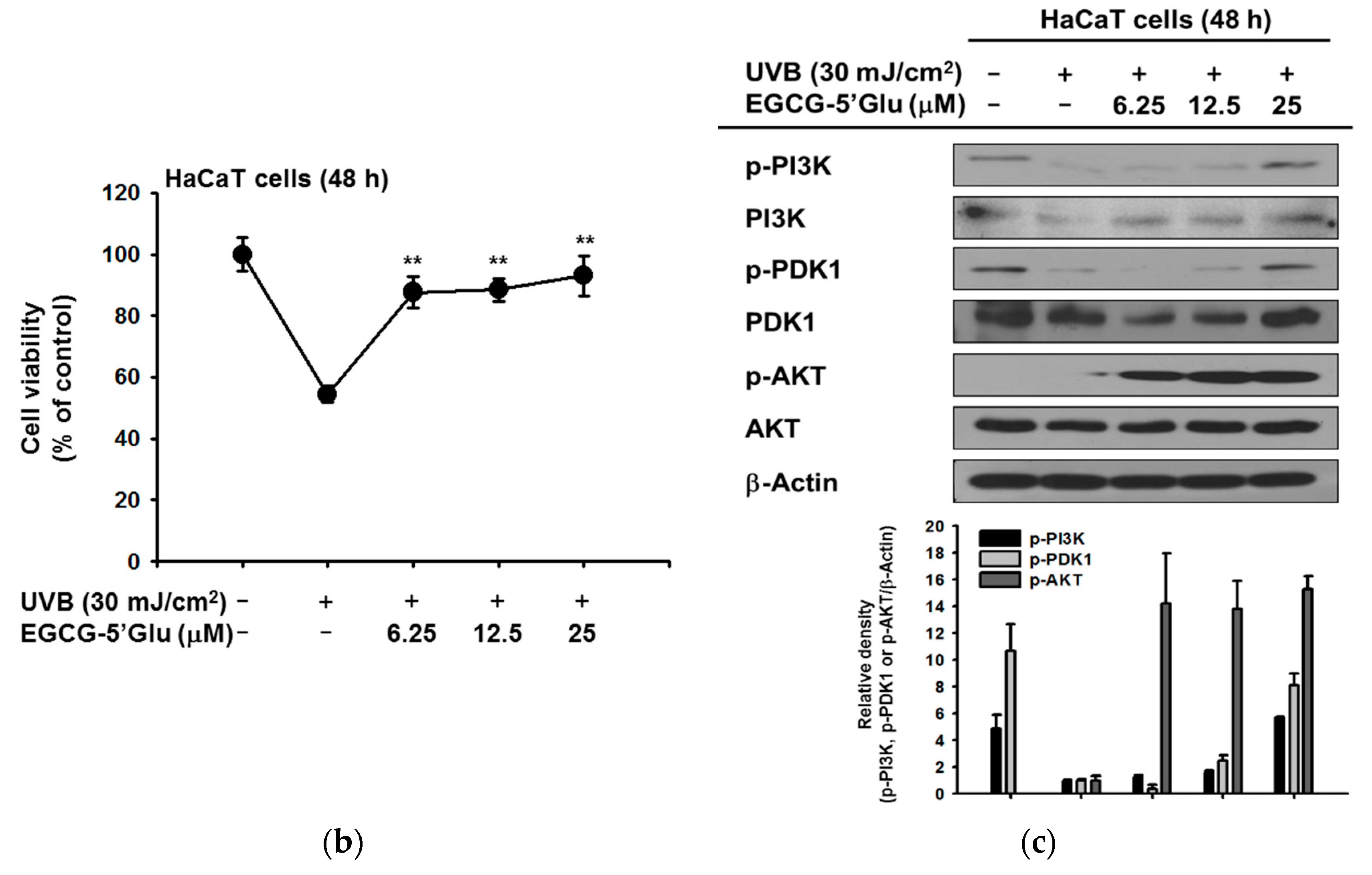
2.3. Cytoprotective Effect of EGCG-5′Glu Against UVB-Induced Damage
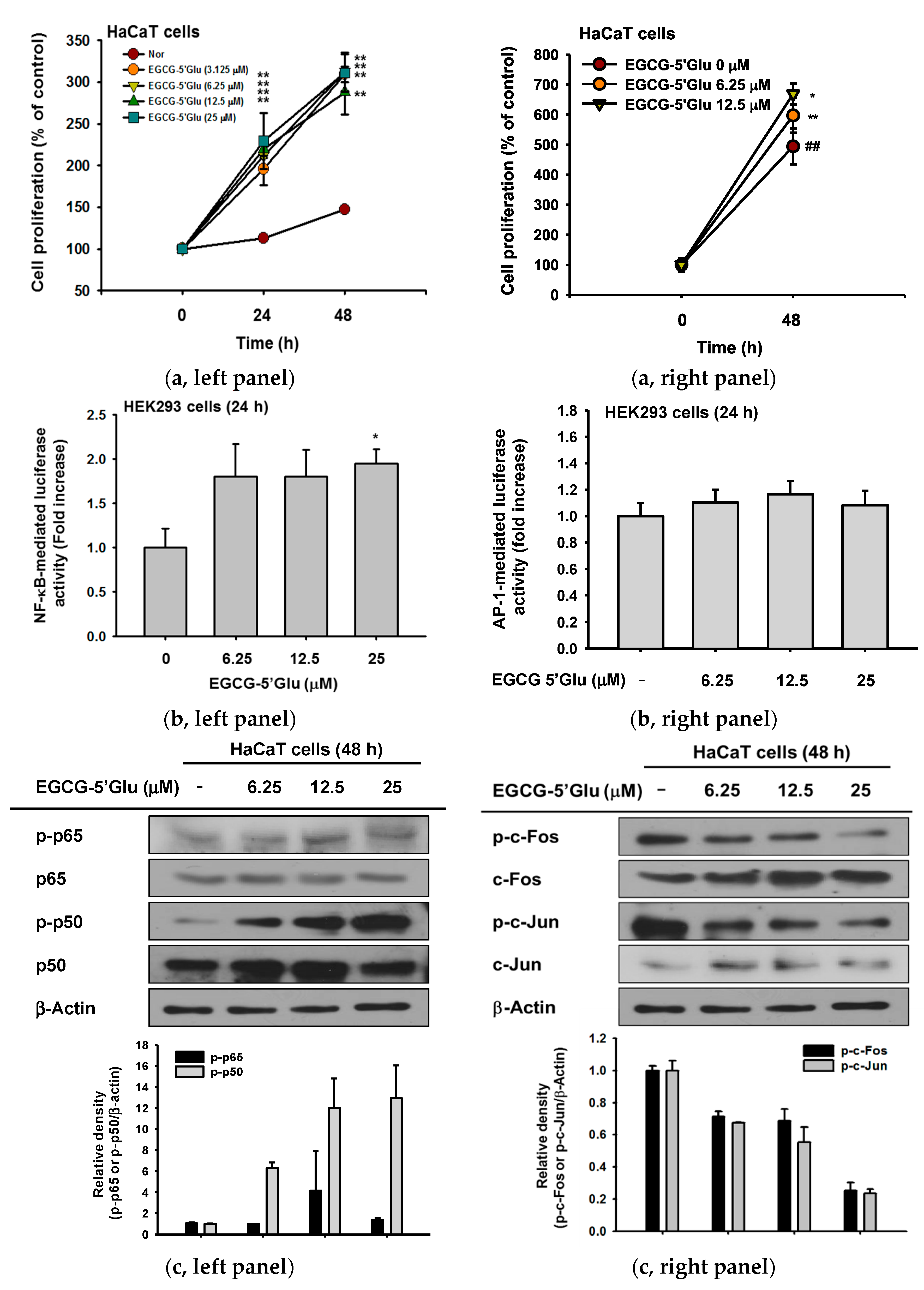
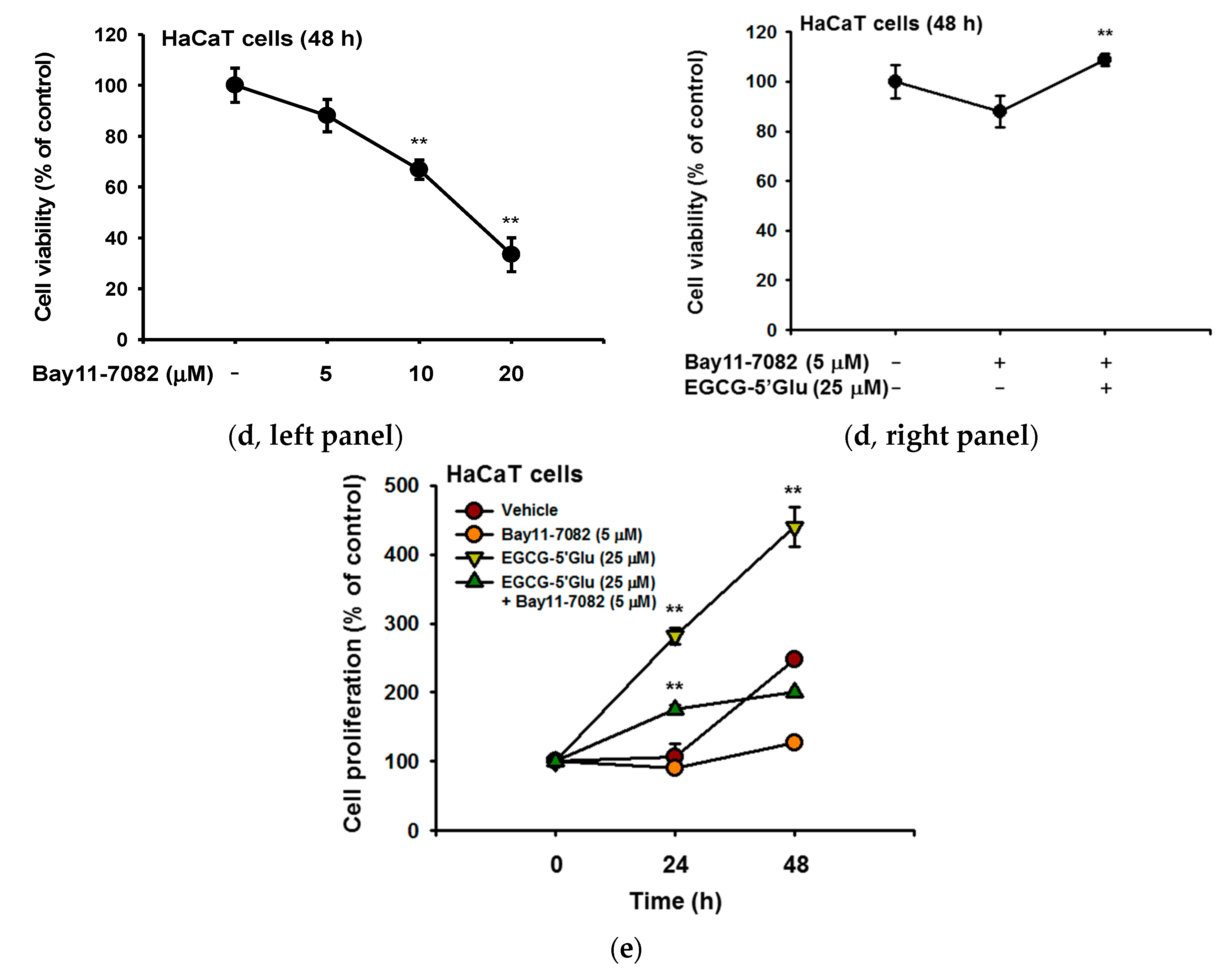
2.4. Cell Proliferative Effect of EGCG-5′Glu
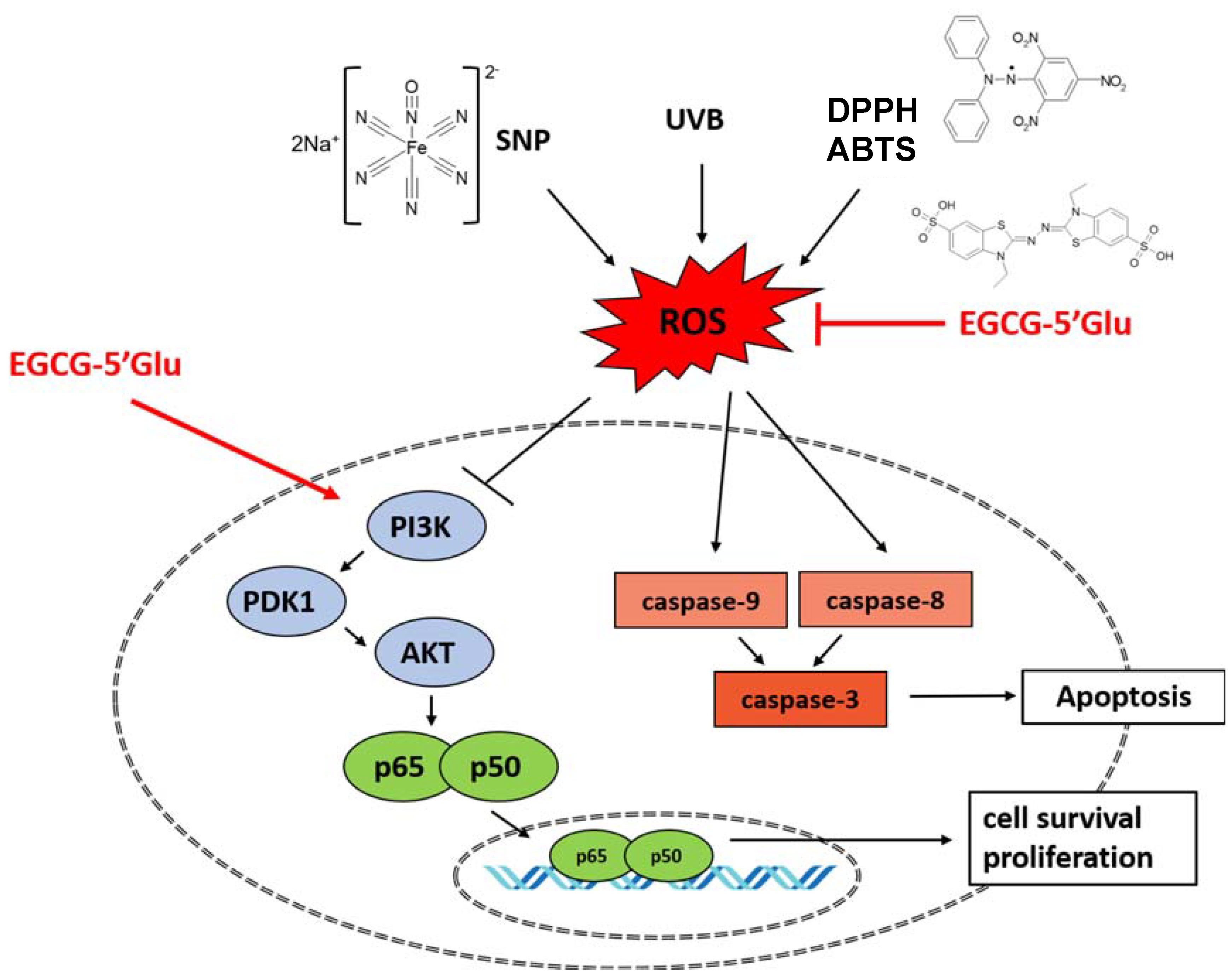
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Viability and Cell Proliferation Assay
4.4. DPPH assay
4.5. ABTS Assay
4.6. ROS Generation
4.7. NO Production and Griess Assay
4.8. Immunoblot Assay
4.9. UVB Irradiation
4.10. Reporter Gene Assay
4.11. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W. Role of reactive oxygen species in cell death pathways. Hanyang Med. Rev. 2013, 33, 77–82. [Google Scholar] [CrossRef]
- Kamogashira, T.; Fujimoto, C.; Yamasoba, T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. BioMed Res. Int. 2015, 2015, 617207. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 270. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Byrne, C. The epidermis: Rising to the surface. Curr. Opin. Genet. Dev. 1994, 4, 725–736. [Google Scholar] [CrossRef]
- Maruoka, Y.; Harada, H.; Mitsuyasu, T.; Seta, Y.; Kurokawa, H.; Kajiyama, M.; Toyoshima, K. Keratinocytes become terminally differentiated in a process involving programmed cell death. Biochem. Biophys. Res. Commun. 1997, 238, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Gandarillas, A. Epidermal differentiation, apoptosis, and senescence: Common pathways? Exp. Gerontol. 2000, 35, 53–62. [Google Scholar] [CrossRef]
- Calautti, E.; Li, J.; Saoncella, S.; Brissette, J.L.; Goetinck, P.F. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J. Biol. Chem. 2005, 280, 32856–32865. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Verzoni, E.; Prinzi, N.; Mennitto, A.; Femia, D.; Grassi, P.; Concas, L.; Vernieri, C.; Lo Russo, G.; Procopio, G. Everolimus treatment for neuroendocrine tumors: Latest results and clinical potential. Ther. Adv. Med. Oncol. 2017, 9, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 2004, 15, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.P.; Shapiro, V.S.; Stokoe, D.; Weiss, A. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 1999, 9, 601–604. [Google Scholar] [CrossRef]
- Barkett, M.; Gilmore, T.D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 1999, 18, 6910. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Falah, R.R.; Talib, W.H.; Shbailat, S.J. Combination of metformin and curcumin targets breast cancer in mice by angiogenesis inhibition, immune system modulation and induction of p53 independent apoptosis. Ther. Adv. Med. Oncol. 2017, 9, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.-H.; Liu, X.; Rennert, O.M. Apoptosis: Reprogramming and the fate of mature cells. ISRN Cell Biol. 2012, 2012, 685852. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.J.; Chow, J.M.; Chuang, S.E.; Chang, C.L.; Yan, M.D.; Lee, H.L.; Lai, I.C.; Lin, P.C.; Lai, G.M. Induction of Forkhead Class box O3a and apoptosis by a standardized ginsenoside formulation, KG-135, is potentiated by autophagy blockade in A549 human lung cancer cells. J. Ginseng Res. 2017, 41, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kinney, C.M.; Chandrasekharan, U.M.; Yang, L.; Shen, J.; Kinter, M.; McDermott, M.S.; DiCorleto, P.E. Histone H3 as a novel substrate for MAP kinase phosphatase-1. Am. J. Physiol. Cell. Physiol. 2009, 296, C242–C249. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tower, J. Programmed cell death and apoptosis in aging and life span regulation. Discov. Med. 2009, 8, 223–226. [Google Scholar] [PubMed]
- Sarlak, G.; Jenwitheesuk, A.; Chetsawang, B.; Govitrapong, P. Effects of melatonin on nervous system aging: Neurogenesis and neurodegeneration. J. Pharmacol. Sci. 2013, 123, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Kitao, S.; Matsudo, T.; Saitoh, M.; Horiuchi, T.; Sekine, H. Enzymatic syntheses of two stable (-)-epigallocatechin gallate-glucosides by sucrose phosphorylase. Biosci. Biotechnol. Biochem. 1995, 59, 2167–2169. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Eckert, R.L. Keratinocyte proliferation, differentiation, and apoptosis—Differential mechanisms of regulation by curcumin, EGCG and apigenin. Toxicol. Appl. Pharmacol. 2007, 224, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.-M.; Park, J.; Cho, J.Y. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.-H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar] [PubMed]
- Zhang, X.; Wang, J.; Hu, J.-M.; Huang, Y.-W.; Wu, X.-Y.; Zi, C.-T.; Wang, X.-J.; Sheng, J. Synthesis and biological testing of novel glucosylated epigallocatechin gallate (EGCG) derivatives. Molecules 2016, 21, 620. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilised epigallocatechin gallate (EGCG) derivatives and their antioxidant potential in food and biological systems. Food Chem. 2012, 131, 22–30. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Yun, M.; Choo, E.J.; Kim, S.H.; Jeong, M.S.; Jung, D.B.; Lee, H.; Kim, E.O.; Kato, N.; Kim, B. A derivative of epigallocatechin-3-gallate induces apoptosis via SHP-1-mediated suppression of BCR-ABL and STAT3 signalling in chronic myelogenous leukaemia. Br. J. Pharmacol. 2015, 172, 3565–3578. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T.T.H.; Kim, N.M.; Moon, Y.-H.; Ha, J.-M.; Park, N.; Lee, D.-G.; Hwang, K.-H.; Park, J.-S.; Kim, D. Functional properties of novel epigallocatechin gallate glucosides synthesized by using dextransucrase from Leuconostoc mesenteroides B-1299CB4. J. Agric. Food Chem. 2016, 64, 9203–9213. [Google Scholar] [CrossRef] [PubMed]
- Dehshahri, S.; Wink, M.; Afsharypuor, S.; Asghari, G.; Mohagheghzadeh, A. Antioxidant activity of methanolic leaf extract of Moringa peregrina (Forssk.) Fiori. Res. Pharm. Sci. 2012, 7, 111–118. [Google Scholar] [PubMed]
- Yoon, H.J.; Kim, C.S.; Lee, K.Y.; Yang, S.Y. Antioxidant activity of Rubus coreanus fruit extract: In comparison to green tea extract. Chonnam Med. J. 2010, 46, 148–155. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Antioxidants Suppress Apoptosis. J. Nutr. 2004, 134, 3179S–3180S. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Sánchez-Jiménez, F.M. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000, 32, 157–170. [Google Scholar] [CrossRef]
- Hiramoto, K.; Sugiyama, D.; Takahashi, Y.; Mafune, E. The amelioration effect of tranexamic acid in wrinkles induced by skin dryness. Biomed. Pharmacother. 2016, 80, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-M.; O’donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-aging potentials of methylene blue for human skin longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.J.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Chung, H.S.; Kim, S.C.; Jung, E. 7, 8-Dihydroxyflavone protects human keratinocytes against oxidative stress-induced cell damage via the ERK and PI3K/Akt-mediated Nrf2/HO-1 signaling pathways. Int. J. Mol. Med. 2014, 33, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Buddenkotte, J.; Stroh, C.; Engels, I.H.; Moormann, C.; Shpacovitch, V.M.; Seeliger, S.; Vergnolle, N.; Vestweber, D.; Luger, T.A.; Schulze-Osthoff, K. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J. Investig. Dermatol. 2005, 124, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Bellemere, G.; Stamatas, G.; Bruere, V.; Bertin, C.; Issachar, N.; Oddos, T. Antiaging action of retinol: From molecular to clinical. Skin Pharmacol. Physiol. 2009, 22, 200–209. [Google Scholar] [PubMed]
- Baek, K.S.; Yi, Y.S.; Son, Y.J.; Jeong, D.; Sung, N.Y.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. Comparison of anticancer activities of Korean Red Ginseng-derived fractions. J. Ginseng Res. 2017, 41, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Aranjani, J.M.; Pai, K.S.; Srinivasan, K.K. Promising anticancer activities of Justicia simplex D. Don. in cellular and animal models. J. Ethnopharmacol. 2017, 199, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Hong, Y.D.; Baek, K.-S.; Yoo, S.; Hong, Y.H.; Kim, J.H.; Lee, J.-O.; Kim, D.; Park, J.; Cho, J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, W.; Kim, J.; Lee, J.; Lee, I.K.; Yun, B.S.; Rhee, M.; Cho, J. Src kinase-targeted anti-inflammatory activity of davallialactone from Inonotus xeranticus in lipopolysaccharide-activated RAW264. 7 cells. Br. J. Pharmacol. 2008, 154, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Yang, W.S.; Kim, J.H.; Park, J.G.; Kim, H.G.; Ko, J.; Hong, Y.D.; Rho, H.S.; Shin, S.S.; Sung, G.-H. Lancemaside A from Codonopsis lanceolata modulates the inflammatory responses mediated by monocytes and macrophages. Mediat. Inflamm. 2014, 2014, 405158. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Hong, J.T.; Han, S.B.; Park, Y.H.; Son, D.J. Effect of Ixeris dentata Nakai extract on nitric oxide production and prostaglandin E2 generation in LPS-stimulated RAW264. 7 Cells. Immune Netw. 2015, 15, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.Y.; Kim, E.; Hwang, K.; Ratan, Z.A.; Hwang, H.; Kim, E.-M.; Kim, D.; Park, J.; Cho, J.Y. Cytoprotective Effect of Epigallocatechin Gallate (EGCG)-5′-O-α-Glucopyranoside, a Novel EGCG Derivative. Int. J. Mol. Sci. 2018, 19, 1466. https://doi.org/10.3390/ijms19051466
Han SY, Kim E, Hwang K, Ratan ZA, Hwang H, Kim E-M, Kim D, Park J, Cho JY. Cytoprotective Effect of Epigallocatechin Gallate (EGCG)-5′-O-α-Glucopyranoside, a Novel EGCG Derivative. International Journal of Molecular Sciences. 2018; 19(5):1466. https://doi.org/10.3390/ijms19051466
Chicago/Turabian StyleHan, Sang Yun, Eunji Kim, Kyeonghwan Hwang, Zubair Ahmed Ratan, Hyunsik Hwang, Eun-Mi Kim, Doman Kim, Junseong Park, and Jae Youl Cho. 2018. "Cytoprotective Effect of Epigallocatechin Gallate (EGCG)-5′-O-α-Glucopyranoside, a Novel EGCG Derivative" International Journal of Molecular Sciences 19, no. 5: 1466. https://doi.org/10.3390/ijms19051466





